Abstract
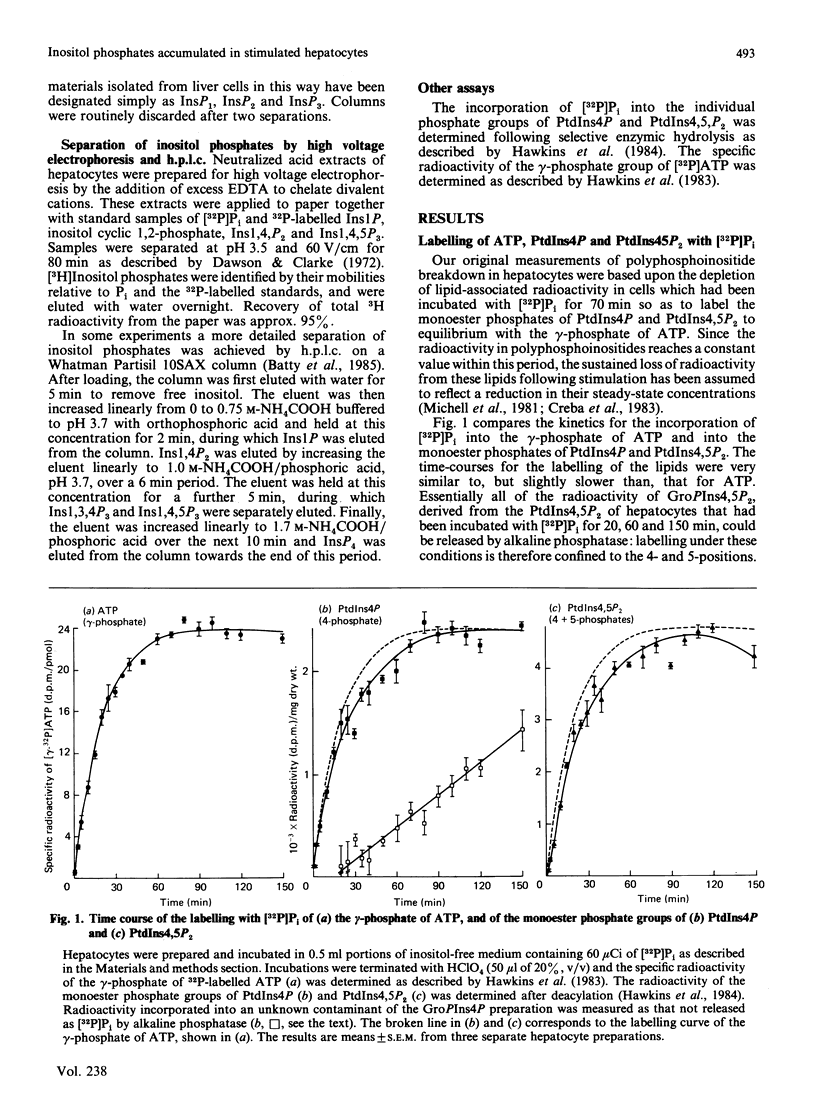
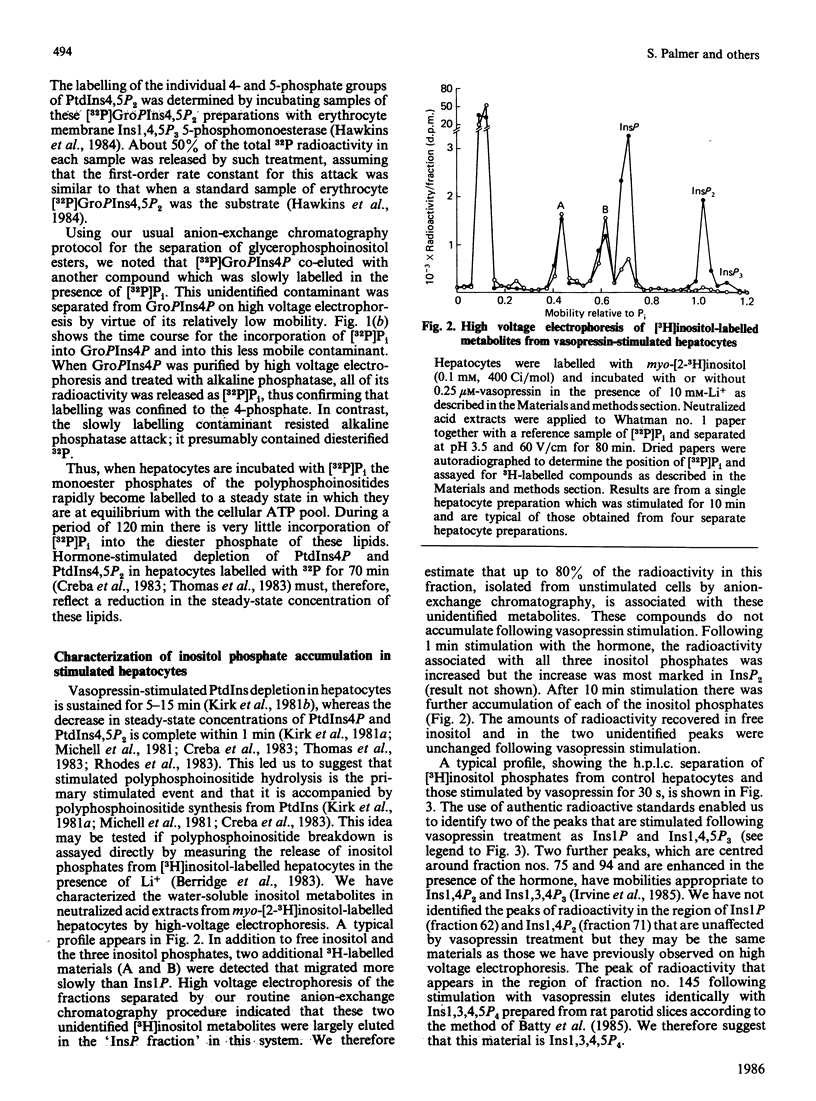
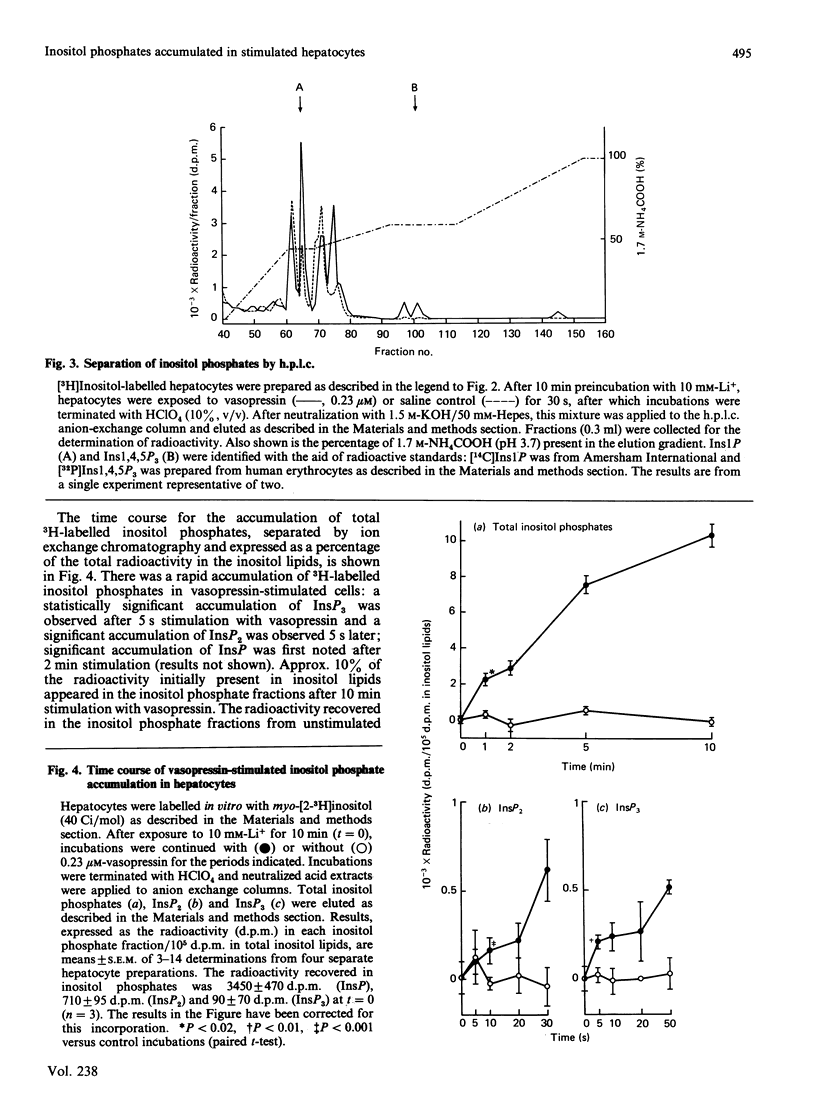
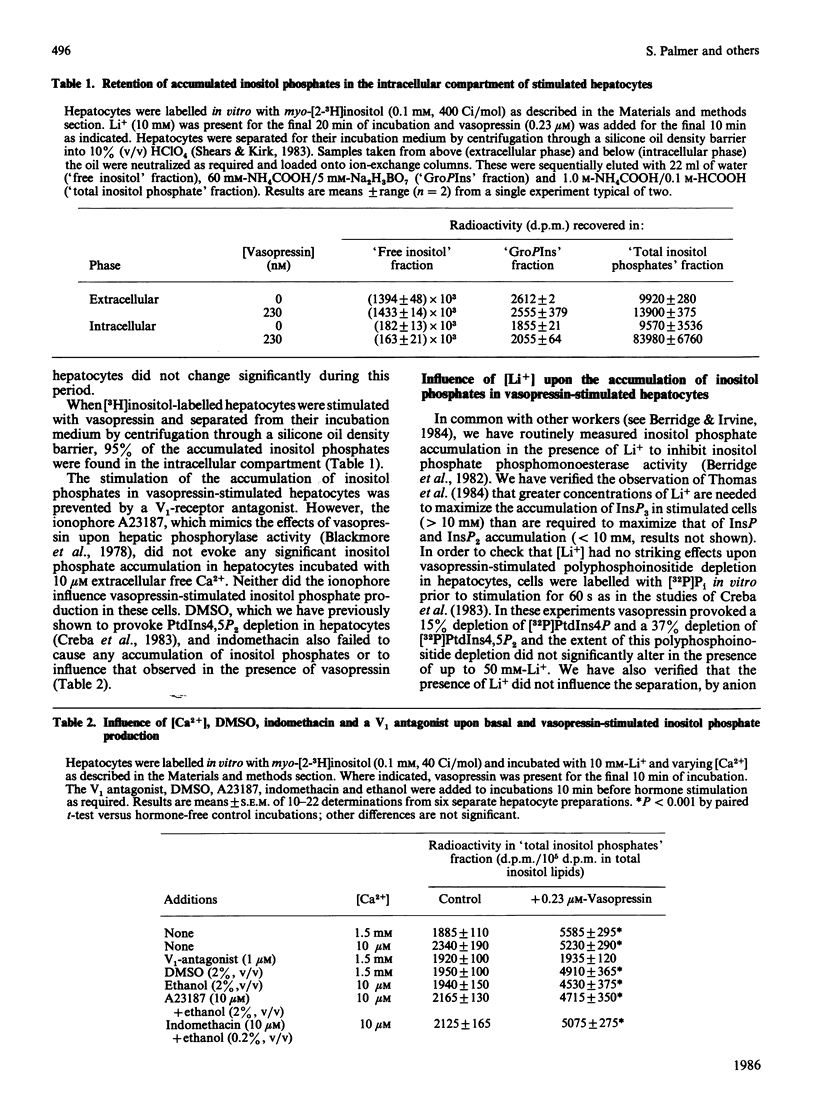
When hepatocytes were incubated with [32P]Pi, the kinetics for the labelling of the monoester phosphate groups of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate were similar to each other and slightly slower than that for the labelling of the gamma-phosphate of ATP. Analysis of the water-soluble 3H-labelled materials derived from [3H]inositol-labelled hepatocytes revealed that, in addition to inositol and its mono-, bis- and tris-phosphates (Ins, InsP, InsP2 and InsP3), these cells contained two unidentified radioactive compounds which co-eluted with InsP on anion-exchange chromatography. When [3H]inositol-labelled hepatocytes were stimulated with 0.23 microM-vasopressin in the presence of 10 mM-Li+, there was an accumulation of radioactivity in InsP, InsP2 and InsP3 but not in Ins or the two unidentified compounds. Further analysis of these inositol phosphates by h.p.l.c. revealed that vasopressin also stimulates the accumulation of inositol tetrakisphosphate (InsP4) in these cells. Vasopressin-stimulated InsP and InsP2 accumulations were maximal in the presence of 1-10 mM-Li+ but InsP3 accumulation continued to increase up to 50 mM-Li+. Accumulated inositol phosphates were retained within the cell. Li+ from 1 to 50 mM did not influence the extent of vasopressin-stimulated inositol lipid degradation in hepatocytes. In the absence of Li+, radioactivity in vasopressin-stimulated hepatocytes accumulated almost entirely in free inositol. The vasopressin-stimulated accumulation of inositol phosphates in the presence of 10 mM-Li+ was abolished by a V1-vasopressin antagonist. Inositol phosphate accumulation was not influenced by ionophore A23187, dimethyl sulphoxide or indomethacin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Durell J., Garland J. T., Friedel R. O. Acetylcholine action: biochemical aspects. Science. 1969 Aug 29;165(3896):862–866. doi: 10.1126/science.165.3896.862. [DOI] [PubMed] [Google Scholar]
- Galliard T., Michell R. H., Hawthorne J. N. Incorporation of phosphate into diphosphoinositide by subcellular fractions from liver. Biochim Biophys Acta. 1965 Dec 2;106(3):551–563. doi: 10.1016/0005-2760(65)90071-8. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Michell R. H., Kirk C. J. A simple assay method for determination of the specific radioactivity of the gamma-phosphate group of 32P-labelled ATP. Biochem J. 1983 Mar 15;210(3):717–720. doi: 10.1042/bj2100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Michell R. H., Kirk C. J. Analysis of the metabolic turnover of the individual phosphate groups of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate. Validation of novel analytical techniques by using 32P-labelled lipids from erythrocytes. Biochem J. 1984 Mar 15;218(3):785–793. doi: 10.1042/bj2180785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Bone E. A., Palmer S., Michell R. H. The role of phosphatidylinositol 4,5 bisphosphate breakdown in cell-surface receptor activation. J Recept Res. 1984;4(1-6):489–504. doi: 10.3109/10799898409042569. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Michell R. H., Hems D. A. Phosphatidylinositol metabolism in rat hepatocytes stimulated by vasopressin. Biochem J. 1981 Jan 15;194(1):155–165. doi: 10.1042/bj1940155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. A membrane-bound activity catalysing phosphatidylinositol breakdown to 1,2-diacylglycerol, D-myoinositol 1:2-cyclic phosphate an D-myoinositol 1-phosphate. Properties and subcellular distribution in rat cerebral cortex. Biochem J. 1973 Mar;131(3):433–442. doi: 10.1042/bj1310433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell B. Oncogenes and inositol lipids. 1984 Apr 26-May 2Nature. 308(5962):770–770. doi: 10.1038/308770a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N., Coleman R., Karnovsky M. L. Extraction of polyphosphoinositides with neutral and acidified solvents. A comparison of guinea-pig brain and liver, and measurements of rat liver inositol compounds which are resistant to extraction. Biochim Biophys Acta. 1970 Jun 9;210(1):86–91. doi: 10.1016/0005-2760(70)90064-0. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol lipid metabolism in dividing and differentiating cells. Cell Calcium. 1982 Oct;3(4-5):429–440. doi: 10.1016/0143-4160(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. Phosphatidylinositol breakdown induced by vasopressin and epinephrine in hepatocytes is calcium-dependent. J Biol Chem. 1982 Oct 10;257(19):11323–11331. [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. myo-Inositol uptake and metabolism in isolated rat liver cells. J Biol Chem. 1982 Oct 10;257(19):11315–11322. [PubMed] [Google Scholar]
- Rhodes D., Prpić V., Exton J. H., Blackmore P. F. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in hepatocytes by vasopressin. J Biol Chem. 1983 Mar 10;258(5):2770–2773. [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J. Characterization of a rapid cellular-fractionation technique for hepatocytes. Application in the measurement of mitochondrial membrane potential in situ. Biochem J. 1984 Apr 15;219(2):375–382. doi: 10.1042/bj2190375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]


