Abstract
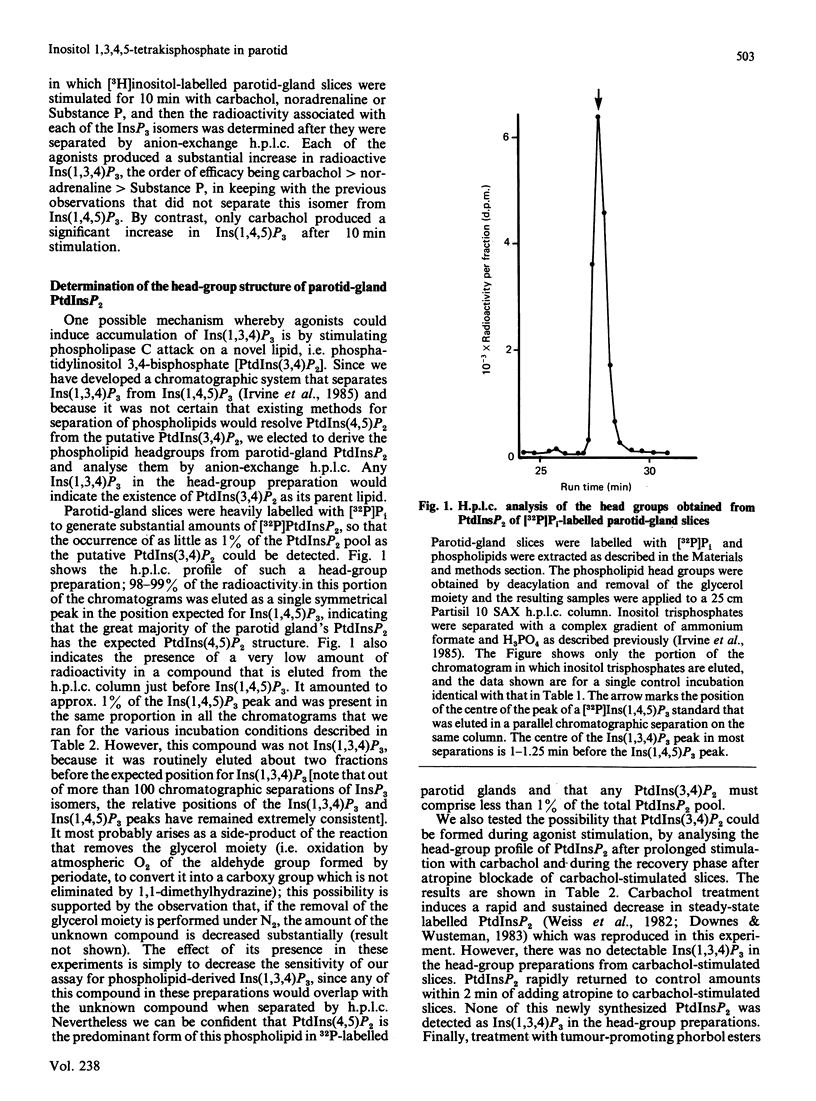
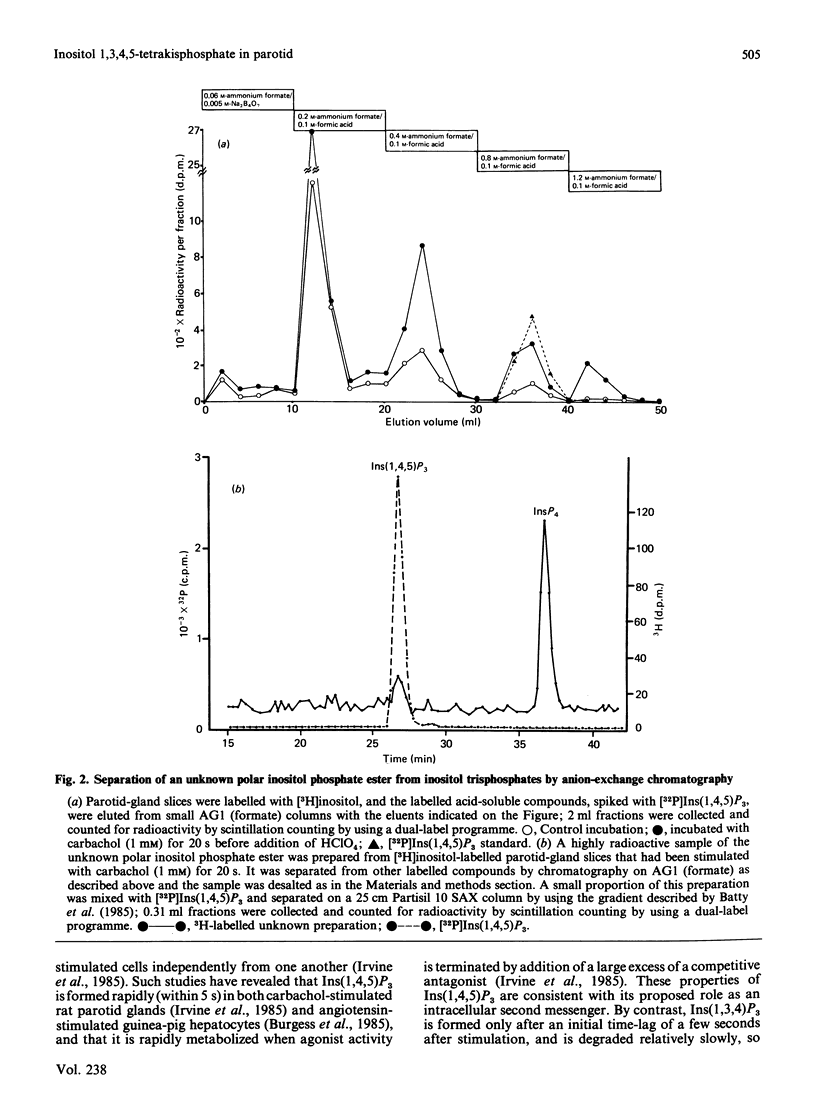
When [3H]inositol-prelabelled rat parotid-gland slices were stimulated with carbachol, noradrenaline or Substance P, the major inositol trisphosphate produced with prolonged exposure to agonists was, in each case, inositol 1,3,4-trisphosphate. Much lower amounts of radioactivity were present in the inositol 1,4,5-trisphosphate fraction separated by anion-exchange h.p.l.c. Analysis of the inositol trisphosphate head group of phosphatidylinositol bisphosphate in [32P]Pi-labelled parotid glands showed the presence of phosphatidylinositol 4,5-bisphosphate, but no detectable phosphatidylinositol 3,4-bisphosphate. Carbachol-stimulated [3H]inositol-labelled parotid glands contained an inositol polyphosphate with the chromatographic properties and electrophoretic mobility of an inositol tetrakisphosphate, the probable structure of which was determined to be inositol 1,3,4,5-tetrakisphosphate. Since an enzyme in erythrocyte membranes is capable of degrading this tetrakisphosphate to inositol 1,3,4-trisphosphate, it is suggested to be the precursor of inositol 1,3,4-trisphosphate in parotid glands.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Stewart J. C. The structure of triphosphoinositide from beef brain. Biochim Biophys Acta. 1966 Dec 7;125(3):413–421. doi: 10.1016/0005-2760(66)90029-4. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- DESJOBERT A., PETEK F. Chromatographie sur papier des esters phosphoriques de l'inositol; application a l'étude de la dégradation hydrolytique de l'inositolhexaphosphate. Bull Soc Chim Biol (Paris) 1956 Sep 4;38(5-6):871–883. [PubMed] [Google Scholar]
- Downes C. P., Dibner M. D., Hanley M. R. Sympathetic denervation impairs agonist-stimulated phosphatidylinositol metabolism in rat parotid glands. Biochem J. 1983 Sep 15;214(3):865–870. doi: 10.1042/bj2140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Putney J. W., Jr Net calcium fluxes in rat parotid acinar cells: evidence for a hormone-sensitive calcium pool in or near the plasma membrane. Pflugers Arch. 1982 Jan;392(3):239–243. doi: 10.1007/BF00584303. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol lipids and cell stimulation in mammalian salivary gland. Cell Calcium. 1982 Oct;3(4-5):369–383. doi: 10.1016/0143-4160(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Seiffert U. B., Agranoff B. W. Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):574–581. doi: 10.1016/0005-2760(65)90154-2. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Receptor-mediated net breakdown of phosphatidylinositol 4,5-bisphosphate in parotid acinar cells. Biochem J. 1982 Sep 15;206(3):555–560. doi: 10.1042/bj2060555. [DOI] [PMC free article] [PubMed] [Google Scholar]


