Abstract
Enhancing the endurance and efficiency of polymer electrolyte membrane fuel cells (PEMFCs) requires efficient thermal management. This comprehensive review examines the primary cooling techniques employed in PEMFC systems, concentrating on techniques for air and liquid cooling. Liquid cooling, which circulates a coolant through channels adjacent to the ability of the fuel cell stack to maintain ideal operating temperatures, is highlighted and significantly reduces temperature variations, thereby improving overall efficiency and lifespan. In contrast, air cooling, while simpler and more cost-effective, is less effective in high-power applications due to its reliance on ambient air for heat dissipation. The review also discusses advancements in thermal management strategies, including innovative designs for heat exchangers and the integration of thermal resistance networks, which enhance heat dissipation efficiency. Furthermore, the paper underscores the importance of developing durable materials to address catalyst and membrane degradation, and it explores the potential for integrating PEMFCs using renewable energy sources to encourage environmentally friendly transportation solutions. By identifying current challenges and proposing future research directions, this review aims to support the continuous creation of effective and reliable PEMFC technologies.
Keywords: PEMFCs, Cooling techniques, Automotive applications, Renewable energy integration (ICE), Sustainable energy solutions
Nomenclature Abbreviations Description
| PEMFCs | Proton exchange membrane fuel cells |
| SOA | Sulfuric oxide acid |
| ICE | Internal combustion engine |
| PFSA | Perfluoro sulfonic acid |
| UVC | Ultra-thin vapor chamber |
| EW | Equivalent weight |
| SEPA | State Environment Protection Administration |
| PBI | Poly benzimidazole |
| RDE | Real driving emission |
| GO | Graphene oxide |
| BP | Bipolar plate |
| MG | Modular Galvano |
| WLTP | World harmonized light vehicle test procedure |
| O2 | Oxygen |
| EU | European Union |
| H2 | Hydrogen |
| Pt | Platinum |
| SGO | Sulfone graphite oxide |
| PGMs | Platinum group metals |
| GDL | Gas diffusion layers |
| USD | United States Department |
| PTFE | Polytetrafluoroethylene |
| Pd | Palladium |
| CL | Catalyst layer |
| Rh | Rhodium |
| MPL | Micro porous layer |
| TWC | Three-way catalysts |
| Rh | Relative humidity |
| DOC | Diesel oxidation catalysts |
| HFR | Resistance to high frequencies |
| DFF | Diesel particulate filters |
| FC | Fuel cell |
| PGS | Sheet of pyrolytic graphite |
| NAC | Nitrogen oxide adsorbed catalysts |
| AM | Additive Manufacturing |
| USDOE | United state Department of Energy |
| GEIS | Galvano Electrochemical Impedance Spectroscopy |
| MA | Mass activity |
| ORR | Oxygen reduction reaction |
| CCL | Cathode catalysts layer |
| ECSA | Electrochemically active surface area |
| ORRMA | Oxygen reduction reaction mass activity |
| ADT | Acceleration degradation testing |
| MEA | Membrane electrode assembly |
| PCMs | Phase change materials |
| IJP | Inkjet Printing |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| HT-PEMFCs | High-temperature Proton exchange membrane fuel cell |
| PEMEW | Proton exchange membrane equivalent weight |
1. Introduction
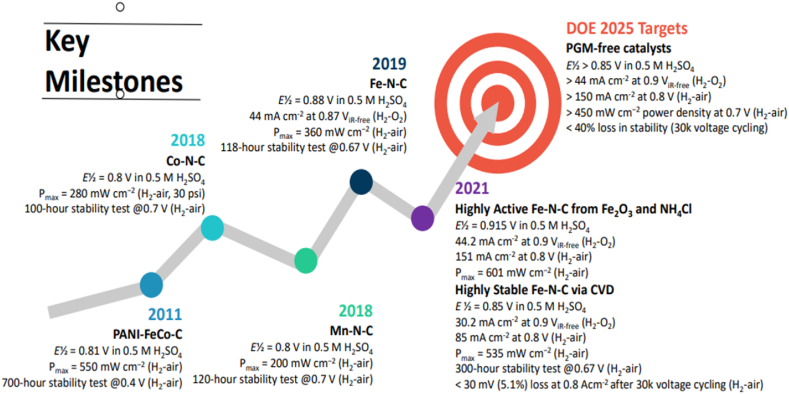
The global search for a fresh wave of alternative energy sources has gained popularity due to the escalating worries regarding environmental contamination and the energy crisis [1,2]. In recent decades, many studies on energy have focused on fuel cells, which are seen as a competitive substitute for internal combustion engines (ICEs). Having a high-power density and efficiency (up to 58 % for PEMFCs) and lacking any moving mechanical parts, Fuel cells' primary benefits are their wide operating temperature range and zero pollution [3]. Hydrogen fuel's chemical energy can be transformed into electricity by the PEMFCs. PEMFCs greatly benefit from using hydrogen as a fuel since it has a high energy content per unit mass; throughout several decades, it has been produced and managed, and the technology and well-developed rules for its secure transportation and storage [[4], [5], [6]]. Hydrogen energy includes its wide availability, renewable nature, high utilization rate, cleanliness, and environmental preservation, making it a novel energy source garnering a lot of interest; now, it is the most promising power generation device that protects the environment and has high efficiency [7]. A green energy source with PEMFCs has a high-power density and efficiency [8]. One advantage of PEMFCs is that the only products of the oxygen and hydrogen redox reaction are heat and water., which produces electricity [9]. Thus, the PEMFCs are a high-power density, high-efficiency, environmentally friendly energy source (see Fig. 4, Fig. 5).
Fig. 4.
Developing platinum group metals -free oxygen-reduction catalysts for polymer electrolyte membrane fuel cells.
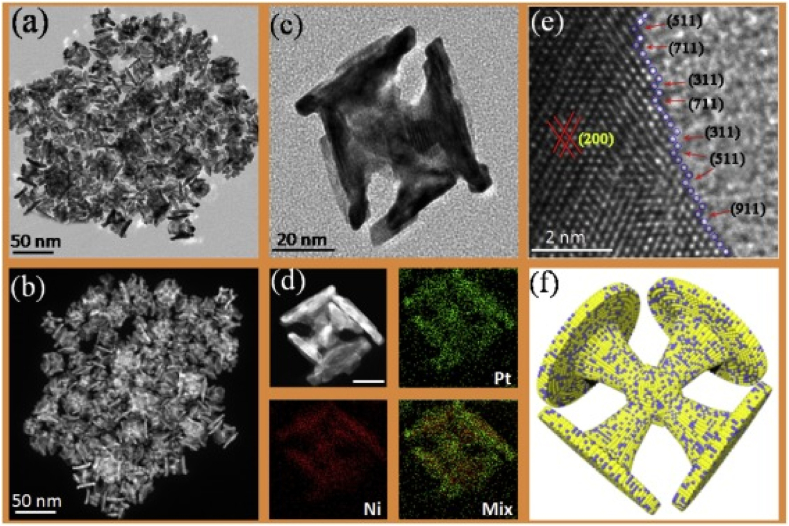
Fig. 5.
shows Pt-Ni N.P.s in the form of a dumbbell recorded by their 3D structural model (f), Stem [(b) and (d)], and Tem [(a), (c), and (e)]. The Pt-Ni/C catalyst's oxygen reduction reaction mass activity was higher than 1.3 A/mg Pt.21 [89].
On the other hand, there are certain technical problems with PEMFC application and commercialization. Water management in the PEMFCs that is appropriate has garnered significant attention as one of the major issues [10,11]. Water has two effects on the functionality of cells, making water management a crucial topic. Water hydrates increase the membrane's ionic conductivity; however, flooding results from an abundance of liquid water, which reduces the quantity of maldistribution of gases, reactant starvation, and reaction sites, all of which contribute to the cell's decreased performance [9,12,13]. The buildup of water vapor in the gas channels to an excessive degree, or GDL, is known as flooding and causes a decrease in or obstruction among the reaction sites [10]. Two effects of flooding on cell performance are an increase in pumping force and a decrease in cell voltage, lessening the impact of pressure drop, both of which result in a considerable loss of cell power [14]. Characterizing and researching PEMFC floods and other two-phase flow phenomena is essential for effective water management [9,15,16]. However, a number of obstacles have prevented their widespread use, including issues with cost, the requirement for a strong hydrogen infrastructure, and durability. For instance, a PEMFC's thermal management system has been a major obstacle to fuel cell engines being used widely [17].
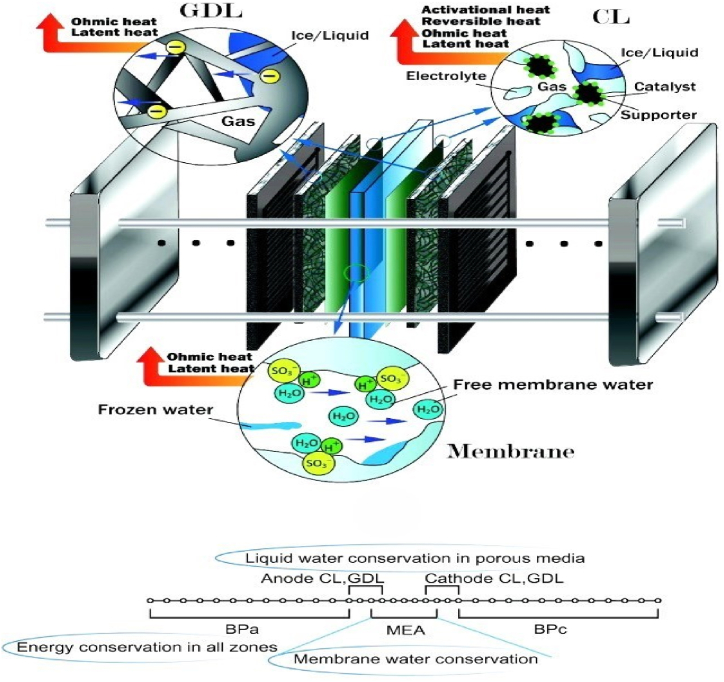
One important component of electricity is thermal management. Compared to combustion (PEMFC) operations, they are more efficient, because it has an immediate effect on efficacy, longevity, and performance. To ensure peak performance, a number of issues related to thermal management must be addressed [[18], [19], [20]]. Solving these thermal management problems calls for an all-encompassing strategy that incorporates design optimization, robust operational protocols, sophisticated materials, and system-level control techniques. Through efficient control of the PEMFC stack's temperature, moisture content, humidity, and thermal stresses, performance, efficiency, and durability can all be improved by engineers and researchers while ensuring consistent, secure performance across a range of applications [21]. This also includes heating systems, characteristics of the substance, cold start, cooling, and two-phase non-isothermal flow. Additionally, thermal analysis and fundamental ideas like variations in temperature and time constants, dependability on content qualities and related durability issues are crucial for creating and creating fuel cells with different uses in mind [22]. Two general approaches can be used to model a PEMFC two-dimensionally, as shown in Fig. 1.
Fig. 1.
Diagram showing the coolant channel proton exchange membrane fuel cell system [251].
Many researchers have recently looked into water management in PEMFCs through experimental means. Owejan et al. [23] constructed a PEMFC and examined the water distribution in the cell's channels using neutron imaging. Ali et al. [24] employed the amount utilizing a triple-serpentine PEMFC; the anode and cathode channels can be directly visualized to manually determine the amount of water accumulation in the cell's channels. Hussaini and Wang [14] investigated air stoichiometry's effects, relative humidity, and current density on cathode side flow patterns. Ous and Arcoumanis [25] observed droplets in a transparent PEMFC; they concluded that droplets originate in the middle of channels, and there isn't any visible buildup of water at the channel's end. Based on gas velocity, they also categorized droplet detachment velocities. Akhtar et al. [26] examined a number of cross-sections related to the fields of flow. Their results showed that triangle channels increased pressure drop in addition to having no positive effect on the removal of water from channels. But rectangular cross sections remove water better and result in a moderate pressure drop.
Mohammadreza et al. [6] revealed a dynamic droplet with a straight investigation of serpentine flow fields and PEM fuel cell channels. It was recommended to use filled-in and tapered channels to improve water removal from channels for the serpentine and straight flow fields, respectively. To investigate the fuel cell's performance, one must comprehend how these parameters affect both the cell's operation and the way in which water management is carried out [6]. Jeon et al. [27] used CFD to examine how relative humidity at the cathode influenced PEMFC performance for use in automobiles. Wang et al. [11] investigated how different Performance of PEMFCs was impacted by flow channel configurations. Perng et al. [28] investigated how a changed flow field could increase cell power. Comparing the cell performance to the traditional flow channel, there was an 8 % increase. Ting et al. [29] installed an Au-coated nickel foam in place of the PEMFCs' traditional flow field. They deduced that the temperature at which cells operate is the most important operating condition for cell performance, cathode stoichiometry, and cathode humidification temperature.
Nguyan et al. [30] claimed that in order to reduce ohmic loss, At the anode side, a fully humidified reactant is required and that a humidified reactant should only be used at the cathode side of the oxidant is being used, which is air. However, Cai et al. [31] research showed that membrane resistance was not significantly affected by anode humidification. However, by keeping MEA from drying out, humidification of inlet gases enhances cell performance [32]. In addition to temperature, flow field design, pressure, and stoichiometry rate, other operating conditions also have an impact on PEMFC performance when it comes to relative humidity [33]. Santarelli and Torchi [34] examined a PEMFC's behavior to make changes to six operational variables. They demonstrated how raising the humidity and temperature enhances the functionality of cells. They also concluded that the operating pressure only really matters when both sides of the cell are humidified. Williams et al. [35] stated that when the dry state in which the anode and cathode are functioning, the operating temperature and inlet flow rate are critical factors that affect cell performance. By improving electrode kinetics for anode and cathode reactions and membrane proton conductivity, the temperature is another significant influencing factor in fuel cell performance [36]. However, Increasing the cell's temperature could cause more hydrogen to cross over and membrane dehydration [37,38]. Yan et al. [39] demonstrated through experimentation that greater cell performance is correlated with higher cell temperature.
Additionally, they indicated that if the temperature inside the cell matches or exceeds the temperature at the inlet, raising it would result in a decrease in cell performance. One essential operating factor is the condition of the inlet gas flow rate that significantly affects electrode flooding, membrane dry-out, and current density distribution [40]. Performance for both single cells and stacks would improve as the cathode flow rate increases, in accordance with several experimental and numerical studies [41,42]. Several researchers have previously looked into water's behavior in PEMFCs' operational channels. However, those primarily concentrated on the analysis of flow in two phases qualitatively. Additional investigation is required to rectify the glaring lack of comprehensive and adequate quantitative characterization in the literature, two-phase flow has been evident. The validation of numerical models and single-cell/stack design both benefit from the quantitative information on two-phase flow. Refs. [43,44], the authors selected optical images of PEMFC channels by hand in order to identify channel regions, including water. Hussaini et al. [14] investigated a PEM fuel cell that uses direct optical visualization and a parallel flow field with manual selection to measure how much water accumulates in the cathode channel. To measure the amount of liquid water that accumulates in channels, they developed the "wetted area ratio" parameter. It was explained as the proportion of the entire length of the channel that held distilled water. Sugiura et al. [45] examined the direct visualization of the single-serpentine and parallel cathode channels of PEM fuel cells utilized to investigate the impact of the water absorption layer on the development of water in the flooding and channels. Yamauchi et al. [46] utilized PEMFCs with three serpentines that utilized the ability to see the anode and cathode channels directly. They manually determined the flow field's water content in order to account for inherent error and uncertainty. The authors of [14,45,46] calculated the amount of water that accumulated in channels manually.
Furthermore, they recommended using optical images and software-based image processing to increase the precision of two-phase flow quantification because this method was time-consuming. To quantify the water content in PEMFC channels and improve the accuracy of the visualization results, a few other authors employed image processing. Nirunsin et al. [47] employed image processing and optical visualization to measure the amount of water in a PEMFC's single-serpentine cathode channel. They investigated the effects of stoichiometry and temperature on the volume of water encased in the channels. Ous et al. [48] investigated the water content directly in the anode/cathode serpentine channel of the transparent PEMFC, observing variances in cell temperature, current density, and stoichiometry. They calculated each droplet's diameter, the contact angle, and Young's equation to determine how much liquid water was in the channel that was acquired through image processing.
The review of primary cooling techniques and thermal management control of the development of PEMFCs, or PEM fuel cells, will have a big impact on this field's future research. The role that effective heat management plays in improving PEMFC performance is emphasized in the paper. Focus on developing innovative cooling systems, such as advanced heat exchangers and ultra-thin vapor chambers, which can improve fuel cells' longevity and efficiency, particularly in automotive applications [49,50]. For example [51], found that an ultrathin vapor chamber (UVC) could significantly increase the PEMFC output voltage when there is a high current density; UCV improves water management, maintains temperature stability, effectively controls heat, and offers a compact design, all of which help the PEMFCs system function better for automotive applications. Similarly [52], The main advancements in this field are thoroughly examined in this review, which also offers insights into how these developments will affect sustainable transportation in the future, the explosion of advanced materials and designs that optimize water transport and retention, thereby improving overall fuel cell performance and reliability [22,52]. Commercial viability also focuses on economic aspects, including the development of cost-effective manufacturing processes and materials that can make PEMFC technology more accessible for widespread commercial use [49,50]. While advancements in materials and designs are being made, translating these innovations into economically viable production methods is still a challenge [8]. To explore market dynamics, consumer acceptance, and the impact of government policies on the commercialization of fuel cell vehicles [12].
Underscore the need for improved durability in PEMFC systems, particularly concerning catalyst and membrane degradation. Research could be directed towards developing more resilient materials and accelerated testing protocols that simulate real-world conditions, allowing for quicker identification of weaknesses and enhancements in fuel cell design [48]. Current modeling approaches for PEMFCs often do not account for all operational variables, such as humidity, temperature fluctuations, and varying load conditions. Developing more comprehensive models that can predict performance under diverse conditions is essential for optimizing fuel cell designs [4]. There is a need for improved data sharing and collaboration among researchers, manufacturers, and policymakers. Establishing databases and platforms for sharing research findings and best practices can accelerate advancements in PEMFC technology [16]. However, examine how PEMFCs can be combined with sustainable energy sources, such as solar and wind power, to that can create hybrid systems that use less fossil fuel and are more energy efficient. The scope of this research can be narrowed to specifically investigate the development and optimization of advanced cooling techniques for automotive applications using polymer electrolyte membrane fuel cells (PEMFCs). This focused approach could include a detailed analysis of innovative liquid cooling and heat exchanger designs, as well as the integration of thermal resistance networks to enhance heat dissipation efficiency. Furthermore, the study could focus on how these cooling techniques affect the robustness and efficiency of PEMFCs under varying operational conditions, thereby addressing the critical challenges of overheating and efficiency loss. By honing in on these specific areas, the study can provide targeted insights and practical solutions that contribute to the commercial viability and sustainability of fuel cell technology within the vehicle industry.
The statement emphasizes that the insights gained from reviewing the technology behind (PEMFCs) are essential for researchers. It suggests that by understanding and addressing the current challenges and limitations faced by PEMFCs, such as issues related to thermal management, water management, durability, and cost, researchers can make significant progress in improving this technology. These developments' ultimate objective is to improve the efficiency and cleanliness of energy solutions used in transportation. This suggests that improved PEMFC technology may result in more environmentally friendly automobiles that burn less fuel and emit fewer emissions, thus supporting the development of a greener and more ecologically friendly transportation industry.
2. Primary cooling techniques
The primary cooling techniques used for fuel cells with polymer electrolyte membranes (PEMFCs) are essential for maintaining optimal operating temperatures and ensuring efficient performance.
-
i
Liquid cooling: involves circulating a coolant through channels near the fuel cell stack, usually water or a water-glycol mixture. This method effectively removes heat produced in the fuel cells during the electrochemical reactions. Liquids have a high heat capacity and can be designed to fit within the limited space available in automotive applications. It makes it possible to regulate temperature precisely, which is necessary for preserving fuel cell performance and longevity and is commonly used in automotive fuel cell systems, where efficient thermal management is critical due to high power outputs. The liquid cooling system maintained optimal operating temperatures, allowing the vehicle to achieve a cold-start performance time of 5 min at a temperature of 253.15 K 18 [21]. Zhang et al. [33] reported in the study, results found that liquid cooling systems can reduce the temperature variation across fuel cell stack by as much as 30 %, significantly extending the PEMFCs' lifespan and overall efficiency.
-
ii
Air cooling: Using surrounding air, air cooling lowers the fuel cell stack temperature. Usually, fans help achieve this, or blowers that increase airflow over the stack, facilitating heat dissipation. Air cooling systems are generally simpler and less expensive to implement than liquid cooling systems and eliminate the need for a coolant reservoir and associated plumbing, reducing system complexity. Air cooling is generally less effective than liquid cooling, especially in high-power applications where significant heat generation occurs, and ambient temperature fluctuations can affect cooling efficiency. During operation, the stack temperature was kept within ideal bounds by the efficient heat dissipation provided by the air-cooling system and the creative flow field design [21]. Research indicated that air cooling systems could achieve a temperature reduction of approximately 15–20 % compared to passive cooling methods, although they are less effective than liquid cooling in high-power applications [31].
-
iii
Phase change cooling: employs materials that absorb heat during phase transitions (e.g., from solid to liquid) to manage temperature. These materials can store and release heat as they change states. Under a variety of operating conditions, it offers consistent temperature control because the phase change process can absorb substantial heat without significantly raising the temperature. Which energy storage can act as a thermal energy storage system, helping to manage heat loads during peak demand. Cai et al. [31] performance metrics study demonstrated that integrating PCMs into the cooling system could absorb up to 200 W of heat during phase transitions, maintaining the fuel cell temperature within a narrow range of ±2 °C during peak load conditions. The implementation of PCMs resulted in a 25 % increase in the thermal stability of the fuel cell stack, leading to improved efficiency and reduced degradation rates over time [32].
-
iv
Thermoelectric cooling: Utilizes Peltier elements, or thermoelectric devices, to remove heat from the fuel cell stack. When an electric current passes through these devices, it creates a temperature difference, permitting heat to escape from one side and be absorbed from another. Precise temperature control can provide precise control over the stack's fuel cell temperature. Generally, less efficient than traditional cooling methods, and the cost can be higher. The cooling process itself generates heat on the opposite side, which must also be managed. A study showed that thermoelectric cooling could keep the temperature of the fuel cell stack at 70 °C, with a 15 °C variation throughout the stack, effectively managing heat generation during high current operations [31]. According to reports, thermoelectric cooling systems' efficiency is roughly 5–10 % less than traditional liquid cooling systems, but they provide a compact solution for specific applications where space is limited [31].
-
v
Hybrid cooling systems: These cooling systems integrate several different cooling methods. (e.g., liquid and air cooling) to optimize thermal management. This approach leverages the strengths of each method to improve overall cooling efficiency [19]. By utilizing the benefits of different cooling methods, hybrid systems can achieve better thermal management than single-method systems and can be tailored to specific application requirements, allowing for more effective heat removal under varying operating conditions. A hybrid cooling system that uses both air and liquid cooling was tested in a prototype FCEV, achieving a 50 % reduction in cooling unit volume while maintaining a consistent stack temperature of 70 °C under full load conditions; this approach allowed for a significant increase in stack density and overall vehicle efficiency [33]. Zhang et al. [33] reported that the hybrid system demonstrated a 20 % improvement in thermal management efficiency compared to using liquid cooling alone, highlighting the benefits of integrating multiple cooling strategies. Each of these primary cooling techniques is essential to the successful thermal management of PEM fuel cells. Through the careful selection and optimization of the most suitable cooling technique, engineers can improve the fuel cell's longevity, performance, and efficiency, making them more viable for various applications, particularly in the automotive sector.
2.1. Heat transfer mechanisms
Heat transfer mechanisms in fuel cells with polymer electrolyte membranes (PEMFCs) are essential for maintaining optimal operating temperatures and ensuring efficient performance. The primary mechanisms in PEMFCs are conduction, convection, and radiation methods of heat transfer.
-
i
Conduction is the process by which heat is transferred through a substance without the substance moving. It happens when the solid fuel cell components experience a temperature gradient. The heat generated during electrochemical reactions is conducted through the membrane, gas diffusion layers (GDLs), and bipolar plates that make up the fuel cell. The thermal conductivity of materials used in PEMFCs, such as GDLs and membranes, significantly affects heat dissipation. Materials with higher thermal conductivity facilitate better heat transfer, reducing temperature gradients and enhancing performance [32]. Anderson et al. [10] conducted a study that optimizing the thermal conductivity of GDL materials can reduce temperature variations by up to 30 %, resulting in increased fuel cell durability and efficiency.
-
ii
Convection: the movement of fluids (gases or liquids) transferring heat. It can be natural (due to buoyancy effects) or forced (due to external means like fans or pumps). In PEMFCs, convection occurs in the coolant channels and the gas flow paths. The coolant removes heat from the fuel cell stack, while the reactant gases (hydrogen and oxygen) also transfer heat to the stack. Effective design of flow channels can enhance convective heat transfer by expanding the heat exchange surface area and promoting turbulent flow, which improves heat dissipation [34]. Ali et al. [8] According to research, convective heat transfer coefficients can be increased by 20–40 % by optimizing flow channel designs, which will greatly increase the fuel cell system's cooling efficiency.
-
iii
Radiation: the transmission of heat via electromagnetic waves. It occurs between surfaces at different temperatures and does not require a medium; radiation is generally less significant than conduction and convection in PEMFCs, but it can still play a role, especially at high temperatures or in systems with exposed surfaces. Radiative heat transfer can be affected by the fuel cell stack's and its individual parts' designs, especially with regard to surface emissivity and geometry [8]. In high-temperature applications, radiative heat transfer can account for up to 10 % of total heat transfer, necessitating consideration in thermal management strategies [8].
-
iv
Phase change: involves the transition of a substance from one state of matter to another (e.g., liquid to gas). This process can absorb or release significant amounts of heat. Phase change materials (PCMs) can be integrated into cooling systems to absorb excess heat during operation, maintaining stable temperatures within the fuel cell. The use of PCMs can help manage thermal spikes during high-load conditions, enhancing the overall thermal stability of the fuel cell [8]. Ali et al. [8] results of the experiments showed that PCMs could efficiently absorb up to 200 W of heat during phase transitions in order to maintain the peak load conditions. Designing efficient thermal management systems for PEMFCs requires an understanding of these heat transfer mechanisms. By optimizing conduction, convection, radiation, and phase change processes, fuel cell performance, efficiency, and durability can be improved by engineers and researchers. They were ultimately contributing to the advancement of clean energy technologies.
2.1.1. Emerging technologies and trends
Putting Internet of Things (IoT) technologies into practice in PEMFC systems allows for real-time monitoring and data analysis. This can lead to improved operational efficiency, predictive maintenance, and enhanced thermal management through adaptive control systems that respond to changing conditions [1]. Machine learning and artificial intelligence (AI) are being used to optimize fuel cell performance and thermal management strategies. These technologies can analyze large datasets to identify patterns and predict failures, leading to more efficient and reliable fuel cell operations.
2.1.2. Propose research questions
-
•
What innovative materials can be developed to enhance thermal conductivity in PEMFC components, and how do these materials affect overall fuel cell performance?
-
•
How can advanced cooling strategies, such as phase change materials or microchannel cooling, be optimized for PEMFC applications in automotive environments?
-
•
What are the specific degradation mechanisms of novel catalyst materials under varying operational conditions?
-
•
What are the optimal configurations for integrating PEMFCs with renewable energy sources (e.g., solar, wind) to maximize efficiency and minimize reliance on fossil fuels?
-
•
How can control algorithms be developed to manage energy flows between PEMFCs and renewable sources in hybrid systems effectively?
-
•
What cost-effective manufacturing processes can be developed for PEMFC components that maintain performance and reduce production costs?
-
•
How do market dynamics and consumer perceptions influence the adoption of PEMFC technology?
-
•
How can machine learning and artificial intelligence be utilized to improve predictive models for PEMFC performance under varying operational conditions?
-
•
What comprehensive modeling approaches can be developed to account for the interactions between different components of PEMFC systems, including thermal, electrical, and fluid dynamics?
-
•
How can the recycling and disposal processes for PEMFC components be improved to reduce their environmental footprint?
-
•
What novel water management strategies can be developed to optimize water transport and retention in PEMFCs, particularly under varying humidity and temperature conditions?
-
•
What alternative materials can effectively replace or reduce the use of platinum in PEMFC catalysts?
-
•
What design innovations can be implemented to reduce the size and complexity of PEMFC systems?
3. Materials component of fuel cells
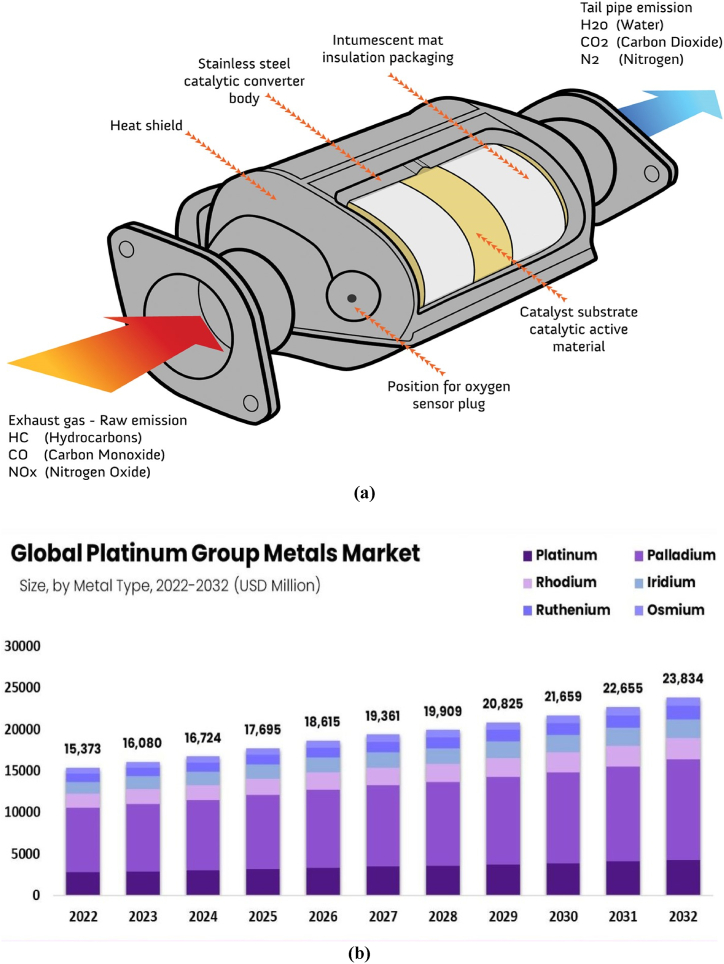
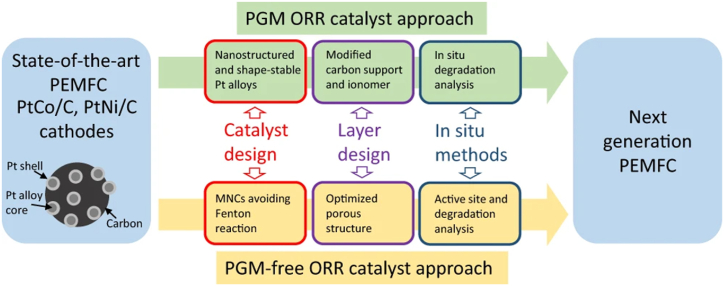
Even though PEMFC vehicles are about to become widely accepted in the commercial market due to increased demand for zero-emission transportation options, such as in China, where, to increase pollution reduction efforts, the State Environmental Protection Administration (SEPA) recently updated the rules governing emission control limits [53,54]. Automotive industries are major platinum (Pt) consumers, with catalytic converters alone accounting for approximately 40 % of the global annual production. The graphical illustration of the price trajectory of platinum group metals (PGMs) over the preceding five years, denominated in USD per troy ounce, with one troy ounce equivalent to 0.0311035 kg [55], and the schematic illustration of the automotive catalytic converter and the platinum global group market is shown in Fig. 2.
Fig. 2.
(a) Illustrates an automotive catalytic converter (b) Exploring the dynamics of the platinum group metals market.
Numerous energies and decarbonization technologies, including thermal catalytic reactors, fuel cells, electrolyzes, and catalytic converters, have been observed to employ platinum group metals (PGM) as catalysts [56,57] (see Fig. 3). Palladium (Pd) is not limited to a reduction catalyst; it can also be used as an oxidation catalyst; catalytic converters meet 80 % of the demand for PGM. In these converters, Pt (platinum) catalyzes oxidation and reduction processes [58]. Rh is indispensable in autocatalytic applications, whereas Pt and Pd can be used interchangeably. Recent research has explored substituting Pd with Pt, an economically favorable and abundant alternative to Pd. This research has gained momentum, particularly due to the rising cost of Pd and the simultaneous improvement in fuel quality, marked by reduced sulfur content [59].
Fig. 3.
Current techniques to improve oxygen reaction reduction catalyst longevity and performance for polymer electrolyte membrane fuel cells.
The two main deposit types from which PGMs are extracted globally are PGM-dominant deposits, in which Ni-Cu sulfide deposits are the main product, and PGMs are obtained as by-products from these deposits [60]. Production centers are mainly located for the former in South Africa, Zimbabwe, the US, Canada, and Russia regarding the latter [61]. The manufacturer, the vehicle's specifications (mass, engine power, fuel type diesel or gasoline), and the specific catalytic tasks that must be completed all affect how PGMs are distributed within the catalyst [59]. There are different types of catalytic converters for different kinds of vehicles. Typically, gasoline-powered vehicles use three-way catalysts (TWC). However, diesel-powered equipment typically contains NOx adsorber catalysts (NAC), particulate filters for diesel (DPF), and catalysts for diesel oxidation (DOC) [55]. By 2020, Platinum group metals (PGMs) should be loaded at a rate of no more than 0.125 mg/cm2. The United States Department of Energy (DOE) established mass activity targets (MAs) for technological goals (PGMs) pertaining to PGM catalysts with a PGM of 0.44 A/mg and catalysts devoid of PGMs; Target activities were at 0.9 % V and 44 mA/cm2 about the RHE (retractable hydrogen electrode) [62]. Even though it is safe to reduce less than 0.05 mg/cm2 of Pt is loaded at the anode [63].
Verifying the technical suitability of Additive Manufacturing (AM) materials for PEM applications requires more investigation. This involves not only comparing their performance against DOE targets but also understanding how they perform under real-world conditions, including their durability, efficiency, and resilience to external elements like corrosion and hydrogen embrittlement. Without this verification, it is challenging to ascertain the competitiveness of AM technologies in the PEM sector [64]. Making certain that the electrocatalyst's performance and stability at the cathode is of utmost importance; thus, the slow cathodic oxygen reaction reduction (ORR) has made a catalyst possessing a greater surface area and an optimized structure necessary [65]; according to Ref. [66], increasing Pt's surface area, concentrated activity, and utilization can reduce ORR losses. Pt-based catalysts are presently used in commercial PEMFCs. PEMFCs face inherent limitations in catalytic activity and stability despite their many benefits, which include outstanding power density, quick reaction times, low operating temperatures, and high energy conversion efficiency [67]. Notable advancements in this field include Pt skin segregating on alloy catalysts, the Pt monolayer development on core-shell nanoparticles, and Pt in nanoparticle form dispersing on carbon black with a large surface area [68,69].
The current generation of ORR catalysts is based on de-alloyed nanoparticles of Pt-Co (platinum cobalt) or Pt-Ni (platinum nickel), which yield an approximate A/mg mass-specific activity for Pt PGM equal to 0.6 [69]. Concurrently, the quest for innovative catalyst materials continues to be a focal point aimed at bolstering overall PEMFC performance [67]. At the anode and cathode of PEMFCs, Pt and its alloys are currently the most commonly used catalysts. Stronger materials for catalysts have been developed, and the creation of stronger catalysts typically leads to improved electrochemical reactions within the fuel cells, resulting in higher power output and efficiency. Too often more resistant to degradation, which can prolong the lifespan of fuel cells and reduce maintenance costs.
The development of advanced catalysts aligns with the growing emphasis on sustainable energy solutions, as more efficient fuel cells can contribute to lower emissions and reduced reliance on fossil fuels; investigation of novel materials to find and create new catalyst materials that are more sustainable and affordable than conventional platinum-based catalysts, such as composite materials or non-precious metal catalysts, and that perform as well or better. Investigating the effects of nano structuring on catalyst performance could lead to enhanced surface area and reactivity, improving the overall efficiency of PEMFCs. Research could explore various nanofabrication methods to optimize catalyst morphology and examine the synergistic effects of combining stronger catalysts with advanced membrane materials that enhance proton conductivity and reduce crossover, leading to improved overall fuel cell performance [14]. Creating uniform testing procedures to assess the stability over the long run and the way novel catalyst materials perform in actual operating environments may offer important new information about their potential uses and explore the potential of hybrid catalyst systems that combine different materials to leverage their unique properties, potentially leading to enhanced functionality and robustness. As the demand for PEMFCs grows, there is a growing emphasis on materials sustainability used in their production. Research into recycling methods for fuel cell components, such as catalysts and membranes, is becoming more prominent, aligning with the principles of a circular economy [14]. Researchers can continue to progress in the field of PEMFC technology by pursuing these, contributing to the creation of sustainable and more effective energy solutions, particularly for the cathode and anode. Although platinum-based catalysts have been widely used, attempts are being made to replace or reduce platinum with more affordable and readily available materials. This not only extends the lifespan of fuel cells but also lowers production costs.
3.1. Potential risks associated with new catalyst materials and their effect on PEMFC functionality
The creation and application of novel catalyst materials for Proton Exchange Membrane Fuel Cells (PEMFCs) hold significant promise for enhancing performance, reducing costs, and improving sustainability. On the other hand, novel catalyst materials might not be as stable as conventional catalysts based on platinum. Performance can deteriorate due to factors like sintering, leaching, or corrosion [10] over time, influencing the fuel cell's overall longevity, and novel catalysts may be more sensitive to variations in temperature, humidity, and other operating conditions, which can lead to inconsistent performance and reduced efficiency under real-world conditions [14]. While new materials may be designed to reduce reliance on precious metals, they might not achieve the same level of catalytic activity as platinum. This may lead to a decrease in fuel cell performance overall, especially when current densities are high and efficiency is crucial [16]. With new catalysts, the electrochemical reactions' kinetics might not be as favorable, increasing overpotentials and decreasing power output [19].
There could be problems like catalyst poisoning or membrane deterioration if new catalyst materials are incompatible with the materials used for the membrane and electrode [35], which may negatively impact the performance of fuel cells. The physical and chemical properties of new catalysts may not align with the design requirements of current PEMFC systems, necessitating redesigns that could introduce additional complexities and costs [35]. The goal of developing new catalysts is often to reduce costs; the initial production processes for novel materials may be expensive or complex, limiting their commercial viability. These challenges reduce end-user acceptance of the technology and impede its commercialization; over the past three decades, significant efforts have been made by industry and academia to address the PEM challenges through research and develop dependable, reasonably priced technology [4,138].
The ability to produce new catalyst materials at scale while maintaining consistent quality and performance can be a significant hurdle, potentially delaying their adoption in PEMFC applications [14]. Trabold et al. [15] stated that new catalyst materials may pose environmental or health risks during production, use, or disposal. This could lead to regulatory challenges and public resistance to their adoption. The availability of raw materials for new catalysts may be limited or subject to geopolitical risks, which could impact supply chains and long-term sustainability [14]. The performance of new catalyst materials may not be fully understood until they are tested in real-world conditions. This uncertainty can lead to unexpected challenges and setbacks in fuel cell development [14]. The process of developing, testing, and commercializing new catalyst materials can be lengthy, potentially delaying advancements in PEMFC technology [18]. While new catalyst materials present exciting opportunities for improving PEMFCs' performance, it is imperative to thoroughly evaluate the possible hazards and disadvantages linked to their utilization. Addressing these challenges through research, testing, and collaboration between material scientists, engineers, and manufacturers will be crucial for ensuring that new catalysts can effectively enhance the performance and viability of PEMFCs in various applications. Balancing innovation with practical considerations will be key to advancing fuel cell technology efficiently and sustainably.
3.1.1. PGM alloys
Pt-based ternary and binary electrocatalysts, as a result of in-depth research on phosphoric acid fuel cells, were created near the end of the 20th century. When using stationary fuel cells for commercial purposes at 190 °C, these electrocatalysts showed remarkable durability, surpassing 40,000 h of use [70]. The most widely used binary alloys today are Pt-Co/C and Pt-Ni/C, as a result of PEMFC's smooth integration of the insights developed from these achievements. Shukla et al. [71] used Inkjet Printing IJP to deposit Pt/C catalyst on the Nafion membrane, resulting in a thin electrode with low platinum loading for the PEMFC anode and cathode. SEM demonstrated the ink-jetted CL to have a porous structure made of Pt/C aggregates bound by the Nafion ionomer; compared to the traditional spray-coated MEA, the MEA that was created using an ink-jetted electrode demonstrated superior catalyst mass activity. Towne et al. [72] work showed that IJP could create MEA for PEMFC. In this work, a desktop inkjet printer was used to deposit a Pt/C catalyst solution onto the Nafion membrane. It has been reported that IJP does not require a post-deposition hot-press step in order to form a mechanically stable CL with excellent adherence performance. Taylor et al. [73] used IJP to deposit Pt/C catalyst onto GDL in order to create an electrode for PEMFC. With the same overall platinum loading, the fabricated electrodes outperformed their conventionally manufactured counterparts. Bezerra et al. [74] An alloying platinum with an effective method to increase oxygen reaction reduction (ORR) is to add cobalt, iron, chromium, and nickel. Process [[75], [76], [77], [78], [79]]. Minimizing the ORR enhancement effect is the aim in Pt-Co alloy catalysts, where base metal leaching during operation is prevented by intentionally keeping the base metal content low (about 8 mol%) [80]. Reducing the concentration of the CCL and altering the proton-conducting ionomer's transport properties inside the CCL, this leaching lowers the catalyst's ORR activity and negatively impacts the ORR kinetics [81].
Furthermore, during fuel cell polarization, within the CCL, the base metal leached may be redistributed [82]. Base metals have an inherent tendency to migrate toward the surface of an alloy; this phenomenon is driven by surface segregation and is challenging to prevent. One major concern for PEMFCs is base metal leaching into the ionomer, as their cathodes have a lower percentage of ionomer (about 30 %) than liquid acid-based fuel cells. Pre-leaching is commonly applied to Pt alloys used in PEMFCs to reduce this worry by removing excess CO from the surface. This pre-leaching step minimizes the long-term increase in catalyst layer resistance, often called protonic resistance. Additionally, PEMFC cathodes can employ smaller Pt particles, given that the operating temperature remains below 90 °C. Several of these catalysts are in commercial use currently, exemplified by Pt-Co catalysts employed in Toyota's fuel cell vehicles [83]. Nevertheless, ongoing research explores diverse Pt-CO nanoparticle structures [[84], [85], [86], [87], [88]]. Present investigations are notably concentrated on advancing Pt-Ni alloys, with numerous studies detailing the synthesis of octahedral nanoparticles [89,90]. [91] Successfully synthesized dumbbell-shaped Pt-Ni particles. Interestingly, these particles did not exhibit an increase in electrochemically active surface area (ECSA). However, they demonstrated superior oxygen reduction reaction mass activity (ORRMA). After accelerated degradation testing (ADT), they maintained a high level of activity retention in comparison to spherical Pt-Ni nanoparticles [91] Pt-Ir [[92], [93], [94], [95]], Pt-Ag [88], Pt-Bi [92], Pt-Cu [85,89], Pt-Fe [[96], [97], [98]], Pt-Se [88], Pt-Te [78], and Pt-Zn [85]. are among the other binary alloys under investigation.
Similar research was done on ternary Pt alloys that contained the Fe, Ni, and CO transition metals. Ultimately, they found that the Pt-Co-Fe combination offered the best possible balance of activity and durability [99]. Furthermore, research has been done on several quaternary alloys [100,101] and ternaries [[102], [103], [104], [105], [106], [107]]. Suggested a Pt-rare earth catalyst [108]. However, this catalyst is not employed in extensive applications because of its relatively low abundance. Other studies focused on Pt nitrogenation, phosphorization [109,110], and alloying.
3.1.2. Improved membrane materials
A PEM fuel cell's membrane is crucial to ion exchange and operation. Novel membrane materials with enhanced chemical stability, mechanical durability, and reduced susceptibility to degradation due to moisture and contaminants have been developed, and these membranes are essential for prolonged stack life. Most current membrane development initiatives have concentrated on various domains, each with a distinct strategy for lowering fuel cell system (FCS) costs. These strategies consist of (i) using more affordable membrane materials, (ii) increasing the number of components of conductive membranes, and (iii) developing membranes suitable for use in drier and hotter environments [111]. Non-perfluorinated ionomer membranes are in the category of economically viable materials. This selection has been propagated for its potential to reduce costs compared to the prevailing perfluorinated membrane alternatives [108,112]. However, it's important to remember that these hydrocarbon-based membranes are generally less strong and have poorer electrical conductivity than SOA perfluoro sulfonic acid (PFSA) membranes, even though they are less expensive [108]. Low equivalent weight (EW) ionomers have been the focus of research into better membrane materials to increase proton conductivity [113]. Creating ionomers with this is demonstrated by multiple acid groups per side chain work [114]. Higher IEC membranes swell more, which poses a mechanical durability challenge for these materials [115], as well as multi-acid side chain ionomers have issues with their chemical stability [116]. The [2,2-(m-phenylene) poly]-5,5-benzimidazole membranes subjected to phosphoric acid treatment (PBI) exhibit exceptional mechanical strength, excellent resistance, and noteworthy thermal stability against chemical degradation. However, this affordable replacement functions flawlessly as high-temperature (HT-PEMFC) membranes [117,118]. Despite its many benefits, one of the disadvantages of HT-PEMFC technology is the slow depletion of phosphoric acid over time. This decline decreases the membrane's proton conductivity, resulting in a decline in cell performance. Over time, this issue may also exacerbate the degradation of other cell components [119,120]. Acid loss in PBI membranes has been effectively mitigated by well-structured composite membranes incorporating inorganic materials, according to reports by Refs. [[121], [122], [123]] (see Table 1).
Table 1.
Advantages and disadvantages of existing cooling techniques and thermal management strategies.
| Cooling techniques | Advantages | Disadvantages |
|---|---|---|
| Air cooling |
|
|
| Liquid cooling |
|
|
| Phase Change Cooling |
|
|
| Hybrid cooling systems |
|
|
According to recent research, graphene oxide, or GO, is promising concerning membrane composites PEMFCs. GO is a soft material with an impressive range of chemical and physical characteristics, a large surface area, and an amphiphilic nature. It combines oxygen (O2) with hydrophilic domains like carboxylic acid, hydroxyl, and epoxy. These hydrophilic domains and acidic groups encourage electron hopping and GO hydration [124]. Additionally, tests revealed that adding GO additives improved the performance compared to using the PBI membrane alone [125]. PBI/Graphite Oxide Sulfonated (GOS)and PBI/GO membranes were created in high-precise PEMFCs [126]. The combined membranes SGO/PBI and GO/PBI outperformed the Phosphoric Acid/PBI membrane in PEMFC tests. PBI/GO and PBI/power densities were found with substantial phosphoric acid loadings between 380 and 600 mW/cm2, respectively, in H2/O2 conditions [126]. Likewise, [127]. A solitary cell experiment compared PBI and PBI/GO membranes using an Alkaline Direct Methanol Fuel Cell (ADMFC) operating between 60 and 80 °C was conducted using PBI/GO membranes [128]. In commercial fuel cells, papers with carbon content are frequently utilized as the substance for gas diffusion layers (GDLs); their composition consists of unwoven carbon fibers, typically with a diameter ranging from 1 to 10 μm [129,130]. Hydrophobic properties are typically induced in GDLs by incorporating Polytetrafluoroethylene (PTFE), effectively reducing electrode flooding [[131], [132], [133]]. Jingke et al. [134,135] created titanium mesh as GDL with high electric and thermal conductivities and exceptional corrosion resistance using EBM for use in PEMWE. Modular Galvano (MG) was used to characterize the GDL's performance in-situ and ex-situ using X-ray diffraction (XRD), scanning electron microscopy (SEM), and Galvano Electrochemical Impedance Spectroscopy (GEIS). When the 3D-printed GDL was compared to the traditional woven and sintered one, it demonstrated an 8 % improvement in electrolysis performance because of the notable decrease in ohmic losses. According to the authors, EBM is a quick and affordable fabrication technique that can create GDL with specialized pore morphology and extremely complex 3D shapes. Research has hypothesized that changes in the spatial distribution of PTFE loading may impact the movement of liquid water [136,137]. A micro-porous layer (MPL) with predominant pore sizes smaller than 0.5 μm needs to be added to improve this interaction between the layer of the (CL) and gas diffusion (GDL) [138]. It's been demonstrated that this MPL reduces electrode flooding and ohmic losses, thereby improving the junction of the graphene dioxide layer (GDL) and the catalyst layer (CL); it comprises PTFE-sealed individual carbon black particles. Removing the need for a cathode humidifier or allowing higher temperatures to be operated at conductivity-exhibiting membranes in dry or anhydrous proton conductors, which enable a smaller radiator, can lower the system costs. These methods include adding highly conductive elements, like acid-functionalized inorganic materials [139,140] or hetero-poly acids, or using additives designed to hold water in low relative humidity (RH) environments [141]. Furthermore, various reasons exist for operating under wet conditions beyond boosting membrane conductivity, including reduced susceptibility to catalyst impurities [142,143] and decreased losses in recoverable voltage [144]. Table 2 summarizes the thermal conductivity range for different materials (see Table 3).
Table 2.
Materials, thermal properties, and heat transfer for fuel cell components.
| S/No | Ref. | Materials | Components | Thickness (mm) | Temperature coefficient (W/m°C) | Heat Capacity () |
|---|---|---|---|---|---|---|
| 1 | Qin et al. [144], Feijie et al. [145] and Andersson et al. [146] | Nafion | PEM | 0.01-0.1 | Nafion 0.2- 0.95 | 1.65 |
| 2 | The porous layer of ionomer, carbon support, and pt or alloy catalyst | CL | 0.00–0.01 | 1.5 - 0.2 | 1.69 | |
| 3 | Qin et al. [144] and Yang Yu et al. [147] | Paper with hydrophobic porous carbon fiber | GDL | 0.3 - 0.1 | Toray paper is 0.3–0.2, while SGL paper is 1.2–0.25 |
|
| 4 | Mancusi et al. [148] Yicheng et al. [149] | A hydrophobic porous layer composed of carbon black and PTFE binders | MPL | 0.05 | 0.3 - 0.05 | – |
| 5 | Yicheng et al. [149] and Ozden et al. [150] | Plate of graphite | BP | 0.3-0.2 | H2∼0.17: Vapor; 30 for graphite; 17 for Titanium; 16.3 for SS316L |
1.57 graphite Titanium H2: 14400; Vapor:2000 |
| 6 | Han et al. [151] | H2 + H2O in the cathode channel and H2O in the anode | GFC | 0.3–1 | 0.024; O2/N2: | N2: 1041; O2:917(J ) |
Table 3.
Mainstream fuel cell vehicle configuration's cold-start performance.
| Type of vehicle | Output power (kW) | Power density of stack (kW/L) | Temperature in Kelvin (K) | Time (min) | Cooling technique | Ref. |
|---|---|---|---|---|---|---|
| Hyundai Tucson | 100 | 1.65 | 253.15 | 5 | Water evaporation | [145,150] |
| Roewe 950 | 36 | 253.15 | 5 | Water evaporation | ||
| Saic MAXUSFCV 80 | 115 | 3.10 | 243.15 | 5 | Water evaporation | [156] |
| Toyota Mirai | 114/128 | 3.10/4.4 | 253.15 | 5 | A three-dimensional mesh flow field and pain plate encircle the flow of coolant water. | [156] |
| Honda FCX Clarity 2015 | 130 | 0.5–1.0 kW per liter (kW/L) or higher | 253.15 | 5 | H2 and O2 GFCs are perpendicular to the coolant water channel. |
[156] |
| Honda FCX Clarity 2017 | 103 | 3.12 | 253.15 | 5 | Channel for coolant water | [144,156] |
4. Thermal resistance networks
Thermal resistance networks are a crucial analytical tool in understanding and optimizing Proton Exchange Membrane Fuel Cells' (PEMFCs') thermal management. These networks are useful for simulating the fuel cell system's internal heat transfer mechanisms, permitting the evaluation of thermal performance and the identification of potential thermal management issues.
-
i
Concept of thermal resistance networks: This is analogous to an electrical circuit, where heat transfer is treated similarly to electrical current flow. In this analogy, thermal resistances represent the opposition to heat flow, similar to electrical resistances, and temperature differences drive the heat flow, akin to voltage differences in electrical circuits.
-
ii
Modelling the thermal resistance network: Generally, the following procedures are taken in order to analyze the thermal resistance network in PEMFC systems:(i) Determine the various paths through which heat is transferred within the PEMFCs, including through the membrane, bipolar plates, and coolant channels. (ii) For each component, calculate the thermal resistances based on material properties (thermal conductivity, specific heat), geometry (thickness, surface area), and operating conditions (temperature, flow rates). (iii) Create a thermal resistance network diagram that visually represents the resistances and their connections. This diagram helps in understanding how heat flows through the system.
-
iii
Solving the thermal resistance network: Once the thermal resistance network is established, it can be analyzed using the following methods: (i) Node Analysis: Apply Kirchhoff's laws to the thermal network, where the sum of heat flows into a node equals the sum of heat flows out. This allows for the calculation of temperature at various points in the network. (ii) Analyze the network under steady-state conditions (where temperatures are constant) and transient conditions (where temperatures change over time). This is crucial for understanding how the PEMFC responds to varying operational conditions. (iii) Utilize computational tools and software to model the PEMFC system's thermal behavior, such as MATLAB, ANSYS fluent, and COMSOL. These tools can handle complex geometries and transient conditions more effectively than manual calculations.
-
iv
Applications of thermal resistance network analysis: By identifying thermal bottlenecks, engineers can optimize the design of PEMFC systems to enhance thermal management, thereby improving efficiency and longevity; the analysis helps in developing effective cooling strategies, such as the choice of suitable coolant fluids and the layout of cooling channels, and understanding the thermal behavior of PEMFCs can aid in predicting potential failure modes related to overheating or thermal cycling, allowing for proactive design improvements, researchers and the thermal dynamics of fuel cells can provide engineers with important new information, leading to improved designs and enhanced performance. This analysis is essential for ensuring that PEMFCs operate efficiently under varying conditions, ultimately contributing to their viability in practical applications.
5. Dynamics control and cold start
The Nafion membrane's ability to absorb water causes it to hydrate over time; depending on the particular process, different phase transitions and liquid drainage time constants apply major power loss [152], and output voltage overshoot or undershoot [152] are caused by the fundamental multi-timescale phenomena. Material qualities, such as species diffusivity, thermal conductivity, porosity, membrane water uptake, and component design, significantly influence the dynamics of fuel cells. Particularly, fast oxygen transport, anode re-hydration, and membrane hydration are characteristics of thin membranes and GDL [153]. Dursch et al. [152,154] explored the fluctuating actions of ice crystals, isothermal galvanostatic cold-starts, and the development and growth of ice crystal ions at PEMFCs' catalytic layer using isothermal differential calorimetry. The isothermal ice nucleation and crystallization rates are derived from measurements of the time of induction and heat flow. The time constant needed to heat a fuel cell to 273.15 K was attained by carefully considering material and fuel cell design.
The study on cold starts also ascertained the ionomer during cold start [104]. Bipolar plate BPs provide a significant amount of the fuel cell's thermal mass. One of the fundamental parameters for evaluating cold-start failures is these two-time constants' ratio. Furthermore, Fuel cells' limited application is due to their cold start. Fuel cell-produced water expands when it freezes, which could impact the stack's internal structure and lead to the collapse and thickening of the catalytic layer's pores [155]. It also looks at how different startup strategies affect the stack's cold start, focusing on the temperature differential between the stack and the ice volume percentage of the catalytic layer [104], examines the important parameters, including the time constants, and obtains the two primary ice-related voltage loss mechanisms' mathematical solutions and contrasted their impacts.
5.1. Water management approach
PEMFCs have been noted as an alternative substitute for energy conversion across various domains, including transportation, portability, and stationary power applications. The things that make them attractive are their high efficiency (>50 %), higher power density, and low operating temperatures (<100 °C) [152]. Nevertheless, the challenges encountered by PEM fuel cells, particularly those employing perfluoro sulfonic acid (PFSA) type membranes where hydration is important, is effective water management, which is crucial to ensure both good ionic conductivity [157,158] and the fuel cell's durability [159,160]. For PEM fuel cells, efficient water management is essential since too much water can cause membrane deterioration and decreased efficiency; improved water management strategies have been developed, including advanced design features and control algorithms. The system may become unstable if water droplets gather in flow field channels [[160], [161], [162], [163]], the flow field's pressure drop increases [164,165], and an imbalance in the flow distribution [166,167]. Following these occurrences, there may be a decline in fuel cell efficiency [168,169] and long-term performance degradation [[170], [171], [172], [173]]. Improving the fuel cell system's performance, efficiency, and control requires optimizing its water balance. Enhancing water resource management increases the membrane's length, avoids pore blockages, and accelerates the catalyst's electrochemical reactions in the GDL. Moreover, cold-start conditions are created by controlling the water levels in PEMFC stacks, is to allow PEMFCs to operate even in extremely cold temperatures [174]. Below 0 °C, inside the cell, the hydrogen-oxygen reaction produces water, which freezes. The stack will become non-reactive, and if ice blocks the diffusion layer or covers the catalytic layer's surface, stopping the catalytic layer (CL) from being reached by gas, the startup will fail. Water possibly impacts the stack's internal structure because it gets bigger when it freezes. Common damages [155] include Pt particle coarsening, agglomeration, and the densification and collapse of the CL's pores.
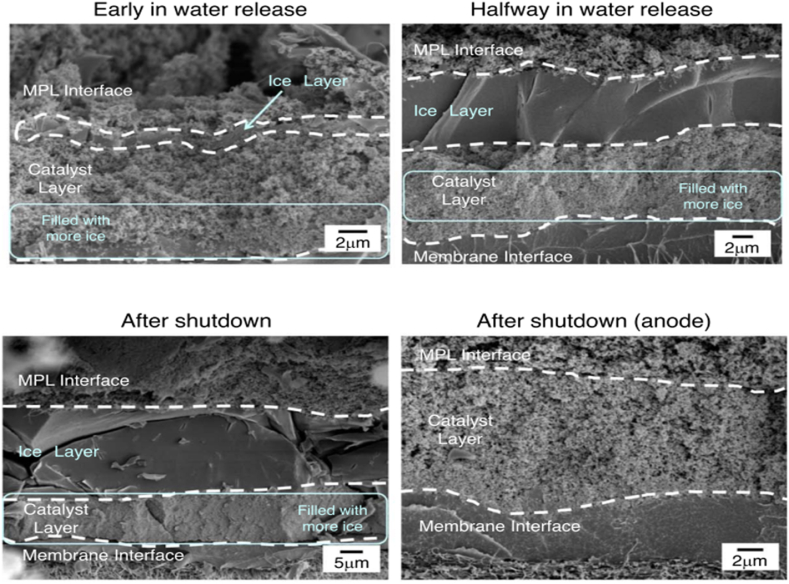
Liquid water that is present or builds up is necessary to move the gas porosity through the electrodes and flow fields GDL into the flow field's flow channels before being exhausted from the system [175]. The flow channels, CL active sites, GDL, and CL pores would all clog if this wasn't done. This flooding phenomenon leads to significantly worse performance from PEM fuel cells. Consequently, electrode flooding is associated with high current density functioning when more water is produced than removed. Despite this, operational factors influence the degree of flooding and its consequences, especially when there is a low gas flow rate or low temperature [176] or when the channels are not immediately cleared of liquid water [177,178]. The interdependent relationship between the performance of cells and coupled mass and heat transfer is one of the primary obstacles to comprehending water management and moisture level; two-phase flow with multiple components that involve phase transition in porous media, the interaction of the gas diffusion layer (GDL) and the gas channel (GC) [146]. The electrochemical reaction produces water and has the potential to condense into liquid, potentially inundating the microporous layer (MPL), catalyst layer, GDL, or GC, reducing catalyst layer performance by obstructing reactant supply. The incomplete knowledge of the fundamental mechanisms underlying the development of PEMFCs is further hampered by the movement and evolution of liquid water [146] (see Fig. 6).
Fig. 6.
Cross-sections of a catalyst layer obtained by cryo-sem with two currents [104,151,152].
Furthermore, the characteristics of the MEA are also important in this case; changing the flow field bipolar plate design of the product is anticipated to be a common solution to the flooding problem of PEM fuel cells. Equal mass movement between the GDL and the catalyst layer, control beyond pressure drops, and a reduction in the requirement for auxiliary systems such as pumps and fans are all made possible by the flow field design of PEMFCs. Fig. 7 shows the coolant flow fields and channel design that efficiently transfer the heat generated by the system for the coolant flow and bipolar plates [179]. PEMFCs are primarily designed with three channel configurations in mind: flow fields that are interdigitated, parallel, and serpentine [180]. Table 4 summarizes all the different designs and their merits and drawbacks, as stated in the literature.
Fig. 7.
Current density's impact on water balance.
Table 4.
lists all the various designs and their merits and drawbacks.
| Ref. | Flow field type | Explanation | Merit | Demerit | Schematic diagram |
|---|---|---|---|---|---|
| Qin et al. [144], Ji et al. [181], Passogulleri et al. [182], Tran et al. [183] | Parallel | The reactant flows through multiple channels that are either horizontally or vertically parallel to one another. |
|
|
 |
| Yang et al. [147] and Ahmad et al [184]. | altered parallel design with a tapering tip | Its tapered inlet channel is a design feature. |
|
Inadequate water management under intense operating circumstances |  |
| Yang et al. [147] | Redesigned parallel design with a crisscrossed channel route | intended to resemble a parallel straight channel with a crisscrossing direction of gas flow |
|
|
 |
| Ahmad et al [184]. | Serpentine | The fuel cell's active area continuously fills with reactive gases flowing through the channel. |
|
|
 |
| Mohammad Reza et al. [185] | Numerous interconnected serpentine passageways | Reactants can reach the catalyst layer and react through various single serpentine channel designs. |
|
|
 |
| Ahmad et al. [184] and Tran et al. [183] | combining parallel and serpentine design | Alteration of several channels with the primary goal of accelerating the rate at which reactants enter the cell's active region |
|
|
 |
| Tang et al. [183] | Serial connections in the serpentine winding channel | This is another serpentine design variation where the channel path splits into multiple sections connected in series. |
|
|
 |
| Qin et al. [144], Ahmad et al. [184] and Yang et al. [147] | Interdigitated | The reactive materials are forced into GDL via flow paths that are dead-ended and irregular. |
|
|
 |
| Tang et al. [183] | The design of diagonally interdigitated | Large channel paths diagonally merge with smaller channel paths. |
|
Problems with water management are still being researched and developed through experimentation. |  |
| Jing et al. [186] | Digitalized spiral design | This represents a change to the interdigitated design. | The humidity load is reduced because of the channels at the inlet flow into the channels at the exit. | Reactants avoid the gas channel at the inlet and outlet. |  |
The advantages of a small channel pressure drop and simplicity are provided by parallel flow-field design [181,182]. However, this design is less effective in high-humidity environments because it may result in excessive flooding without convective gas flow [187]. Liquid water convectively exits the gas channels and the electrode more easily when the serpentine's flow fields produce a high gas velocity and pressure drop [188,189]. Nevertheless, the rise in parasitic power losses, usually connected to compressors or blowers, is one of its drawbacks [190,191]. Eventually, this causes the fuel cell system's efficiency to decrease. On the other hand, in interdigitated flow fields, dead ends in gas channels force the gas to pass through the GDL, which removes any water accumulation in liquid form below the surface [192,193].
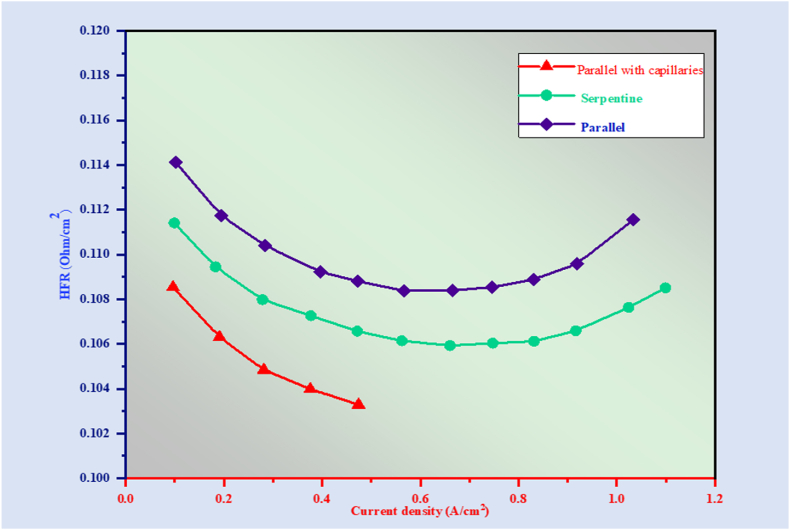
An important pressure drop happens throughout the channel due to the interdigitated geometry directly associated with the serpentine pattern. One of the main causes of this pressure drop is forced convection through the GDL [194,195]. Additionally, the slower gas velocity in interdigitated flow fields typically indicates a lot of water stored in the channel [196]. For this reason, the serpentine configuration is still the suggested choice for commercial fuel cell systems' flow fields [197,198]. Several innovative water management strategies, like flow fields with microgrooves or triangle microchannels, have been developed in response to the difficulty of efficiently using flow-field geometry alone to manage liquid water [199,200]. The integration of water transport channels for dehydration and cooling into porous carbon flow fields has been tested [[201], [202], [203]]. The pressure differential between the two channels causes the pore water to evaporate into the gas channel when porous carbon flow fields are applied in dry environments, supplying humidity within. This is what causes the liquid water to be transferred from the internal water transport channels into the gas channels [204,205]. Wicks designed to stop liquid slug formation are also tested, whether mounted [206,207] or engineered directly onto the surface of a flow field's channel [208,209]. The performance of fuel cells is enhanced by wicking element integration through improved water management; however, flooding still happens, particularly mid-range of current density. This implies that not all of the product water will be able to be transported by the wicks under specific operating circumstances, potentially due to an inadequate gas pressure gradient [210,211]. Capillaries serve two purposes concerning the membrane electrode assembly's (MEA) current local state under saturated gas streams: they can either wick away produced water using the electrode or evaporate to provide humidity. In addition, capillaries offer less resistance to interfacial contact than porous carbon plates and are more readily incorporated into a more diverse range of flow field designs, such as complex stamping plates [[212], [213], [214]]. High-frequency resistance (HFR) measurements ensured uniform membrane hydration and consistent inter-facial contact resistance across all flow-field types. Enough membrane hydration is necessary for efficient oxygen mass transport and appropriate proton conduction [[215], [216], [217]]. The production of liquid water raises the membrane's degree of hydration, which causes the highest rate of return value measured at 0.1 A/cm2 to decline gradually; the local temperature at the MEA was above average [[218], [219], [220]] and enhances water evaporation, which causes the membrane to be twitchily dehydrated when current densities are higher (>0.9 A/cm2). This small change in the HFR value could result in drops of 1.6 mV and 0.5 A/cm2 at 2.7 mV, altering the parallel flow field for the capillary and making changes in the serpentine flow field devoid of capillaries, respectively, as opposed to the field of parallel flow [221].
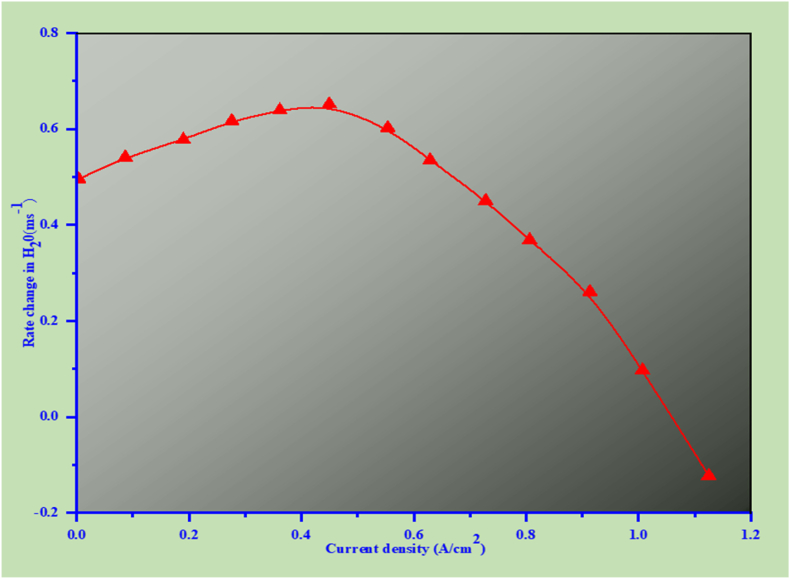
The effect of current density on the water equilibrium in a fuel cell with a polymer electrolyte membrane (PEMFC) is shown in Fig. 7. The illustration of how different current densities impact the flow and control of water within the fuel cell is significant; the relationship between the fuel cell's water transfer rate and current density (measured in A/cm2) is essential for preserving optimal performance. The electrochemical reactions occurring at the cathode cause an increase in water generation as current density does. The water balance is critical because both excess water (flooding) and insufficient water (drying) can lead to performance degradation [4].
Two essential processes are wicking, which is the transfer of water from the transport channels to the electrodes, and evaporation, which is the return of water from the electrodes to the transport channels. A positive mass change rate indicates wicking, where water is effectively transported to the electrodes, enhancing performance. Conversely, a negative mass change rate indicates evaporation, which may cause the membrane to dry out and lose efficiency in the particular current density ranges where water management is especially important [37]. For instance, at low current densities, wicking is more effective, while at mid-range current densities, the balance may shift, leading to potential flooding or drying issues [221]. Understanding these dynamics helps in designing better water management strategies within PEMFCs, ensuring that the fuel cell operates efficiently across various load conditions. Gas diffusion layers (GDLs) and water transport channels can be designed with fuel cell design considerations in mind to maximize water management. For instance, it is possible to create materials and geometries that improve wicking at low current densities or stop flooding at high current densities. Optimizing the lifespan and performance of PEMFCs requires effective water management, particularly in automotive applications where operational conditions can vary widely.
The water generated is drawn via capillaries and the water transport channel (0.5 A/cm2) or lower current densities. The gas and water transport lines have different pressures. Increased water generation at the cathode due to increased wicking rate increases with current density [221]. It is a concern that insufficient water management causes irreversible performance degradation over the whole current density range. It is demonstrated by us that when the wicking direction current density is at mid-range values (0.6 ≤ j ≤ 8 A/cm2), In low-current situations, wicking through capillary channels effectively lessens flooding—additionally, the net rate of water transfer declines. Irreversible performance degradation is primarily caused by ineffective water management, impacting the current density range. This demonstrates that wicking through capillary channels at low current densities successfully addresses flooding concerns [221]. Unfortunately, the intermediate current values are between 0.6 and 0.8 A/cm2, and there is a drop in the total amount of water transferred in the direction of wicking, evaporation which is mostly caused by the cell's high temperature, which is the main process that moves distilled water throughout everything in the cell above 0.8 A/cm2. This observation is consistent with the HFR data [222,223], which show membrane dehydration above 0.8 A/cm2 thresholds. Several studies show that the cell's temperature may increase by 283.15 K or greater when current densities are high, which could result in significant membrane dehydration [223]. By understanding this relationship, researchers and engineers can develop more effective strategies for managing water within fuel cells, ultimately leading to improved performance and durability. Enhanced captions for the figure should include specific details about the processes depicted, the implications for fuel cell operation, and the significance of managing water resources in connection to current density.
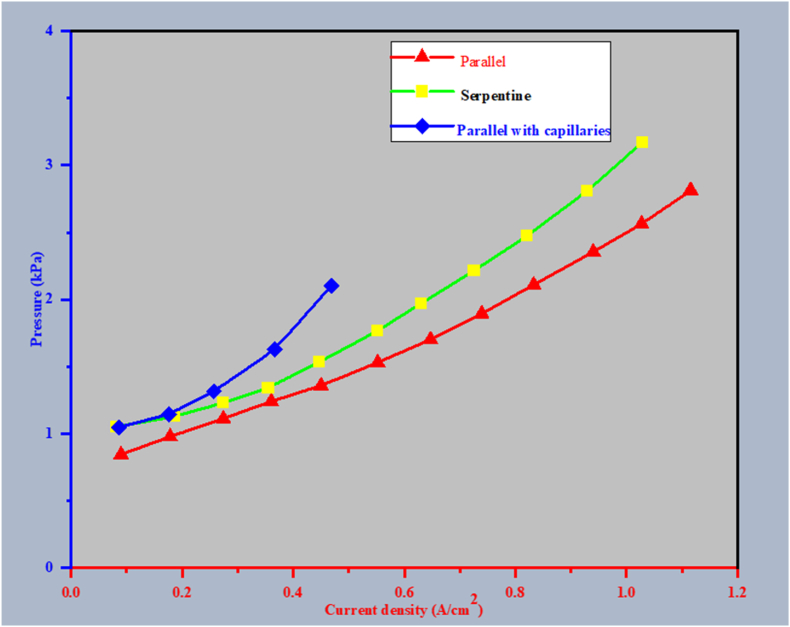
Concerning the decreased pressure, Fig. 8 shows that the maximum value among the unmodified parallel circulation field occurs at low current densities before flooding begins. Previous studies found that coiled passageways had a pressure drop that was much greater than parallel ones [190,191,196], additionally demonstrating the former's longer channel path and faster gas flow. This result contradicts these earlier conclusions; the discrepancy could be credited to the surplus distilled water gathered within the gas channels and bifurcating manifolds, restricting and obstructing the gas flow path [190]. A comparable decrease in pressure was observed at 0.1 A cm2; the flow fields in parallel and serpentine, where flooding issues are significantly reduced. This result implies that the manifolds' additional hydraulic resistance that split apart contributes notably to the pressure decrease and the field of parallel movement. The flow field in parallel displays the least decrease in pressure. The excellent water management maintains the natural benefit of the flow-field design in parallel and, additionally, discrete capillary layout, and the capillaries should ideally be added to the field of parallel flow for controlling liquid water while effectively maintaining a low-pressure decline from the perspective of operating costs (see Fig. 9).
Fig. 8.
Current density's effect on the rate of water change.
Fig. 9.
Current density's impact on pressure.
6. Stack thermal management
PEMFCs have advanced to the early stages of commercialization, especially in the transportation industry, where well-known automakers like Mercedes-Benz, Honda, Toyota, and Audi have released fuel-cell cars [224]. The most challenging problems with car integration usually involve water and heat management [225]. The low durability and high cost of PEMFCs are among the constraints that hinder their widespread commercialization [8,149] and are the primary issues preventing PEMFCs from being commercialized further. The PEMFC, as mentioned earlier, limitations can be addressed with effective heat management, and maintaining the proper operating temperature is essential for maximum efficiency. Innovative techniques, for instance, integrating heat exchangers and cooling systems for thermal control, have been employed to regulate stack temperature efficiently due to significant heat generation. PEMFC thermal management becomes extremely difficult for automotive engines with power over 100 kW [226,227]. As a result of that, there are two main objectives that PEMFC thermal management in automobile vehicles needs to achieve. To improve PEM fuel cell thermal management even more, particularly for FCEV applications that must contend with space, budgetary, and operational variability like a cold start.
Enhancing fuel cell materials is the first step toward reducing temperature variation, reducing thermal stress, and preventing connected deterioration of materials. In the through-plane direction, some commercial GDL materials possess minimal heat conductivity, which could result in a significant regional temperature variation. Additionally, the variation in in-plane temperature is increased by the channel land configuration. Therefore, GDL materials should have thermal conductivity through and in-plane directions, lessening the variation in temperature across space. By enhancing the solid matrix's thermal characteristics, flow fields with porous media have also demonstrated promise in fuel cell electric vehicle (FCEV) applications. This can minimize temperature fluctuations within and between planes in a fuel cell.
Secondly, sophisticated cooling techniques are imperative to enhance heat removal and lower the FCEVs' cooling unit volume. It will take more investigation to find a novel way to use a single cooling system to serve multiple cells. Increasing the number of fuel cells in each cooling system allows for a roughly 50 % reduction in the cooling mechanism's volume. It is leading to a significant increase in stack density of power. Conversely, it has been demonstrated that this kind of cooling technique considerably raises local temperatures in nearby local cells, not reducing the cooling unit's stack and cell efficiency. The fuel cell and cooling strategy design components must be optimized to make the most of this strategy. PEMFCs require less driving energy for heat dissipation because of the heat differential; compared to internal combustion engines, there is less space between the fuel cell stack and the surrounding air (ICEs) [[225], [226], [227], [228]]. To meet thermal management requirements, choosing an appropriate cooling method requires determining the amount of heat produced by PEMFCs.
As the heat produced by the FCs is equivalent to the amount of electric strength generation, the primary issue with heat is being produced and removed from the stacks by PEMFCs, as noted in Refs. [229,230]. Under realistically suitable operating conditions, the usual limit for energy conversion efficiency is 50 % Fig. 2a. Estimating the heat of the PEMFCs output is the first step; the next step is to select the cooling method that best meets the requirements for thermal control. The main problems with PEMFCs are heat generation and removal from stacks because fuel cells produce nearly as much heat as they do electrical power. This results in an approximate 50 % energy conversion efficiency limit under realistic operating conditions, the common limit [231,232] Fig. 2a. It is necessary to remove the nearly 100 kW of heat dissipation rate that an automobile FC engine with 100 kW of power would have a multitude of factors impacts PEMFCs heat generation [233]. These consist of the following: (i) heat from condensation of water vapor; (ii) irreversible reactions; (iii) resistance measured in ohms; (iv) reversible heat coming from electrochemical reactions, sometimes referred to as entropic heat. To keep the temperature of the fuel cells at the proper level, the heat produced by these electrochemical reactions must be efficiently evacuated. Phase change, liquid cooling, cooling with an edge, various airflows, utilizing a cathode air supply, and air cooling are a few methods for managing heat.
Depending on certain factors and situations, these cooling techniques have benefits, drawbacks, and challenges [234]. One of the previously mentioned cooling methods dissipates most heat produced in PEMFCs. Controlling the heat produced by the PEMFCs is not as crucial as maintaining the stack's proper operating temperature and temperature uniformity; cooling systems ensure the PEMFC's long-term survival and safety by maintaining a steady temperature [235,236]. This is true because the temperature at which the system operates and the temperature's constancy inside the piles immediately impact PEMFC functionality and lifespan. Effective heat management requires establishing a constant temperature distribution and preserving an ideal operating temperature. Reliability, energy conversion efficiency, and output performance are all significantly impacted by the PEMFC stack's operating temperature and temperature distribution [237,238]. Adding liquid cooling systems is useful for improving the open-cathode thermal management PEMFC stack. This procedure increases cooling capacity and produces a uniform temperature distribution [239]. They employed pyrolytic graphite sheet (PGS), a thermally conductive material, to enhance the thermal control of PEMFCs. The PGS application resulted in a lower maximum cell temperature and a more even temperature distribution, improving cell functionality at elevated cathode flow rates. In Ref. [240], 3 °C materials whose thermal conductivity was more than 1000 W/(m·K) were employed to maintain a low, typically below. Unfortunately, these incredibly high thermal conductivity materials are often very costly. Due to its high cost, it is not practical for widespread commercial use in PEMFCs.
An open-anode PEMFC stack's heat pipe can be integrated for effective thermal management. Heat pipes have been identified as a possible fuel cell thermal management solution. Mohammad Reza et al. [241] demonstrated how heat pipes can improve fuel cells by promoting a consistent temperature distribution within the stack of PEMFCs and swiftly eliminating waste heat [242] using three steel-mesh heat pipe wicks to keep fuel cells cool. These three pipes for heat effectively fulfilled the necessary dissipation of heat requirements while preserving the operating range of temperatures, according to the results of their experiments. It is crucial to remember that during their investigation, The heat-transmitting pipe was positioned precisely between two graphite plates to replicate the heat-producing capacity of the fuel cell; the heat flow within the PEMFCs stack configuration was not considered. Paula et al. [243] outlined the development of a condominium stainless heating pipe and a heat transfer model for controlling fuel cell temperature; the proposed flat warmth pipe would ensure that the PEMFCs operate as intended by dispersing up to 12 W of thermal energy. Heat pipes improved the energy source stack's energy conversion efficiency, but there were still several problems with the research that needed to be fixed. These three problems are outlined below: To accurately replicate the movement of the heat process within the stack of PEMFCs, it will not be enough to use a skin warmer to replicate the temperature generation within the gasoline cell stack or to look into the integration of fuel cell-equipped heat pipes system using simulation techniques. It still requires extensive experimental research to incorporate heat pipes into stacks of fuel cells; (ii) the mass of the heat pipe system can significantly impede the portability of advancements in thermal management for open-cathode PEMFCs stacks; (iii) Prior research on fuel cell thermal management used cylindrical heat pipes, which may make them challenging to integrate with the most popular flat fuel cell stack design [244]. One unique kind of heat pipe that can assist in resolving these problems is the ultra-thin vapor chamber (UVC). By incorporating the UVC into an open cathode, the system's portability is preserved, and the requirements for thermal management are satisfied [245].
6.1. Enhancing durability
Enhancing fuel cell technology and fuel cell stack durability is essential. Systems for various uses, including mobile electronics, stationary power generation, and the automotive industry, must be dependable, durable, and economically viable. Fuel-related problems in cell stack durability, such as catalyst degradation, membrane degradation, and corrosion of components, can majorly affect the stack's lifetime and performance.
6.2. Accelerated testing protocols
In order to assess the longevity, effectiveness, and durability of these emission control devices under accelerated aging conditions, accelerated testing protocols for catalytic converters are crucial. Accelerated testing allows researchers and manufacturers to simulate years of real-world driving conditions in a shorter time frame, enabling faster development cycles, improved quality control, and a better understanding of degradation mechanisms. There are now accelerated testing protocols in place to evaluate durability more accurately. These protocols simulate long-term operating conditions, allowing researchers to identify potential weaknesses and design improvements in fuel cell stacks.
6.3. Reduction of carbon corrosion
Reduction of carbon corrosion is essential in improving the robustness and functionality of (PEMFCs) for automotive applications. The severe electrochemical environment found inside fuel cells leads to carbon corrosion, which erodes fuel cell components and lowers overall efficiency. To mitigate carbon corrosion, implementation of the following strategies: (i) advanced coatings: introducing modern coatings on carbon-based materials to protect against corrosion and maintain the integrity of fuel cell components [246], (ii) novel materials: Development of novel carbon supports and corrosion-resistant materials to minimize Carbon corrosion's effects on fuel cell performance [246], (iii) Optimized Operating Conditions: Implementing optimized operating conditions and control strategies to reduce the likelihood of carbon corrosion in PEMFC stacks [53]. Addressing carbon corrosion through these strategies improves the durability and longevity of PEMFCs, guaranteeing dependable performance in automotive applications and assisting in the broad fuel cell technology adoption as a clean energy alternative.
6.4. Efficiency gains
When developing (PEMFCs) are crucial for improving fuel economy, reducing waste heat generation, and enhancing overall system performance. The study has focused on various strategies to achieve efficiency gains in PEMFC technology for automotive applications, including optimized cell design; efforts have been directed towards optimizing the configuration of each fuel cell stack cell to enhance reactant dispersion and minimize parasitic losses caused by pressure drops [246]. Reduction of internal resistances by lowering internal resistances within the fuel cell stack through advancements in manufacturing processes and materials, particularly beneficial when catalyst loading is low and enhancements in bipolar plate materials and coatings to control water in the stack, promote reactant flow, and decrease electrical resistance [247]. Efficient handling of oxygen and hydrogen through sophisticated control systems and algorithms to ensure proper reactant supply and timing for peak performance [247]. Implementing these efficiency-enhancing strategies will maximize the performance of PEMFCs, increase energy conversion efficiency, and reduce operational costs, ultimately making fuel cell technology more competitive and sustainable for automotive applications.
6.5. Optimized cell design
Optimized Optimizing the cell design is crucial for improving performance additionally efficiency (PEMFCs) for automotive applications. Optimizing cell design enhances reactant distribution, minimizes parasitic losses, and improves overall fuel cell stack performance. The key aspects of optimized cell design in PEMFCs include designing patterns and structures in the flow field to guarantee the consistent dispersal of reactants (oxygen and hydrogen) across the membrane electrode assembly, promoting efficient electrochemical reactions [247]. Implementing design modifications to reduce pressure drops and optimize flow paths within the fuel cell stack, minimizing energy losses and improving overall efficiency. Configuring the layout and arrangement of individual cells within the stack to maximize power output, minimize internal resistances, and enhance overall system performance [247]. Incorporating advanced materials for Membranes, gas diffusion layers, and catalyst layers are examples of components that enhance the fuel cell stack's durability, conductivity, and overall efficiency [1]. This will enhance the performance, durability, and efficiency of PEMFCs, making them more competitive and reliable for automotive applications. These design advancements generally advance the potential application of fuel cell technology's application in the transportation sector.
6.6. Reducing internal resistances
Reducing internal resistances is the major strategy to enhance the efficiency and performance of (PEMFCs) for automotive applications. They minimized internal resistances significantly better the electrochemical reactions inside the stack of fuel cells, resulting in enhanced system functionality and energy conversion efficiency. Key approaches to reducing internal resistances in PEMFCs include advancements in manufacturing techniques and materials selection to lower internal resistances and strengthen the conductivity of the fuel cell stacks' constituent parts. Fine-tuning the catalyst layers, membrane properties, and electrode interfaces promotes efficient electrochemical reactions and reduces resistive losses within the fuel cell stack [248]. Designing electrodes with improved conductivity and surface area facilitates faster reaction kinetics and minimizes internal resistances in the fuel cell stack [91,92]. Implementing effective thermal management systems to maintain the fuel cell stack's operating temperatures at their ideal levels, reducing resistive losses and improving overall efficiency [101]. Addressing the internal resistances through these strategies, the performance, durability, and efficiency of PEMFCs make them more competitive and reliable for promoting automotive applications. These efforts help fuel cell technology progress and increase its ability to produce energy sustainably solutions in the transportation sector. Considerable industrial and academic research has been dedicated to addressing the PEM fuel cell challenges over the last thirty years and creating dependable, affordable technology. The majority of scholarly research concentrated on design development elements where different experimental, to determine the ideal configuration and operational parameters for these technologies, modeling and optimization techniques were applied [4,66,249]. Conversely, there hasn't been much research done on the manufacturing and assembly procedures for PEM components. Actually, it's thought that the primary obstacles preventing PEM technologies from being widely commercialized are the manufacturing difficulties [250].
6.7. Reactant management
Efficient reactant Management is necessary to maximize the effectiveness and performance of (PEMFCs) in-vehicle applications. Proper handling of reactants, such as oxygen and hydrogen, ensures that Reactant management in PEMFCs primarily focuses on maximizing power output when the fuel cell is operating at maximum efficiency, implementing control systems and algorithms to ensure the appropriate fuel cell stack reactant supply at the appropriate flow rate rates and timings for efficient electrochemical reactions [88]. We are designing flow field structures and gas distribution systems to disperse reactants throughout the membrane electrode assembly uniformly, promoting uniform reaction rates and minimizing concentration gradients [246], monitoring and controlling the pressure of reactant gases to preserve ideal functioning circumstances inside the fuel cell stack, preventing flooding or starvation issues that may affect output. Managing the stack of fuel cells' water content to avoid flooding the membrane and maintain proton conductivity is crucial for efficient reactant transport and electrochemical reactions [3]. They effectively manage reactants in PEMFCs, improving power output and prolonging fuel cell components' lifespan. These initiatives support the general progress within fuel cell technology for car use, providing the transportation industry with a more viable and long-lasting energy source.
7. Challenges of cooling PEMFC
The fuel cell stack and its surroundings have very little temperature differences, and cooling proton exchange membrane fuel cells (PEMFCs) pose special challenges. These difficulties may result in decreased performance, overheating, and inefficiencies. It is challenging to transfer heat effectively because of the coolant's small temperature difference from the fuel cell stack. This may result in the stack having hotspots and an uneven temperature distribution [46]. Traditional cooling methods may not be effective due to the low driving force for heat transfer, resulting in inadequate cooling performance. High heat generation PEMFCs can generate significant heat, especially under high power output conditions. Managing this heat is critical to maintaining optimal operating temperatures; rapid changes in temperature can cause thermal stress, resulting in material deterioration and decreased fuel cell component durability. The size and complexity of cooling systems are restricted by the compact design of automotive applications, making it challenging to implement effective cooling solutions. PEMFCs are sensitive to low temperatures, which can hinder performance during cold starts. Effective heating and cooling strategies are needed to manage this.
7.1. Proposed solutions and strategies
Incorporating phase change materials, PCMs can help absorb excess heat during operation and release it when temperatures drop, maintaining a more stable temperature within the fuel cell. By expanding the heat exchange surface area, the use of microchannel heat exchangers can improve heat transfer efficiency and improving fluid flow dynamics, designing compact and efficient heat exchangers can improve the cooling capacity without requiring significant space. This can include using finned or plate-type heat exchangers to maximize surface area. Implementing active cooling systems, such as liquid cooling with pumps and fans, can enhance heat removal, especially during peak power demands; using materials with high thermal conductivity for components like bipolar plates and gas diffusion layers can facilitate better heat distribution and reduce hotspots and proper insulation of the fuel cell stack can help maintain optimal operating temperatures and reduce heat loss to the environment.
Optimizing cooling performance can be achieved by implementing sophisticated control algorithms that modify cooling in response to real-time temperature data. This includes varying coolant flow rates and temperatures based on operational conditions, utilizing sensors and data analytics to predict thermal behavior and potential overheating can help in proactive management of cooling systems. However, Optimizing the design of flow fields to enhance coolant distribution and minimize thermal gradients can improve overall cooling efficiency, and developing modular cooling systems that can be easily integrated and scaled in accordance with the particular needs of the fuel cell application can improve performance and flexibility. Effectively cooling PEMFCs with minimal temperature differences requires a multifaceted approach that combines advanced cooling techniques, material innovations, and optimized design strategies. By addressing the challenges associated with heat management, these solutions can enhance the performance, durability, and reliability of PEMFC systems, making them more viable for automotive and other applications.
7.1.1. Limitations of current thermal management practices
There are a number of issues with the way that proton exchange membrane fuel cells (PEMFCs) are currently managed that could compromise their longevity, effectiveness, and performance. Addressing these limitations is crucial for advancing PEMFC technology, particularly in automotive and other high-demand applications. Ineffective heat transfer may result from the coolant's and the fuel cell stack's slight temperature differences, resulting in hotspots and uneven temperature distribution [46]; many existing cooling methods, such as conventional liquid cooling, may not provide sufficient heat removal under high power output conditions and a few of the materials that go into making the bipolar plates and gas diffusion layers (GDLs) of PEMFCs, have low thermal conductivity, which can impede effective heat distribution and management. Current materials may not withstand the thermal stresses associated with rapid temperature changes, resulting in deterioration and a shorter fuel cell component lifespan; the size and complexity of thermal management systems are constrained by the compact nature of automotive applications, making it challenging to implement effective cooling solutions, and integrating thermal management systems with other vehicle systems can complicate design and operation, leading to potential inefficiencies. Improving thermal management practices in PEMFCs is essential for enhancing their performance, efficiency, and durability. By addressing the limitations of current practices and exploring potential areas for improvement, researchers and engineers can develop more effective thermal management solutions that support the widespread adoption of PEMFC technology in various applications, particularly in the automotive sector.
8. Conclusion
The conclusion synthesizes the advancements discussed throughout the study and identifies specific avenues for further investigation into the field of polymer electrolyte membrane fuel cells (PEMFCs). The review summarizes the substantial advancements in cooling methods and thermal control techniques that are crucial for enhancing PEMFC longevity and performance. The creation of novel cooling techniques is one of the breakthroughs, the incorporation of cutting-edge materials, and improved heat transfer mechanisms, all of which contribute to enhanced operational efficiency and longevity of fuel cells. Future research will take more into passive and active cooling systems to find more efficient ways to dissipate heat. Research should aim to develop hybrid cooling solutions that combine the benefits of both approaches to maximize efficiency. Investigating new materials with superior thermal conductivity and durability is crucial. This should prioritize the development of materials that can withstand thermal stresses and improve overall heat management in PEMFCs; additionally, investigate the creation of hybrid systems that improve energy efficiency and lessen dependency on fossil fuels by integrating PEMFCs with renewable energy sources like solar and wind. Implementing accelerated testing protocols that simulate real-world conditions will be vital for identifying weaknesses in current designs and materials. This strategy can address the financial aspects of PEMFC technology and expedite the development of fuel cell technology, focusing on cost-effective manufacturing processes and materials that can make PEMFCs more accessible for widespread commercial use. By addressing these areas, researchers can significantly contribute to the advancement of PEMFC technology, ensuring its viability as a sustainable alternative to internal combustion engines (ICE). The ongoing development in this field holds the promise of delivering high-efficiency, low-emission energy solutions that align with global sustainability goals.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Abubakar Unguwanrimi Yakubu: Writing – original draft. Jiahao Zhao: Data curation, Huang Wangsen, Methodology. Qi Jiang: Conceptualization. Xuanhong Ye: Project administration. Junyi Liu: Data curation. Qinglong Yu: Resources. Shusheng Xiong: Supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work and that there is no professional or other personal interest of any nature or kind in any product, service, or company that could be construed as influencing the position presented in the review of the manuscript entitled “A comprehensive review of primary cooling techniques and thermal management strategies for polymer electrolyte membrane fuel cells PEMFCs”
Acknowledgment
2022YFB2502403 is the project number for the New Energy Vehicle National Key Research and Development Program. The same applies to the Provincial Cutting-Edge, Leading Talent Research and Development Program for 2023, proposal ID: 2023C01239. The People's Republic of China provides funding for the project.
References
- 1.Zhao Jing, Qifei Jian, Huang Zipeng. Experimental study on heat transfer performance of vapor chambers with potential applications in thermal management of proton exchange membrane fuel cells. Appl. Therm. Eng. 2020;180 [Google Scholar]
- 2.Thompson A.G. Olabi. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC) Energy. 2019;179:246–267. [Google Scholar]
- 3.Hasheminasab Mohammadreza, et al. vol. 46278. American Society of Mechanical Engineers; 2014. Simultaneous investigation of PEMFC performance and water content at different flow rates and relative humidities. (International Conference on Nanochannels, Microchannels, and Minichannels). [Google Scholar]
- 4.Baroutaji A., et al. PEMFC poly-generation systems: developments, merits, and challenges. Sustainability. 2021;13 (2021. [Google Scholar]
- 5.Olabi A.G., Bahri A., Abdlghafar A.A., Baroutaji A., Taha Sayed E., Hai Alami A., Rezk H., Abdelkarim M. Large-scale hydrogen production and storage technologies: current status and future directions. Int. J. Hydrogen Energy. 2021;46:23498–23528. [Google Scholar]
- 6.Mazloomi K., Gomes C. Hydrogen as an energy carrier: prospects and challenges. Renew. Sustain. Energy Rev. 2012;16:3024–3033. [Google Scholar]
- 7.Zhang T., Wang P., Chen H., Pei P. A review of automotive proton exchange membrane fuel cell degradation under start-stop operating condition. Appl. Energy. 2018;223:249–262. [Google Scholar]
- 8.Bozorgnezhad, Ali, et al. "The experimental study of water accumulation in PEMFC cathode channel." Fluids engineering division summer meeting. Vol. 57212. (American Society of Mechanical Engineers).
- 9.Srinivasan S. Springer Science Business Media, LLC; New York: 2006. Fuel Cells: Fundamentals to Applications. [Google Scholar]
- 10.Anderson R., Zhang L., Ding Y., Blanco M., Bi X., Wilkinson D.P. A critical review of two-phase flow in gas flow channels of proton exchange membrane fuel cells. J. Power Sources. 2010;195:4531e53. [Google Scholar]
- 11.Xiao-Dong Wang, Wei-Mon Yan, Yuan-Yuan Duan, Fang-Boor Weng, Jung Gouin, Chi-Yuan Lee. Numerical study on channel size effect for proton exchange membrane fuel cell with serpentine flow field. Energy Convers Manage. 2010;51:959–968. [Google Scholar]
- 12.Mench M.M. John Wiley & Sons, Inc; New Jersey: 2008. Fuel Cell Engines. [Google Scholar]
- 13.Pasaogullari U., Wang C.-Y. Two-phase modeling and flooding prediction of polymer electrolyte fuel cells. J. Electrochem. Soc. 2005;152 [Google Scholar]
- 14.Hussaini I.S., Wang C.-Y. Visualization and quantification of cathode channel flooding in PEM fuel cells. J. Power Sources. 2009;187 [Google Scholar]
- 15.Trabold T.A. Mini channels in polymer electrolyte membrane fuel cells. Heat Transf Eng. 2005;26 [Google Scholar]
- 16.Li H., Tang Y., Wang Z., Shi Z., Wu S., Song D., et al. A review of water flooding issues in the proton exchange membrane fuel cell. J. Power Sources. 2008;178:103e17. [Google Scholar]
- 17.Cao Tao-Feng, et al. Numerical investigation of the coupled water and thermal management in PEM fuel cell. Appl. Energy. 2013;112:1115–1125. [Google Scholar]
- 18.Jeong Kwi Seong, Oh Byeong Soo. Fuel economy and life-cycle cost analysis of a fuel cell hybrid vehicle. J. Power Sources. 2002;105(1):58–65. [Google Scholar]
- 19.Hoogers Gregor., editor. Fuel Cell Technology Handbook. CRC Press; 2002. [Google Scholar]
- 20.O'hayre Ryan, et al. John Wiley & Sons; 2016. Fuel Cell Fundamentals. [Google Scholar]
- 21.Bargal Mohamed HS., et al. Liquid cooling techniques in proton exchange membrane fuel cell stacks: a detailed survey. Alex. Eng. J. 2020;59(2):635–655. [Google Scholar]
- 22.Luo Lizhong, Qifei Jian. Experimental study and mitigation of abnormal behavior of cell voltage in a proton exchange membrane fuel cell stack. Int. J. Energy Res. 2019;43(5):1912–1923. [Google Scholar]
- 23.Owejan J.P., Gagliardo J.J., Sergi J.M., Kandlikar S.G., Trabold T.A. Water management studies in PEM fuel cells, part I: fuel cell design and in situ water distributions. Int. J. Hydrogen Energy. 2009;34(8):3436–3444. doi: 10.1016/j.ijhydene.2008.12.100. [DOI] [Google Scholar]
- 24.Bozorgnezhad Ali, et al. Experimental study of the effect of inlet flow parameters on the operation of PEMFC. Modares Mechanical Engineering. 2014;14(5):33–43. [Google Scholar]
- 25.Ous T., Arcoumanis C. Visualization of water droplets during the operation of PEM fuel cells. J. Power Sources. 2007;173(1):137–148. doi: 10.1016/j.jpowsour.2007.04.075. [DOI] [Google Scholar]
- 26.Akhtar N., Qureshi A., Scholta J., Hartnig C., Messerschmidt M., Lehnert W. Investigation of water droplet kinetics and optimization of channel geometry for PEM fuel cell cathodes. Int. J. Hydrogen Energy. 2009;34(7):3104–3111. doi: 10.1016/j.ethylene.2009.01.022. [DOI] [Google Scholar]
- 27.Jeon D.H., et al. The effect of relative humidity of the cathode on the performance and the uniformity of PEM fuel cells. Int. J. Hydrogen Energy. 2011;36(19):12499–12511. [Google Scholar]
- 28.Shiang-Wuu Perng, Wu Horng-Wen, Ren-Hung Wang. Effect of modified flow field on non-isothermal transport characteristics and cell performance of a PEMFC. Energy Convers Manage. 2014;80:87–96. [Google Scholar]
- 29.Ting F., Hsieh C.-W., Weng W.-H., Lin J.-C. Effect of operational parameters on the performance of PEMFC assembled with Au-coated Ni-foam. Int. J. Hydrogen Energy. 2012;37:13691–13703. [Google Scholar]
- 30.Nguyen T.V., White R.E. A water and heat management model for proton exchange membrane fuel cells. J. Electrochem. Soc. 1993;140:2178–2186. [Google Scholar]
- 31.Cai Y., et al. Effect of water transport properties on a PEM fuel cell operating with dry hydrogen. Electrochim. Acta. 2006;51(28):6361–6366. [Google Scholar]
- 32.Iranzo A., et al. Experimental fuel cell performance analysis under different operating conditions and bipolar plate designs. Int. J. Hydrogen Energy. 2010;35(20):11437–11447. [Google Scholar]
- 33.Zhang J., et al. Pem Fuel Cell Testing and Diagnosis. Elsevier; Amsterdam: 2013. Chapter 8 – relative humidity (RH) effects on PEM fuel cells; pp. 201–223. [Google Scholar]
- 34.Santarelli M.G., Torchio M.F. Experimental analysis of the effects of the operating variables on the performance of a single PEMFC. Energy Convers Manage. 2007;48:40–51. [Google Scholar]
- 35.Williams M.V., Kunz H.R., Fenton J.M. Operation of NafionÒ-based PEM fuel cells with no external humidification: influence of operating conditions and gas diffusion layers. J. Power Sources. 2004;135(1–2):122–134. [Google Scholar]
- 36.Zhang J., et al. Pem Fuel Cell Testing and Diagnosis. Elsevier; Amsterdam: 2013. Chapter 4 – the effects of temperature on PEM fuel cell kinetics and performance; pp. 121–141. [Google Scholar]
- 37.Kanani Homayoon, et al. Model development and optimization of operating conditions to maximize PEMFC performance by response surface methodology. Energy Convers. Manag. 2015;93:9–22. [Google Scholar]
- 38.Yu W.-L., Wu S.-J., Shiah S.-W. Experimental analysis of dynamic characteristics on the PEM fuel cell stack by using the Taguchi approach with neural networks. Int. J. Hydrogen Energy. 2010;35(20):11138–11147. [Google Scholar]
- 39.Yan W.-M., et al. Analysis of thermal and water management with temperature-dependent diffusion effects in the membrane of proton exchange membrane fuel cells. J. Power Sources. 2004;129(2):127–137. [Google Scholar]
- 40.Cheng X., et al. Hydrogen crossover in high-temperature PEM fuel cells. J. Power Sources. 2007;167(1):25–31. [Google Scholar]
- 41.Yan M., Chen Y., Sheng M., Soong Y., Chen F. Effects of operating conditions on cell performance of PEM fuel cells with conventional or interdigitated flow field. J. Power Sources. 2006;162(2):1157–1164. [Google Scholar]
- 42.Guvelioglu G.H., Stenger H.G. Flow rate and humidification effects on a PEM fuel cell performance and operation. J. Power Sources. 2007;163(2):882–891. [Google Scholar]
- 43.Scholta J., et al. Development and performance of a 10 kW PEMFC stack. J. Power Sources. 2004;127(1–2):206–212. [Google Scholar]
- 44.Santarelli M.G., et al. Experimental analysis of cathode flow stoichiometry on the electrical performance of a PEMFC stack. Int. J. Hydrogen Energy. 2007;32(6):710–716. [Google Scholar]
- 45.Sugiura K., Nakata M., Yodo T., Nishiguchi Y., Yamauchi M., Itoh Y. Evaluation of a cathode gas channel with a water absorption layer/waste channel in a PEFC by using visualization technique. J. Power Sources. 2005;145:526e33. [Google Scholar]
- 46.Yamauchi M., Sugiura K., Yamauchi T., Taniguchi T., Itoh Y. Proposal for an optimum water management method using two-pole simultaneous measurement. J. Power Sources. 2009;193:1e8. [Google Scholar]
- 47.Nirunsin S., Khun Torn Y. Quantification of liquid water saturation in a transparent single-serpentine cathode flow channel of PEM fuel cell by using image processing. J Sustain Energy Environ. 2010;1 [Google Scholar]
- 48.Ous T., Arcoumanis C. Visualization of water accumulation in the flow channels of PEMFC under various operating conditions. J. Power Sources. 2009;187:182e9. [Google Scholar]
- 49.Grilli Maria Luisa, et al. Platinum group metals: green recovery from spent auto-catalysts and reuse in new catalysts—a review. Crystals. 2023;13(4):550. [Google Scholar]
- 50.Utomi Allison, et al. 2023. "Trends in US Merchandise Trade, 2022.". [Google Scholar]
- 51.Huang Lei, et al. Molybdenum-modified and vertex-reinforced quaternary hexapod nano-skeletons as efficient electrocatalysts for methanol oxidation and oxygen reduction reaction. Appl. Catal. B Environ. 2019;258 [Google Scholar]
- 52.Shi Dongcai, et al. Fabrication methods, structure design, and durability analysis of advanced sealing materials in proton exchange membrane fuel cells. Chem. Eng. J. 2023;454 [Google Scholar]
- 53.Mann Margaret, Putsche Vicky. USDOE Office of Policy (PO); 2022. Semiconductor-Supply Chain Deep Dive Assessment. [Google Scholar]
- 54.Badgett Alex, et al. USDOE Office of Policy; 2022. Water Electrolyzers and Fuel Cells Supply Chain-Supply Chain Deep Dive Assessment. [Google Scholar]
- 55.Utomi Allison, et al. 2023. "Trends in US Merchandise Trade, 2022.". [Google Scholar]
- 56.Xia Jinsong, Ahmad Ghahreman. Platinum group metals recycling from spent automotive catalysts: metallurgical extraction and recovery technologies. Separation and Purification Technology. 2023 [Google Scholar]
- 57.Centre, Alternative Fuels Data . US Department of Energy; 2022. Fuel Cell Electric Vehicles. [Google Scholar]
- 58.Moschkowitsch Wenjamin, Lori Oran, Elbaz Lior. Recent progress and viability of PGM-free catalysts for hydrogen evolution and oxidation reaction. ACS Catal. 2022;12(2):1082–1089. [Google Scholar]
- 59.Kolliopoulos Georgios, et al. Behavior of platinum group metals during their pyrometallurgical recovery from spent automotive catalysts. Open Access Lib. J. 2014;1:1–9. [Google Scholar]
- 60.Omrani Mehrazin, et al. Platinum group elements study in automobile catalysts and exhaust gas samples. Environmental Pollution. 2020;257 doi: 10.1016/j.envpol.2019.113477. [DOI] [PubMed] [Google Scholar]
- 61.Lead, Overall, Team. "US Department of Energy 2022 Collegiate Wind Competition Technical Design Report.".
- 62.Saternus M., Fornalczyk A., Cebulski J. Analysis of platinum content in used auto catalytic converter carriers and the possibility of its recovery. Arch. Metall. Mater. 2014;59(2):557–564. [Google Scholar]
- 63.Ton Dan T., Wang WT Paul. A more resilient grid: the US Department of Energy joins with stakeholders in an R&D plan. IEEE Power Energy Mag. 2015;13(3):26–34. [Google Scholar]
- 64.Sanchez-Molina M., Amores E., Rojas N., Kunowsky M. Additive manufacturing of bipolar plates for hydrogen production in proton exchange membrane water electrolysis cells. Int. J. Hydrogen Energy. Nov. 2021;46(79):38983e91. doi: 10.1016/J.IJHYDENE.2021.09.152. [DOI] [Google Scholar]
- 65.Shao Minhua, et al. Recent advances in electrocatalysts for the oxygen reduction reaction. Chem. Rev. 2016;116(6):3594–3657. doi: 10.1021/acs.chemrev.5b00462. [DOI] [PubMed] [Google Scholar]
- 66.Wang X.X., Swihart M.T., Wu G. Nat. Catal. 2019;2:578. [Google Scholar]
- 67.Evangelisti Sara, et al. Life cycle assessment of a polymer electrolyte membrane fuel cell system for passenger vehicles. J. Clean. Prod. 2017;142:4339–4355. [Google Scholar]
- 68.Zhao Jing, et al. Thermal performance enhancement of air-cooled proton exchange membrane fuel cells by vapor chambers. Energy Convers. Manag. 2020;213 [Google Scholar]
- 69.Li Hongda, et al. Pt-based oxygen reduction reaction catalysts in proton exchange membrane fuel cells: controllable preparation and structural design of catalytic layer. Nanomaterials. 2022;12(23):4173. doi: 10.3390/nano12234173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai Yeh-Hung, et al. 2009. "Viscoelastic Stress Analysis of Constrained Proton Exchange Membranes under Humidity cycling.". [Google Scholar]
- 71.Shukla S., et al. Analysis of inkjet printed PEFC electrodes with varying platinum loading. J. Electrochem. Soc. 2016;163(7) [Google Scholar]
- 72.Towne S., Viswanathan V., Holbery J., Rieke P. Fabrication of polymer electrolyte membrane fuel cell MEAs utilizing inkjet print technology. J Power Sources Sep. 2007;171(2):575e84. doi: 10.1016/j.jpowsour.2007.07.017. [DOI] [Google Scholar]
- 73.Taylor A.D., Kim E.Y., Humes V.P., Kizuka J., Thompson L.T. Inkjet printing of carbon-supported platinum 3-D catalyst layers for use in fuel cells. J Power Sources Sep. 2007;171(1):101e6. doi: 10.1016/J.JPOWSOUR.2007.01.024. [DOI] [Google Scholar]
- 74.Bezerra C.A.G., Deiner L.J., Tremiliosi-Filho G. Inkjet printed double-layered cathodes for PEM fuel cells. J Electrochem Soc Aug. 2020;167(12) doi: 10.1149/1945-7111/ABA6CA. [DOI] [Google Scholar]
- 75.Pollet Bruno G., Kocha Shyam S., Iain Staffell. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019;16:90–95. [Google Scholar]
- 76.Gröger Oliver, Gasteiger Hubert A., Suchsland Jens-Peter. Electromobility: batteries or fuel cells. J. Electrochem. Soc. 2015;162(14) [Google Scholar]
- 77.Chen Shuo, et al. Platinum-alloy cathode catalyst degradation in proton exchange membrane fuel cells: nanometer-scale compositional and morphological changes. J. Electrochem. Soc. 2009;157(1):A82. [Google Scholar]
- 78.Kongkanand Anusorn, Mathias Mark F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. The journal of physical chemistry letters. 2016;7(7):1127–1137. doi: 10.1021/acs.jpclett.6b00216. [DOI] [PubMed] [Google Scholar]
- 79.Strasser Peter, et al. Lattice-strain control of the activity in unalloyed core-shell fuel cell catalysts. Nat. Chem. 2010;2(6):454–460. doi: 10.1038/nchem.623. [DOI] [PubMed] [Google Scholar]
- 80.Cai Xin, et al. Gram-scale synthesis of well-dispersed shape-controlled Pt− Ni/C as high-performance catalysts for the oxygen reduction reaction. ACS applied materials & interfaces. 2019;11(33):29689–29697. doi: 10.1021/acsami.9b03590. [DOI] [PubMed] [Google Scholar]
- 81.Wang Yun, et al. Materials, technological status, and fundamentals of PEM fuel cells–a review. Mater. Today. 2020;32:178–203. [Google Scholar]
- 82.Wang Hongjing, et al. Direct synthesis of superlong Pt| Te mesoporous nanotubes for electrocatalytic oxygen reduction. J. Mater. Chem. A. 2019;7(4):1711–1717. [Google Scholar]
- 83.Sui Sheng, et al. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A. 2017;5(5):1808–1825. [Google Scholar]
- 84.Myers Deborah J., et al. Degradation of platinum-cobalt alloy PEMFC cathode catalysts in catalyst-ionomer inks. J. Electrochem. Soc. 2021;168(4) [Google Scholar]
- 85.Greszler T.A., Moylan T.E., Gasteiger H.A. Modeling the impact of cation contamination in a polymer electrolyte membrane fuel cell. Handbook of Fuel Cells. 2010 doi: 10.1002/9780470974001. [DOI] [Google Scholar]
- 86.Cho J.I.S., Neville T.P., Trogadas P., Bailey J., Shearing P., Brett D.J.L., Coppens M.-O. Capillaries for water management in polymer electrolyte membrane fuel cells. International Journal of Hydration Energy. 2018;4 3 (2 0 1 8) 2 1 9 4 9 e2 1 9 5 8. [Google Scholar]
- 87.Yoshida Toshihiko, Kojima Koichi. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Electrochem. Soc. Interface. 2015;24(2):45. [Google Scholar]
- 88.Li Junrui, et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule. 2019;3(1):124–135. [Google Scholar]
- 89.Gao H., Liao S., Zhang Y., Jia X., Zhou L., Zheng Z., Yang Y. Methanol-tolerant Se^Pt/C: effects of Se content on the structure and electrocatalytic performance for oxygen reduction reaction. Ionics. 2020;26:1315. doi: 10.1007/s11581-019-03336-3GoogleScholarCrossref. [DOI] [Google Scholar]
- 90.Hu Bin, et al. Facile synthesis of synergistic Pt/(Co-N) @ C composites as alternative oxygen-reduction electrode of PEMFCs with attractive activity and durability. Compos. B Eng. 2020;193 [Google Scholar]
- 91.Sriwannaboot Jittima, Kannan Arunachala, Tantavichet Nisit. Pulse-reverse electrodeposition of Pt-Co bimetallic catalysts for oxygen reduction reaction in acidic medium. Int. J. Hydrogen Energy. 2020;45(11):7025–7035. [Google Scholar]
- 92.Tian Xinlong, et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science. 2019;366(6467):850–856. doi: 10.1126/science.aaw7493. [DOI] [PubMed] [Google Scholar]
- 93.Zhao Qing, et al. H2‐induced thermal treatment significantly influences the development of a high-performance low‐platinum core‐shell PtNi/C alloyed oxygen reduction catalyst. Int. J. Energy Res. 2020;44(6):4773–4783. [Google Scholar]
- 94.Mardle Peter, et al. Comparative study of PtNi nanowire array electrodes toward oxygen reduction reaction by half-cell measurement and PEMFC test. ACS Appl. Mater. Interfaces. 2020;12(38):42832–42841. doi: 10.1021/acsami.0c11531. [DOI] [PubMed] [Google Scholar]
- 95.Choi Juhyuk, et al. Highly durable fuel cell catalysts using cross-linkable block copolymer-based carbon supports with ultralow Pt loadings. Energy Environ. Sci. 2020;13(12):4921–4929. [Google Scholar]
- 96.Zhu Jiawei, et al. Facet-controlled Pt–Ir nanocrystals with substantially enhanced activity and durability towards oxygen reduction. Mater. Today. 2020;35:69–77. [Google Scholar]
- 97.Park Jin-Young, et al. Organic ligand-free PtIr alloy nanostructures for superior oxygen reduction and evolution reactions. J. Ind. Eng. Chem. 2019;77:105–110. [Google Scholar]
- 98.Bak Junu, et al. Boosting the role of Ir in mitigating corrosion of carbon support by alloying with Pt. ACS Catal. 2020;10(20):12300–12309. Gatalo, Matija, et al. "Insights into thermal annealing of highly-active PtCu3/C Oxygen Reduction Reaction electrocatalyst: An in-situ heating transmission Electron microscopy study." Nano Energy 63 (2019): 103892. [Google Scholar]
- 99.Zhang Lu, et al. Facile solvothermal synthesis of Pt71Co29 lamellar nanoflowers as an efficient catalyst for oxygen reduction and methanol oxidation reactions. J. Colloid Interface Sci. 2019;536:556–562. doi: 10.1016/j.jcis.2018.10.080. [DOI] [PubMed] [Google Scholar]
- 100.Yang Zhaojun, et al. Hollow PtFe alloy nanoparticles derived from Pt‐Fe3O4 dimers through a silica‐protection reduction strategy as efficient oxygen reduction electrocatalysts. Chem.--Eur. J. 2020;26(18):4090–4096. doi: 10.1002/chem.201904208. [DOI] [PubMed] [Google Scholar]
- 101.Gong Wenhao, et al. Cross-double dumbbell-like Pt–Ni nanostructures with enhanced catalytic performance toward the reactions of oxygen reduction and methanol oxidation. Appl. Catal. B Environ. 2019;246:277–283. [Google Scholar]
- 102.Sriphathoorat Rinrada, Wang Kai, Pei Kang Shen. Trimetallic hollow PtNi–Co nano dendrites as efficient anodic electrocatalysts. ACS Appl. Energy Mater. 2019;2(2):961–965. [Google Scholar]
- 103.Tu Wenzhe, et al. Tungsten‐doping‐induced surface reconstruction of porous ternary Pt‐based alloy electrocatalyst for oxygen reduction. Adv. Funct. Mater. 2019;29(7) [Google Scholar]
- 104.Gao Yanyan, et al. Performance-and durability-enhanced carbon-skeleton nanofiber electrode with Pt3Co/C for PEMFCs. ACS Sustain. Chem. Eng. 2020;8(34):13030–13038. [Google Scholar]
- 105.Wang Zhongxiang, et al. Rational development of structurally ordered platinum ternary intermetallic electrocatalysts for the oxygen reduction reaction. Catalysts. 2019;9(7):569. (J) [Google Scholar]
- 106.Liang Z. Zhao, Li N., Wang X., Li S., Liu X., Wang T., Lu G., Wang D., Hwang B.J., Huang Y., Su D., Li Q. Biaxial strains mediated oxygen reduction electrocatalysis on Fenton reaction resistant L10-PtZn fuel cell cathode. Adv. Energy Mater. 2020;10 doi: 10.1002/aenm.202000179GoogleScholarCrossref. [DOI] [Google Scholar]
- 107.Dionigi Fabio, et al. Controlling near-surface Ni composition in octahedral PtNi (Mo) nanoparticles by Mo doping for a highly active oxygen reduction reaction catalyst. Nano Lett. 2019;19(10):6876–6885. doi: 10.1021/acs.nanolett.9b02116. [DOI] [PubMed] [Google Scholar]
- 108.Cao Liang, et al. Differential surface elemental distribution leads to significantly enhanced stability of PtNi-based ORR catalysts. Matter. 2019;1(6):1567–1580. [Google Scholar]
- 109.Shen Xiaochen, et al. Tuning electronic structure and lattice diffusion barrier of ternary Pt–In–Ni for improved activity and stability properties in oxygen reduction electrocatalysis. ACS Catal. 2019;9(12):11431–11437. [Google Scholar]
- 110.Tu Wenzhe, et al. Tungsten as "adhesive" in Pt2CuW0. 25 ternary alloy for highly durable oxygen reduction electrocatalysis. Adv. Funct. Mater. 2020;30(6) [Google Scholar]
- 111.Wang Xiaoran, et al. Nickel-introduced structurally ordered PtCuNi/C as high-performance electrocatalyst for oxygen reduction reaction. Prog. Nat. Sci.: Mater. Int. 2020;30(6):905–911. [Google Scholar]
- 112.He Caimei, et al. Promoting the ORR catalysis of Pt-Fe intermetallic catalysts by increasing atomic utilization and electronic regulation. Electrochim. Acta. 2020;330 [Google Scholar]
- 113.Chu Tiankuo, et al. Highly active and durable carbon support Pt-rare earth catalyst for proton exchange membrane fuel cell. Int. J. Hydrogen Energy. 2020;45(51):27291–27298. [Google Scholar]
- 114.Guo Ruiyun, et al. Phosphorization treatment improves the catalytic activity and durability of platinum catalysts toward oxygen reduction reaction. Chem. Mater. 2019;31(19):8205–8211. [Google Scholar]
- 115.Pu Zonghua, et al. Anion-modulated platinum for high-performance multifunctional electrocatalysis toward HER, HOR, and ORR. Science. 2020;23:12. doi: 10.1016/j.isci.2020.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiong Yunjie, et al. N-doping induced tensile-strained Pt nanoparticles ensuring an excellent durability of the oxygen reduction reaction. J. Catal. 2020;382:247–255. [Google Scholar]
- 117.Gubler Lorenz, et al. Perspective—prospects for durable hydrocarbon-based fuel cell membranes. J. Electrochem. Soc. 2018;165(6):F3100. [Google Scholar]
- 118.Goto Kohei, et al. Development of aromatic polymer electrolyte membrane with high conductivity and durability for fuel cell. Polym. J. 2009;41(2):95–104. [Google Scholar]
- 119.Miyahara Takahiro, et al. Sulfonated poly benzophenone/poly (arylene ether) block copolymer membranes for fuel cell applications. ACS applied materials & interfaces. 2012;4(6):2881–2884. doi: 10.1021/am300821v. [DOI] [PubMed] [Google Scholar]
- 120.Hamrock Steven J., Yandrasits Michael A. Proton exchange membranes for fuel cell applications. J. Macromol. Sci. Polym. Rev. 2006;46(3):219–244. [Google Scholar]
- 121.Yandrasits Michael, et al. Increasing fuel cell efficiency by using ultra-low equivalent weight ionomers. Electrochem. Soc. Interface. 2017;26(1):49. [Google Scholar]
- 122.Yandrasits Michael, et al. Chemical stability of perfluorobis (sulfonyl) imide-acid (PFIA) ionomers in open circuit voltage (OCV) accelerated test conditions. J. Electrochem. Soc. 2018;165(6):F3261. [Google Scholar]
- 123.Chandan Amrit, et al. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources. 2013;231:264–278. [Google Scholar]
- 124.Bose Saswata, et al. Polymer membranes for high-temperature proton exchange membrane fuel cell: recent advances and challenges. Prog. Polym. Sci. 2011;36(6):813–843. [Google Scholar]
- 125.Kerr Robert, et al. Lifetime and degradation of high-temperature PEM membrane electrode assemblies. Int. J. Hydrogen Energy. 2015;40(46):16860–16866. [Google Scholar]
- 126.Shojaeefard M.H., et al. A review on microstructure reconstruction of PEM fuel cells porous electrodes for pore-scale simulation. Int. J. Hydrogen Energy. 2016;41(44):20276–20293. [Google Scholar]
- 127.Berchtold Kathryn A., et al. Polybenzimidazole composite membranes for high-temperature synthesis gas separations. J. Membr. Sci. 2012;415:265–270. [Google Scholar]
- 128.Ozdemir Yağmur, Üregen Nurhan, Devrim Yılser. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high-temperature PEM fuel cells. Int. J. Hydrogen Energy. 2017;42(4):2648–2657. [Google Scholar]
- 129.Lobato Justo, et al. A novel titanium PBI-based composite membrane for high-temperature PEMFCs. J. Membr. Sci. 2011;369(1–2):105–111. [Google Scholar]
- 130.Farooqui Usaid R., Ahmad Abdul Latif, Hamid N.A. Graphene oxide: a promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018;82:714–733. [Google Scholar]
- 131.Üregen Nurhan, et al. Development of polybenzimidazole/graphene oxide composite membranes for high-temperature PEM fuel cells. Int. J. Hydrogen Energy. 2017;42(4):2636–2647. [Google Scholar]
- 132.Xu Chenxi, et al. A polybenzimidazole/ionic-liquid-graphite-oxide composite membrane for high-temperature polymer electrolyte membrane fuel cells. J. Power Sources. 2015;274:922–927. [Google Scholar]
- 133.Yu Bor-Chern, et al. Hydroxide-ion selective electrolytes based on a polybenzimidazole/graphene oxide composite membrane. Energy. 2017;134:802–812. [Google Scholar]
- 134.Mo Jingke, et al. Additive manufacturing of liquid/gas diffusion layers for low-cost and high-efficiency hydrogen production. Int. J. Hydrogen Energy. 2016;41(4):3128–3135. [Google Scholar]
- 135.Baroutaji Ahmad, et al. Additive manufacturing for Proton Exchange Membrane (PEM) hydrogen technologies: merits, challenges, and prospects. Int. J. Hydrogen Energy. 2024;52:561–584. [Google Scholar]
- 136.Mathias M.F., et al. Diffusion media materials and characterization. Handbook of fuel cells—fundamentals, technology and applications 3. Part. 2003;1:517–537. [Google Scholar]
- 137.Wang Yun, et al. Stochastic modeling and direct simulation of the diffusion media for polymer electrolyte fuel cells. Int. J. Heat Mass Tran. 2010;53(5–6):1128–1138. [Google Scholar]
- 138.Niblett Daniel, et al. Two-phase flow dynamics in a gas diffusion layer-gas channel-microporous layer system. J. Power Sources. 2020;471 [Google Scholar]
- 139.Niu Zhiqiang, et al. Two-phase flow and oxygen transport in the perforated gas diffusion layer of proton exchange membrane fuel cell. Int. J. Heat Mass Tran. 2019;139:58–68. [Google Scholar]
- 140.Liu Xunliang, et al. Liquid water transport characteristics of porous diffusion media in polymer electrolyte membrane fuel cells: a review. J. Power Sources. 2015;299:85–96. [Google Scholar]
- 141.Benner Jingru, Mortazavi Mehdi, Santamaria Anthony D. Numerical simulation of droplet emergence and growth from gas diffusion layers (GDLs) in proton exchange membrane (PEM) fuel cell flow channels. ASME International Mechanical Engineering Congress and Exposition. 2018;52071 American Society of Mechanical Engineers. [Google Scholar]
- 142.Carrère Pierre, Prat Marc. Impact of non-uniform wettability in the condensation and condensation-liquid water intrusion regimes in the cathode gas diffusion layer of proton exchange membrane fuel cell. Int. J. Therm. Sci. 2019;145 [Google Scholar]
- 143.Wang Yun, et al. A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research. Appl. Energy. 2011;88(4):981–1007. [Google Scholar]
- 144.Chen Qin, et al. Thermal management of polymer electrolyte membrane fuel cells: a review of cooling methods, material properties, and durability. Appl. Energy. 2021;286 [Google Scholar]
- 145.Wang Feijie, et al. Investigation of the recoverable degradation of PEM fuel cell operated under drive cycle and different humidities. Int. J. Hydrogen Energy. 2014;39(26):14441–14447. [Google Scholar]
- 146.Andersson M., et al. Modeling and synchrotron imaging of droplet detachment in gas channels of polymer electrolyte fuel cells. J. Power Sources. 2018;404:159–171. [Google Scholar]
- 147.Yu Yang, et al. Thermal management system for liquid-cooling PEMFC stack: from primary configuration to system control strategy. ETransportation. 2022;12 [Google Scholar]
- 148.Mancusi Erasmo, et al. Numerical study of two-phase flow patterns in the gas channel of PEM fuel cells with tapered flow field design. Int. J. Hydrogen Energy. 2014;39(5):2261–2273. [Google Scholar]
- 149.Huang Yicheng, et al. Thermal management of polymer electrolyte membrane fuel cells: a critical review of heat transfer mechanisms, cooling approaches, and advanced cooling techniques analysis. Energy Convers. Manag. 2022;254 [Google Scholar]
- 150.Ozden Adnan, et al. A review of gas diffusion layers for proton exchange membrane fuel cells—with a focus on characteristics, characterization techniques, materials, and designs. Prog. Energy Combust. Sci. 2019;74:50–102. [Google Scholar]
- 151.Han Chaoling, Chen Zhenqian. Numerical simulations of two-phase flow in a proton-exchange membrane fuel cell based on the generalized design method. Energy Sources, Part A Recovery, Util. Environ. Eff. 2019;41(10):1253–1271. [Google Scholar]
- 152.Dursch T.J., et al. Ice-crystallization kinetics in the catalyst layer of a proton-exchange-membrane fuel cell. J. Electrochem. Soc. 2013;161(3):F199. [Google Scholar]
- 153.Luo Yueqi, Jiao Kui. Cold start of proton exchange membrane fuel cell. Prog. Energy Combust. Sci. 2018;64:29–61. [Google Scholar]
- 154.Wang Yun, et al. Cold start of polymer electrolyte fuel cells: three-stage startup characterization. Electrochim. Acta. 2010;55(8):2636–2644. [Google Scholar]
- 155.Xiong Shusheng, et al. Research on cold start of proton-exchange membrane fuel cells based on model predictive control. Membranes. 2023;13(2):184. doi: 10.3390/membranes13020184. 12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ji W.C., Lin R. Strategy optimization of proton exchange membrane fuel cell cold start. Chin. Sci. Bull. 2022;67 (in Chinese) [Google Scholar]
- 157.Luo Yueqi, Jiao Kui. Cold start of proton exchange membrane fuel cell. Prog. Energy Combust. Sci. 2018;64:29–61. [Google Scholar]
- 158.Wang Yun. Analysis of the key parameters in the cold start of polymer electrolyte fuel cells. J. Electrochem. Soc. 2007;154(10) [Google Scholar]
- 159.Mishler Jeffrey, et al. Subfreezing operation of polymer electrolyte fuel cells: ice formation and cell performance loss. Electrochim. Acta. 2012;65:127–133. [Google Scholar]
- 160.Tabe Yutaka, et al. Ice formation processes in PEM fuel cell catalyst layers during cold startup analyzed by cryo-SEM. J. Electrochem. Soc. 2016;163(10) [Google Scholar]
- 161.White Robin T., et al. 3D printed flow field and fixture for visualization of water distribution in fuel cells by X-ray computed tomography. J. Electrochem. Soc. 2016;163(13):F1337. [Google Scholar]
- 162.Matos Bruno R., Santiago Elisabete I., Fonseca Fabio C. Irreversibility of proton conductivity of Nafion and Nafion–Titania composites at high relative humidity. Materials for Renewable and Sustainable Energy. 2015;4:1–9. [Google Scholar]
- 163.Zawodzinski Thomas A., et al. Water uptake by and transport through Nafion® 117 membranes. J. Electrochem. Soc. 1993;140(4):1041. [Google Scholar]
- 164.Sethuraman Vijay A., et al. Durability of perfluorosulfonic acid and hydrocarbon membranes: effect of humidity and temperature. J. Electrochem. Soc. 2007;155(2):B119. [Google Scholar]
- 165.Karpenko-Jereb L., et al. Membrane degradation model for 3D CFD analysis of fuel cell performance as a function of time. Int. J. Hydrogen Energy. 2016;41(31):13644e56. [Google Scholar]
- 166.Bachman John, et al. Experimental investigation of the effect of channel length on performance and water accumulation in a PEMFC parallel flow field. Int. J. Hydrogen Energy. 2012;37(22):17172–17179. [Google Scholar]
- 167.Liu Xuan, et al. Water flooding and pressure drop characteristics in flow channels of proton exchange membrane fuel cells. Electrochim. Acta. 2007;52(11):3607–3614. [Google Scholar]
- 168.Wu Y., et al. Effect of serpentine flow-field design on the water management of polymer electrolyte fuel cells: an in-operando neutron radiography study. J. Power Sources. 2018;399:254–263. [Google Scholar]
- 169.Zhang Xu, et al. Effects of carbon corrosion on mass transfer losses in proton exchange membrane fuel cells. Int. J. Hydrogen Energy. 2017;42(7):4699–4705. [Google Scholar]
- 170.Kandlikar S.G., et al. Measurement of flow maldistribution in parallel channels and its application to ex-situ and in-situ experiments in PEMFC water management studies. Int. J. Heat Mass Tran. 2009;52(7–8):1741–1752. [Google Scholar]
- 171.Zhang Lifeng, et al. Gas flow rate distributions in parallel mini channels for polymer electrolyte membrane fuel cells: experiments and theoretical analysis. J. Power Sources. 2010;195(10):3231–3239. [Google Scholar]
- 172.Liu Xuan, et al. Water flooding and pressure drop characteristics in flow channels of proton exchange membrane fuel cells. Electrochim. Acta. 2007;52(11):3607–3614. [Google Scholar]
- 173.Trogadas P., et al. A lung-inspired approach to scalable and robust fuel cell design. Energy Environ. Sci. 2018;11(1):136–143. [Google Scholar]
- 174.Taniguchi Akira, et al. Analysis of electrocatalyst degradation in PEMFC caused by cell reversal during fuel starvation. J. Power Sources. 2004;130(1–2):42–49. [Google Scholar]
- 175.Schmittinger Wolfgang, Vahidi Ardalan. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources. 2008;180(1):1–14. [Google Scholar]
- 176.Choa Min Kyung, et al. Analysis of the spatially distributed performance degradation of a polymer electrolyte membrane fuel cell stack. Int. J. Hydrogen Energy. 2014;30(1):e8. [Google Scholar]
- 177.Borup Rod, et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007;107(10):3904–3951. doi: 10.1021/cr050182l. [DOI] [PubMed] [Google Scholar]
- 178.Li Hui, et al. A review of water flooding issues in the proton exchange membrane fuel cell. J. Power Sources. 2008;178(1):103–117. [Google Scholar]
- 179.Wu Horng-Wen. A review of recent development: transport and performance modeling of PEM fuel cells. Appl. Energy. 2016;165:81–106. [Google Scholar]
- 180.Pourrahmani Hossein, et al. A review on the proton exchange membrane fuel cells (PEMFCs) water/thermal management: from theory to the current challenges and real-time fault diagnosis methods. Energy Rev. 2022 [Google Scholar]
- 181.Ji Mengbo, Zidong Wei. A review of water management in polymer electrolyte membrane fuel cells. Energies. 2009;2(4) 1057-1106057-1. [Google Scholar]
- 182.Pasaogullari Ugur, Wang Chao-Yang, Chen Ken S. Two-phase transport in polymer electrolyte fuel cells with bilayer cathode gas diffusion media. J. Electrochem. Soc. 2005;152(8):A1574. [Google Scholar]
- 183.Duy Duc Tran, et al. Anode and cathode flow field design and optimization of the parametric performance of PEMFC. Int. J. Electrochem. Sci. 2021;16(10) [Google Scholar]
- 184.Baroutaji Ahmad, et al. Advancements and prospects of thermal management and waste heat recovery of PEMFC. International Journal of Thermofluids. 2021;9 [Google Scholar]
- 185.Asadi Mohammad Reza, et al. The optimization of an innovative interdigitated flow field proton exchange membrane fuel cell by using artificial intelligence. Energy. 2024;290 [Google Scholar]
- 186.Sun Jing, et al. Redox flow batteries and their stack-scale flow fields. Carbon Neutrality. 2023;2(1):30. [Google Scholar]
- 187.Liu Xuan, Guo Hang, Ma Chongfang. Water flooding and two-phase flow in cathode channels of proton exchange membrane fuel cells. J. Power Sources. 2006;156(2):267–280. [Google Scholar]
- 188.Liu X., Guo H., Ye F. Ma, CF Water flooding and pressure drop characteristics in flow channels of proton exchange membrane fuel cells. Electrochim. Acta. 2007;52:3607–3614. [Google Scholar]
- 189.Zakaria Irnie, et al. A review of nanofluid adoption in polymer electrolyte membrane (PEM) fuel cells as an alternative coolant. J. Mech. Eng. Sci. 2015;8:1351–1366. [Google Scholar]
- 190.Cai Yun, et al. Effects of cobalt cation on low Pt-loaded PEM fuel cell performance. ECS Trans. 2015;69(17):1047. [Google Scholar]
- 191.á Cai Y., et al. áKongkanand A., áMathias M.F., áMukundan R., áBorup R.L. J. Electrochem. Soc. 2018;165:F3132. [Google Scholar]
- 192.Su Ay, et al. Studies on flooding in PEM fuel cell cathode channels. Int. J. Hydrogen Energy. 2006;31(8):1031–1039. [Google Scholar]
- 193.Li Shian, Sundén Bengt. Effects of gas diffusion layer deformation on the transport phenomena and performance of PEM fuel cells with interdigitated flow fields. Int. J. Hydrogen Energy. 2018;43(33):16279–16292. [Google Scholar]
- 194.Park J., Li X. An experimental and numerical investigation on the cross-flow through gas diffusion layer in a PEM fuel cell with a serpentine flow channel. J. Power Sources. 2007;163(2) [Google Scholar]
- 195.Hsieh Shou-Shing, Her Bing-Shyan, Huang Yi-Ji. Effect of pressure drop in different flow fields on water accumulation and current distribution for a micro-PEM fuel cell. Energy Convers. Manag. 2011;52(2):975–982. [Google Scholar]
- 196.Hsieh Shou-Shing, Huang Yi-Ji, Her Bing-Shyan. Pressure drop on water accumulation distribution for a micro-PEM fuel cell with different flow field plates. Int. J. Heat Mass Tran. 2009;52(23–24):5657–5659. [Google Scholar]
- 197.Wood I.I.I., David L., Yi Jung S., Nguyen Trung V. Effect of direct liquid water injection and interdigitated flow field on the performance of proton exchange membrane fuel cells. Electrochim. Acta. 1998;43(24):3795–3809. [Google Scholar]
- 198.Owejan J.P., et al. In situ investigation of water transport in an operating PEM fuel cell using neutron radiography: Part 2 e transient water accumulation in an interdigitated cathode flow field. Int. J. Heat Mass Tran. 2006;49(25) [Google Scholar]
- 199.Li Junrui, et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule. 2019;3(1):124–135. [Google Scholar]
- 200.Chen Shizhong, Zhang Xuyang, Liu Hongtan. Effect of the pressure difference between adjacent channels in an adjustable flow field in PEM fuel cells. Int. J. Hydrogen Energy. 2017;42(7):4667–4672. [Google Scholar]
- 201.Spernjak Dusan, Prasad Ajay K., Advani Suresh G. In situ comparison of water content and dynamics in parallel, single-serpentine, and interdigitated flow fields of polymer electrolyte membrane fuel cells. J. Power Sources. 2010;195(11):3553–3568. [Google Scholar]
- 202.Belhadj Mariem, et al. Current density distributions in polymer electrolyte fuel cells: a tool for characterization of gas distribution in the cell and its state of health. Chem. Eng. Sci. 2018;185:18–25. [Google Scholar]
- 203.Iranzo Alfredo, et al. Water build-up and evolution during the startup of a PEMFC: visualization employing Neutron Imaging. Int. J. Hydrogen Energy. 2017;42(19):13839–13849. [Google Scholar]
- 204.Metz Tobias, et al. Passive water removal in fuel cells by capillary droplet actuation. Sensor Actuator Phys. 2008;143(1):49–57. [Google Scholar]
- 205.Koresawa Ryo, Utaka Yoshio. Water control by employing microgrooves inside gas channel for performance improvement in polymer electrolyte fuel cells. Int. J. Hydrogen Energy. 2015;40(25):8172–8181. [Google Scholar]
- 206.Yi Jung S., Deliang Yang J., Constance King. Water management along the flow channels of PEM fuel cells. AIChE J. 2004;50(10):2594–2603. [Google Scholar]
- 207.Wang Zhiqiang, et al. Improvement of PEMFC water management by employing water transport plate as a bipolar plate. Int. J. Hydrogen Energy. 2017;42(34):21922–21929. [Google Scholar]
- 208.Weber Adam Z., Darling Robert M. Understanding porous water-transport plates in polymer-electrolyte fuel cells. J. Power Sources. 2007;168(1):191–199. [Google Scholar]
- 209.Litster S., et al. Active water management for PEM fuel cells. J. Electrochem. Soc. 2007;154(10) [Google Scholar]
- 210.Fabian T., et al. Passive water management at the cathode of a planar air-breathing proton exchange membrane fuel cell. J. Power Sources. 2010;195(10):3201–3206. [Google Scholar]
- 211.Ge, Shan-Hai, Li Xu-Guang, Hsing I-Ming. Water management in PEMFCs using absorbent wicks. J. Electrochem. Soc. 2004;151(9) [Google Scholar]
- 212.Ge S., Li X., Hsing I.M. Internally humidified polymer electrolyte fuel cells using water water-absorbing sponge. Electrochim. Acta. 2005;50(9):1909e16. [Google Scholar]
- 213.Strickland Daniel G., Santiago Juan G. In situ polymerized wicks for passive water management and humidification of dry gases. ECS Trans. 2009;25(1):303. [Google Scholar]
- 214.Strickland D.G., Santiago J.G. In situ-polymerized wicks for passive water management in proton exchange membrane fuel cells. J. Power Sources. 2010;195(6) [Google Scholar]
- 215.Brett Daniel JL., Nigel P. Brandon. 2007. Review of Materials and Characterization Methods for Polymer Electrolyte Fuel Cell Flow-Field Plates; pp. 29–44. [Google Scholar]
- 216.Haase S., et al. Current density and catalyst-coated membrane resistance distribution of hydro-formed metallic bipolar plate fuel cell short stack with 250 cm2 active area. J. Power Sources. 2016;301:251–260. [Google Scholar]
- 217.Jung Shiauh-Ping, et al. Development of novel proton exchange membrane fuel cells using stamped metallic bipolar plates. J. Power Sources. 2015;283:429–442. [Google Scholar]
- 218.Zhang Jianlu, et al. PEM fuel cell relative humidity (RH) and its effect on performance at high temperatures. Electrochim. Acta. 2008;53(16):5315–5321. [Google Scholar]
- 219.Novitski David, Holdcroft Steven. Determination of O2 mass transport at the Pt| PFSA ionomer interface under reduced relative humidity. ACS applied materials & interfaces. 2015;7(49):27314–27323. doi: 10.1021/acsami.5b08720. [DOI] [PubMed] [Google Scholar]
- 220.Neyerlin K.C., et al. Effect of relative humidity on oxygen reduction kinetics in a PEMFC. J. Electrochem. Soc. 2005;152(6):A1073. [Google Scholar]
- 221.Fabian Tibor, et al. The role of ambient conditions on the performance of a planar, air-breathing hydrogen PEM fuel cell. J. Power Sources. 2006;161(1):168–182. [Google Scholar]
- 222.Raileanu Ilie V.A., Martemianov S., Thomas A. Investigation of the local temperature and overheating inside the membrane electrode assembly of PEM fuel cell. Int. J. Hydrogen Energy. 2016;41(34):15528e37. [Google Scholar]
- 223.Meyer Quentin, et al. Optimisation of air-cooled, open-cathode fuel cells: current of lowest resistance and electro-thermal performance mapping. J. Power Sources. 2015;291:261–269. [Google Scholar]
- 224.Cho J.I.S., et al. Capillaries for water management in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy. 2018;43(48):21949–21958. [Google Scholar]
- 225.Trabold T.A., et al. In situ investigation of water transport in an operating PEM fuel cell using neutron radiography: Part 1e experimental method and serpentine flow field results. Int. J. Heat Mass Tran. 2006;49(25) [Google Scholar]
- 226.Gemmen R.S., Johnson C.D. Evaluation of fuel cell system efficiency and degradation at development and during commercialization. J. Power Sources. 2006;159(1):646–655. [Google Scholar]
- 227.Kurnia Jundika Candra, Pulung Sasmito Agus. Energy Efficiency in Mobility Systems. 2020. Hydrogen fuel cell in vehicle propulsion: performance, efficiency, and challenge; pp. 9–26. [Google Scholar]
- 228.Hu Bin, et al. Facile synthesis of synergistic Pt/(Co-N) @ C composites as alternative oxygen-reduction electrode of PEMFCs with attractive activity and durability. Compos. B Eng. 2020;193 [Google Scholar]
- 229.Qiu Diankai, et al. Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2019;113 [Google Scholar]
- 230.Balogun Emmanuel O., et al. Performance and durability studies of perfluorosulfonic acid ionomers as binders in PEMFC catalyst layers using Electrochemical Impedance Spectroscopy. Int. J. Hydrogen Energy. 2019;44(60):32219–32230. [Google Scholar]
- 231.Yu Yang, et al. Thermal management system for liquid-cooling PEMFC stack: from primary configuration to system control strategy. ETransportation. 2022;12 [Google Scholar]
- 232.Moreno Nayibe Guerrero, et al. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015;52:897–906. [Google Scholar]
- 233.Asghari Saeed, Akhgar Hooman, Imani Bagher Faghih. Design of thermal management subsystem for a 5-kW polymer electrolyte membrane fuel cell system. J. Power Sources. 2011;196(6):3141–3148. [Google Scholar]
- 234.Amirfazli Amir, Asghari Saeed, Koosha Morteza. Mathematical modeling and simulation of thermal management in polymer electrolyte membrane fuel cell stacks. J. Power Sources. 2014;268:533–545. [Google Scholar]
- 235.Zhang Guangsheng, Kandlikar Satish G. A critical review of cooling techniques in proton exchange membrane fuel cell stacks. Int. J. Hydrogen Energy. 2012;37(3):2412–2429. [Google Scholar]
- 236.Bargal Mohamed HS., et al. Liquid cooling techniques in proton exchange membrane fuel cell stacks: a detailed survey. Alex. Eng. J. 2020;59(2):635–655. [Google Scholar]
- 237.Shahsavari Setareh, et al. Thermal analysis of air-cooled PEM fuel cells. Int. J. Hydrogen Energy. 2012;37(23):18261–18271. [Google Scholar]
- 238.He Guangli, Yamazaki Yohtaro, Abudula Abuliti. A three-dimensional analysis of the effect of anisotropic gas diffusion layer (GDL) thermal conductivity on the heat transfer and two-phase behavior in a proton exchange membrane fuel cell (PEMFC) J. Power Sources. 2010;195(6):1551–1560. [Google Scholar]
- 239.Chen Huicui, et al. Influencing sensitivities of critical operating parameters on PEMFC output performance and gas distribution quality under different electrical load conditions. Appl. Energy. 2019;255 [Google Scholar]
- 240.Jia Yuxin, Sunden Bengt, Xie Gongnan. A parametric comparison of temperature uniformity and energy performance of a PEMFC having serpentine wavy channels. Int. J. Energy Res. 2019;43(7):2722–2736. [Google Scholar]
- 241.Asadi Mohammad Reza, et al. The optimization of an innovative interdigitated flow field proton exchange membrane fuel cell by using artificial intelligence. Energy. 2024;290 [Google Scholar]
- 242.Burke Kenneth A., Jakupca Ian J., Colozza Anthony J. 2010. Development of Passive Fuel Cell Thermal Management Heat Exchanger. No. NASA/TM-2010-216892. [Google Scholar]
- 243.Silva Ana Paula, et al. A combined capillary cooling system for fuel cells. Appl. Therm. Eng. 2012;41:104–110. [Google Scholar]
- 244.Oro Marcos Vinício, Bazzo Edson. Flat heat pipes for potential application in fuel cell cooling. Appl. Therm. Eng. 2015;90:848–857. [Google Scholar]
- 245.Chen Yangyang, et al. Improvement of thermal management of proton exchange membrane fuel cell stack used for portable devices by integrating the ultrathin vapor chamber. Int. J. Hydrogen Energy. 2021;46(74):36995–37006. [Google Scholar]
- 246.Chen Cong, et al. Performance and parameter sensitivity analysis of the PEMFC flow channel with porous baffles. Appl. Sci. 2021;11(24) [Google Scholar]
- 247.Huang Lei, et al. Molybdenum-modified and vertex-reinforced quaternary hexapod nano-skeletons as efficient electrocatalysts for methanol oxidation and oxygen reduction reaction. Appl. Catal. B Environ. 2019;258 [Google Scholar]
- 248.Meng Zhengong, et al. Direct synthesis of L1 0-FePt nanoparticles from single-source bimetallic complex and their electrocatalytic applications in oxygen reduction and hydrogen evolution reactions. Nano Res. 2019;12:2954–2959. [Google Scholar]
- 249.Chen Zhijie, et al. Multi-objective optimization of proton exchange membrane fuel cells by RSM and NSGA-II. Energy Convers. Manag. 2023;277 [Google Scholar]
- 250.Kampker A., et al. Challenges towards large-scale fuel cell production: results of an expert assessment study. Int. J. Hydrogen Energy. 2020;45(53):29288–29296. [Google Scholar]
- 251.Luo Yueqi, Jiao Kui, Jia Bin. Elucidating the constant power, current and voltage cold start modes of proton exchange membrane fuel cell. Int. J. Heat Mass Tran. 2014;77:489–500. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.