Abstract
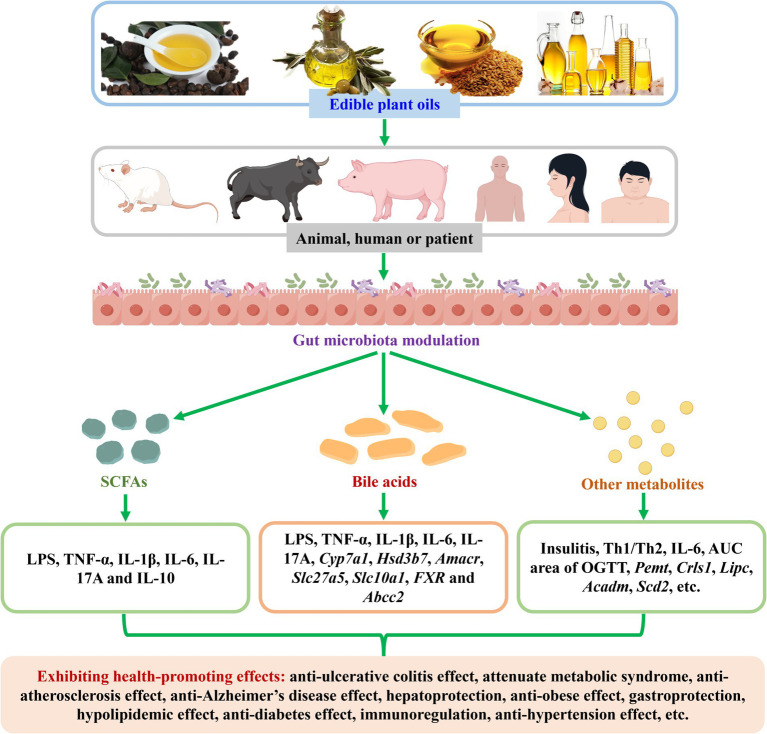
Edible plant oils are widely used in cooking, cosmetics, health supplement capsules, and other industries, due to their various health-promoting effects. There is increasing evidence that edible plant oils can modulate gut microbiota during their health-promoting effects in animal experiments and cohort or clinical studies. However, the information concerning the gut microbiota modulation of edible plant oils during their health-promoting effects is scattered. In this article, the research progress on gut microbiota modulation of edible plant oils (especially camellia oil, olive oil, and flaxseed oil) is summarized. Meanwhile, a summary on correlations between modulated gut microbiota and changed biochemical indexes is provided. The alterations of edible plant oils on gut microbiota-derived metabolites and the correlations between altered metabolites and modulated gut microbiota as well as changed biochemical indexes are reviewed. Furthermore, the prospects for gut microbiota modulation of edible plant oils during their health-promoting effects are put forward. Existing literature has shown that edible plant oils could modulate gut microbiota during their health-promoting effects, and some differential gut microbiota biomarkers were gained. Some similarities and differences existed while the oils exhibited health-promoting actions. Dosage and treatment time have influences on gut microbiota modulation of edible plant oils. Different edible plant oils exhibited different behaviors in modulating gut microbiota, and edible plant oils were mostly different in modulating gut microbiota compared to edible animal oils. Moreover, the modulated gut microbiota was significantly correlated with the changed biochemical indexes. Furthermore, edible plant oils altered SCFAs and other gut microbiota-derived metabolites. The altered metabolites were obviously correlated with the modulated gut microbiota and changed biochemical indexes. This review is helpful to the future research and application of edible plant oils in health-promoting effects from the perspective of gut microbiota.
Keywords: edible plant oils, gut microbiota, health-promoting effects, metabolites, correlations, nutritional foods
1. Introduction
Edible plant oils, obtained from the seeds, pulps, fruits, or plumules of certain plants, are an important source of dietary fat and represent as much as 25% of human caloric intake in developed countries (1). They are widely used in cooking, food, pharmaceutical, cosmetics, and other industries (2). With the increase in the world population, the demand for edible oils is increasing, showing the annual growth rate of global demand for edible plant oils as 5.14% from 2020 to 2025 (3). In 2022, the global consumption of edible plant oils was 212.82 million tons, showing a 1.25-fold increase compared to 2014 (4). A more important reason behind this was the health-promoting effects of them, due to their special fatty acid composition and abundant bioactive components.
Compared with most animal oils, edible plant oils are rich in unsaturated fatty acids and lacking cholesterol. Moreover, there are a diversity of bioactive compounds in edible plant oils, such as sterol, squalene, polyphenols, and tocopherols. As research continues, more attention has been increasingly paid to the edible plant oils’ health-promoting effects, including antioxidant (5), anti-inflammatory (6), regulation of lipid metabolism (7), anti-Alzheimer’s disease (8), and anti-cancer (9) activities, as well as other activities. For example, camellia oil has been reported to possess antioxidant, anti-cancer, anti-inflammatory, antibacterial, antihypertensive, hypoglycemic, cardioprotective, and immunoregulatory activities (10). Olive oil has been reviewed to have anti-inflammatory, chemopreventive, antimicrobial, hepatoprotective, kidney protection, and anti-neurodegenerative effects (11). Flaxseed oil has been summarized to have antioxidant, anti-inflammatory, anti-obesity, and bone osteoporosis improvement actions (12). Coconut oil has been reported to possess hypocholesterolemic, anti-cancer, antihepatosteatotic, antidiabetic, antioxidant, anti-inflammatory, antimicrobial, and skin moisturizing properties (13).
The trillions of microorganisms in the human intestine are important regulators of health (14). Gut microbiota is considered as an environmental factor that interacts with diet and may also have an impact on health outcomes, many of which involve metabolites produced by the microbiota from dietary components that can impact the host (15). In recent years, a lot of edible plant oils have been demonstrated to modulate gut microbiota during their health-promoting effects in numerous animal experiments (16–18) and cohort or clinical studies (19–21). For instance, camellia oil has been demonstrated to exhibit anti-fatigue properties by modulating the gut microbial composition of mice, which were received with Rotarod test and Treadmill test (16). Olive oil-enriched diet has been indicated to increase the abundance of lactic acid bacteria in overweight/obese subjects (19). Perilla oil has been found to relieve constipation and enhance diversity of gut microbiota in sedentary healthy female (21). Among them, camellia oil, olive oil, and flaxseed oil are three of the common edible plant oils on the market. Especially, these three oils have been extensively reported to modulate gut microbiota during their health-promoting effects.
Currently, the research progress on health-promoting effects of edible plant oils has been summarized in some literature (10–12, 22). Moreover, there were several reviews that partly summarized some findings about the modulations of edible plant oils (such as olive oil, flaxseed oil, safflower oil, and palm oil) on gut microbiota during their health-promoting effects (23–25). However, the information concerning the gut microbiota modulation of edible plant oils during their health-promoting effects is still scattered. It is necessary to conduct a comprehensive review on this aspect for better understanding of the health-promoting effects of edible plant oils from the perspective of gut microbiota.
Herein, the research progress on gut microbiota modulation of edible plant oils (especially camellia oil, olive oil, and flaxseed oil) is reviewed. Meanwhile, the correlations between modulated gut microbiota and changed biochemical indexes are summarized. The alterations of edible plant oils on gut microbiota-derived metabolites and the correlations between altered metabolites and modulated gut microbiota as well as changed biochemical indexes are reviewed. Furthermore, the prospects for gut microbiota modulation of edible plant oils in exhibiting health-promoting effects are put forward.
2. Overview of chemical composition in edible plant oils
Camellia oil is extracted from the seeds of Camellia oleifera Abel. The unsaturated fatty acid content of camellia oil is as high as 85–97%, consisting mainly of oleic (71.42–90%) and linoleic (7–14%) acids. And the saturated fatty acid content is usually approximately 10–13%, consisting mainly of palmitic acid (7–9%), stearic acid (1–3%), and a small amount of palmitoleic acid (26). Meanwhile, it also contains a multitude of bioactive components, such as sterol (2860.18–4748.39 mg/kg), squalene (122.02–248.24 mg/kg), polyphenols (20.56–88.56 mg/kg), sasanquasaponin (38.5 mg/kg), tocopherols (α-tocopherol, 153–771 mg/kg; γ-tocopherol, 9.4–59 mg/kg; δ-tocopherol, 0.27–28 mg/kg) and other functional substances (26, 27).
Olive oil is obtained from the fruit of Olea europaea L. It is composed of ~98–99% of fatty acids, mainly triacylglycerol esters of oleic acid (55–83%), palmitic acid (7.5–20%), linoleic acid (3.5–21%), and other fatty acids such as stearic acid (0.5–5%) (28). The unsaponifiable fraction of olive oil includes triterpenic dialcohols and acids (20–200 mg/kg), sterols (1000–5,000 mg/kg), squalene (1,000–8,000 mg/kg), pigments (5–30 mg/kg), and phenolic compounds (50–1,000 mg/kg) (9, 29).
Flaxseed oil is gained from the seeds of Linum usitatissimum L. It has high unsaturated fatty acids (>70%), mainly composed of linolenic acid (53.36–65.84%), linoleic acid (10.14–16.39%), oleic acid (10.03–12.37%), stearic acid (3.98–9.85%), and palmitic acid (2.41–7.97%) (12, 30). In addition, it contains many bioactive compounds, including sterols (0.25–0.3%), polyphenols (15.69–47.68 mg/kg), cyclic polypeptides (188.6–643.8 mg/kg), tocopherol (374–563.7 mg/kg), lignans (0–32, 28 mg/kg), chlorophyll (1.45–2.08 mg/kg), carotenoid (2.89–3.45 mg/kg), and other compounds (12).
Other edible plant oils are also rich in unsaturated fatty acids, mainly composed of oleic acid (soybean oil, 15–36%; peony seed oil, 20.5–45.1%; walnut oil, 10–20%; etc.), linoleic acids (soybean oil, 42.8–56.1%; peony seed oil, 16.5–33.6%; walnut oil, 55–70%; etc.) and linolenic acids (soybean oil, 2–14%; peony seed oil, 28.1–46.9%; walnut oil, 10–18%; etc.) (2). Meanwhile, other edible plant oils contain many bioactive ingredients, including phytosterols (safflower oil, 243.7 mg/100 g; peony seed oil, 154.5 mg/100 g; walnut oil, 115.7 mg/100 g; etc.), phenolic compounds (safflower oil, 231.40 mg/kg; Torreya grandis seed oil, 12,630 mg/kg; almond oil, 644.54 mg/kg; etc.), tocopherol (sea buckthorn seed oil, 898.1 mg/kg; tomato seed oil, 345.8 mg/kg; rice bran oil, 322.7 mg/kg; etc.), squalene (walnut oil, 12 mg/100 g; peony seed oil, 4 mg/100 g; rice bran oil, 24 mg/100 g; etc.), and β-carotene (tomato seed oil, 765.7 mg/kg; sea buckthorn seed oil, 55.3 mg/kg; corn oil, 0.07 mg/kg; etc.) (22).
3. Gut microbiota modulation of edible plant oils
3.1. Camellia oil modulation
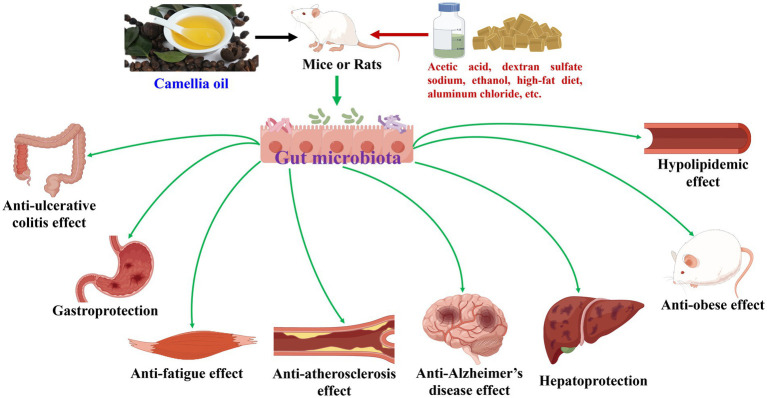
Camellia oil has been demonstrated to modulate gut microbiota during its health-promoting effects (8, 16, 31–42), as shown in Table 1 and Figure 1. In terms of anti-ulcerative colitis effect, supplementation of camellia oil increased the abundances of Firmicutes and/or Firmicutes/Bacteroidetes ratio, and/or decreased that of Bacteroidetes in acetic acid and dextran sulfate sodium-induced rats or mice, at phylum level (32, 33). And, at the genus level, camellia oil upregulated the amounts of Bacteroides and Lactobacillus and downregulated those of Alistipes and Lachnospiraceae NK4A136 group in dextran sulfate sodium-induced colitis mice (33). Moreover, Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria were differential gut microbiota biomarkers for camellia oil in anti-ulcerative colitis against acetic acid-induced colitis rats (32).
Table 1.
Gut microbiota modulation of camellia oil during its health-promoting effects.
| Health-promoting effect | Experimental model | Oil dosage and treatment time | Gut microbiota modulation | Gut microbiota biomarker | References |
|---|---|---|---|---|---|
| Anti-ulcerative colitis | Acetic acid-induced rats | 2 mL/kg BW, 3 weeks | Lactobacillus spp. and Bifidobacterium spp. (+) | NA | (31) |
| Anti-ulcerative colitis | Acetic acid-induced rats | 2 mL/kg BW, 20 d | Phylum level: Firmicutes (+); Bacteroidetes (−) | Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria | (32) |
| Anti-ulcerative colitis | Dextran sulfate sodium-induced mice | 0.5, 1 and 2 mL/kg BW, 3 weeks | Phylum level: Firmicutes and Firmicutes/Bacteroidetes ratio (+) | NA | (33) |
| Genus level: Bacteroides and Lactobacillus (+); Alistipes and Lachnospiraceae NK4A136 group (−) | |||||
| Gastroprotection | Ethanol-induced mice | NA, 2 weeks | Phylum level: Bacteroidetes (+); Actinobacteria and Verrucomicrobia (−) | Dorea, Olsenella and Bosea | (41) |
| Genus level: Bacteroides (+) | |||||
| Anti-fatigue | Rotarod test and Treadmill test in mice | 2, 4, and 6 mL/kg BW, 4 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Actinobacteria (−) | Faecalibacterium, Muribaculaceae, Coriobacteriaceae_UCG_002, Desulfobacteria and Faecalibaculum | (16) |
| Genus level: Alistipes, Alloprevotella, Lactobacillus, g_UCG-010 and Muribaculaceae (+); Dubosiella (−) | |||||
| Anti-atherosclerosis | High-fat diet-induced ApoE−/− mice | 3 and 6 mL/kg BW, 8 weeks | Phylum level: Bacteroidetes and Tenericutes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | Faecalibaculum, Defluviitaleaceae UCG, Streptococcus, Enterorhabdus, Bilophila and Leuconostoc | (40) |
| Genus level: Alloprevotella and Lachnospiraceae NK4A136 groups (+); Faecalibaculum, Dubosiella, Coriobacteriaceae UCG-002 and Lactobacillus (−) | |||||
| Anti-Alzheimer’s disease | Aluminum chloride-induced rats | 1.5 and 3 mL/kg BW, 7 weeks | Lactobacillales (+); Enterobacteriaceae (−) | Lactobacillus | (34) |
| Anti-Alzheimer’s disease | Aβ25-35-induced mice | 0.5 and 1 mL/kg BW, 4 weeks | Phylum level: Bacteroidetes (+); Firmicutes (−) | NA | (35) |
| Anti-Alzheimer’s disease | Aluminum chloride-induced rats | 2 mL/kg BW, 7 weeks | Family level: Muribaculaceae (+); Lachnospiraceae (−) | NA | (8) |
| Genus level: [Eubacterium]_coprostanoligenes_group, Lactobacillus_johnsonii (+); Lachnospiraceae_ND3007_group and Bacteroides (−) | |||||
| Anti-Alzheimer’s disease | Aluminum chloride-induced rats | 3 mL/kg BW, 7 weeks | Phylum level: Bacteroidetes and Proteobacteria (+); Firmicutes (−) | NA | (36) |
| Genus level: Bacteroides pectinophilus group, Eubacterium xylanophilum group, and Intestinimonas (+); Lachnospira and Ruminiclostridium (−) | |||||
| Hepatoprotection | Ethanol-induced mice | NA, 4 weeks | Phylum level: Bacteroidota (+); Firmicutes (−) | NA | (42) |
| Genus level: Alloprevotella and Bacteroides (+); Turicibacter (−) | |||||
| Anti-obese | High-fat diet-induced mice | 3 and 6 mL/kg BW, 8 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | Actinobacteria, Lactobacillaceae, Coriobacteriaceae, Bacilli, Coriobacteriia, Coriobacteriales, Lactobacillales, Anoxybacillus, Limnobacter, Perlucidibaca, Lactobacillus and Finegoldia | (37) |
| Genus level: Lactobacillus, Bacteroides and Alloprevotella (+); Allobaculum, Lachnospiraceae NK4A136 group and Helicobacter (−) | |||||
| Anti-obese | High-fat diet-induced mice | 2 g/kg BW, 8 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | NA | (38) |
| Genus level: Alistipes, Blautia, Alloprevotella and Akkermansia (+); Lactobacillus, Bacteroides, Helicobacter and Parabacteroides (−) | |||||
| Hypolipidemic effect | High-fat diet-induced mice | 9 g/kg BW, 6 weeks | Phylum level: Bacteroidota and Desulfobacterota (+); Firmicutes and Proteobacteria (−) | NA | (39) |
| Genus level: Dubosiella, Lactobacillus and Alistipes (+); Staphylococcus and Aerococcus (−) |
Figure 1.
Camellia oil modulates gut microbiota during its health-promoting effects.
Regarding anti-Alzheimer’s disease action, camellia oil added Lactobacillales and reduced Enterobacteriaceae, with Lactobacillus as the differential gut microbiota biomarker, toward aluminum chloride-induced rats (34). At the phylum level, camellia oil enhanced Bacteroidetes and Proteobacteria and lowered Firmicutes (36). At genus level, camellia oil elevated the abundances of Bacteroides pectinophilus group, Eubacterium xylanophilum group, Intestinimonas, [Eubacterium]_coprostanoligenes_group and Lactobacillus_johnsonii, and decreased those of Lachnospira, Ruminiclostridium, Lachnospiraceae_ND3007_group and Bacteroides (8, 36). On the other hand, camellia oil intervention upregulated Bacteroidetes and downregulated Firmicutes while exerted anti-Alzheimer’s disease action on Aβ25–35-induced mice (35).
To anti-obese activity, camellia oil enhanced Bacteroidetes and reduced Firmicutes and Firmicutes/Bacteroidetes ratio in high-fat diet-induced mice, at the phylum level (37, 38). At the genus level, camellia oil increased the abundances of six bacteria (Lactobacillus, Bacteroides, Alloprevotella, Alistipes, Blautia, etc.), and decreased those of six bacteria (Allobaculum, Lachnospiraceae NK4A136 group, Helicobacter, Lactobacillus, Bacteroides, etc.) in high-fat diet-induced mice (37, 38). Moreover, 12 types of bacteria (Actinobacteria, Lactobacillaceae, Coriobacteriaceae, Bacilli, Coriobacteriia, etc.) were differential gut microbiota biomarkers for camellia oil that exerted anti-obese effect against high-fat diet-induced mice (37).
Other effects included anti-atherosclerosis (40), gastroprotection (41), hepatoprotection (42), anti-fatigue (16), and hypolipidemic effects (39). Camellia oil upregulated the abundances of Bacteroidetes, Tenericutes, Alloprevotella, and/or Desulfobacterota, and downregulated those of Firmicutes, Actinobacteria, Verrucomicrobia, Turicibacter, and Proteobacteria along with Firmicutes/Bacteroidetes ratio at phylum level, in high-fat diet or ethanol-induced ApoE−/− mice and/or normal mice. At the genus level, camellia oil enhanced the abundances of nine bacteria (Bacteroides, Alistipes, Alloprevotella, Lactobacillus, g_UCG-010, etc.) and lowered those of seven bacteria (Dubosiella, Faecalibaculum, Coriobacteriaceae UCG-002, Lactobacillus, Turicibacter, etc.) (16, 39–42). Moreover, Dorea, Olsenella, and Bosea were identified to be differential gut microbiota biomarkers for camellia oil that exhibited gastroprotection on ethanol-induced mice (41). Six bacteria (Faecalibaculum, Defluviitaleaceae UCG, Streptococcus, Enterorhabdus, Bilophila, etc.) were differential gut microbiota biomarkers for camellia oil exerted anti-atherosclerosis effect against high-fat diet-induced ApoE−/− mice (40).
Some differences and similarities in gut microbiota modulation could be found while camellia oil exhibited health-promoting effects in animal experiments. In terms of the similarities, at the phylum level, camellia oil treatment mostly caused increment in Bacteroidetes (16, 35–39, 41, 42) and reduction in Firmicutes (16, 35–39, 42). These led to the decrease of Firmicutes/Bacteroidetes ratio (37–40). Meanwhile, the abundance of Actinobacteria was lowered while camellia oil exerted gastroprotection (41) and anti-fatigue (16) effects. At the genus level, the amount of Bacteroides was raised as camellia oil exhibiting anti-ulcerative colitis (33), gastroprotection (41), hepatoprotection (42), and anti-obese (37) effects. The abundance of Alloprevotella was elevated, while camellia oil showed anti-fatigue (16), anti-atherosclerosis (40), hepatoprotection (42), and anti-obese (37, 38) activities. That of Alistipes was enhanced as camellia oil displayed anti-fatigue (16), anti-obese (38), and hypolipidemic (39) effects. On the contrary, the abundance of Dubosiella was decreased as camellia oil revealed anti-fatigue (16) and anti-atherosclerosis (40) activities. That of Lachnospiraceae NK4A136 group was reduced, while camellia oil showed anti-ulcerative colitis (33) and anti-obese (37) actions. The amount of Lactobacillus was downregulated while camellia oil generated anti-atherosclerosis and anti-obese effects (37, 38).
Regarding the differences, at the phylum level, the abundance of Firmicutes was increased and/or that of Bacteroidetes was decreased, while camellia oil exerted anti-ulcerative colitis action (32, 33). Whereas, opposite phenomena were observed as camellia oil showed gastroprotection (41), anti-fatigue (16), anti-atherosclerosis (40), anti-Alzheimer’s disease (35, 36), hepatoprotection (42), anti-obese (37, 38), and hypolipidemic effects (39). Correspondingly, the Firmicutes/Bacteroidetes ratio was enlarged while camellia oil exerted anti-ulcerative colitis action (33), while it was shrunk as the oil revealed anti-atherosclerosis (40) and anti-obese (37, 38) effects. Meanwhile, the amount of Proteobacteria was added and reduced while camellia oil exhibited anti-Alzheimer’s disease action (36) and hypolipidemic effect (39), respectively. At the genus level, the abundance of Bacteroides was decreased while camellia oil showed anti-Alzheimer’s disease (8) and anti-obese (38) effects, whereas it was increased as this oil displayed anti-ulcerative colitis (33), gastroprotection (41), hepatoprotection (42) and anti-obese (37) activities. Meanwhile, the abundance of Lactobacillus was upregulated while camellia oil displayed anti-ulcerative colitis (33), anti-fatigue (16), and hypolipidemic (39) effects, and it was downregulated as camellia oil exerted anti-atherosclerosis action (40). That of Lachnospiraceae NK4A136 group was decreased while camellia oil exhibited anti-ulcerative colitis (33) and anti-obese (37) activities, and it was increased as the oil showed anti-atherosclerosis action (40). The amount of Alistipes was reduced while camellia oil revealed anti-ulcerative colitis effect (33), while it was enhanced as this oil exerted anti-fatigue (16), anti-obese (38), and hypolipidemic (39) effects. The abundance of Dubosiella was decreased while camellia oil showed anti-fatigue (16) and anti-atherosclerosis (40) activities, whereas it was increased as the oil exerted hypolipidemic effect (39).
Overall, camellia oil could modulate gut microbiota during their many health-promoting effects. Moreover, differential gut microbiota biomarkers have been screened for it and showed anti-ulcerative colitis, anti-Alzheimer’s disease, anti-obese, and gastroprotection activities. Furthermore, some differences and similarities in gut microbiota modulation have been found while this oil exhibited different health-promoting effects.
3.2. Olive oil modulation
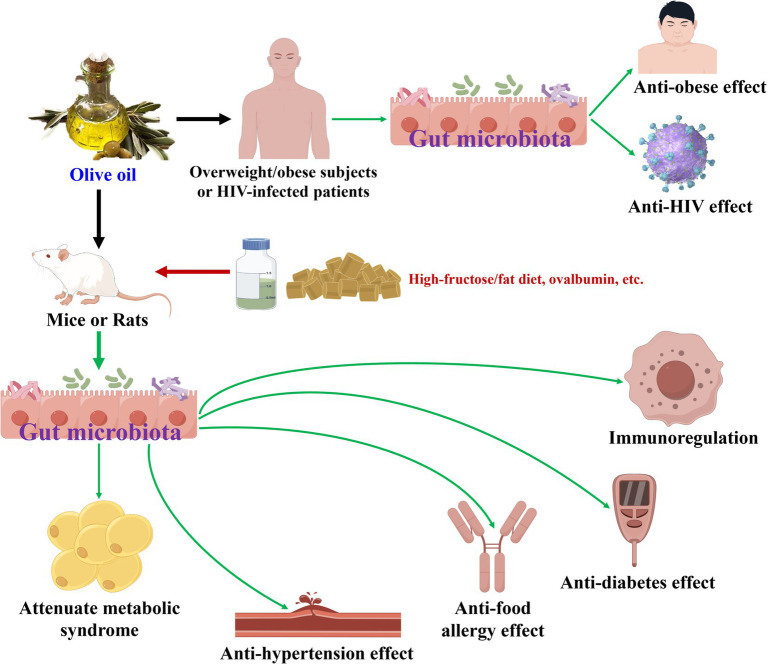
Olive oil has been proven to modulate gut microbiota during its health-promoting effects (19, 43–50), as illustrated in Table 2 and Figure 2. In terms of attenuate metabolic syndrome action, olive oil increased the abundances of six bacteria (Eubacteriaceae, Bifidobacteriaceae, Anaeroplasmataceae, Erysipelotrichaceae, Clostridiaceae_1, etc.) and decreased those of seven bacteria (Corynebacteriaceae, Enterococcaceae, Aerococcaceae, Staphylococcaceae, Coriobacteriaceae, etc.) at family level in high-fructose/fat diet-induced rats or normal mice (43, 45). At the phylum level, olive oil enhanced the amount of Proteobacteria in normal mice (44). At the genus level, olive oil elevated the amounts of eight bacteria (Olsenella, Bifidobacterium, [Eubacterium]_fissicatena_group, Ruminococcaceae_UCG-014, Allobaculum, etc.), and lowered those of 12 bacteria (Bilophila, Aerococcus, Staphylococcus, uncultured_bacterium_f_Coriobacteriaceae, Faecalitalea, etc.) (43–45). Moreover, six types of bacteria (c__Gammaproteobacteria, o__Enterobacteriales, f__Enterobacteriaceae, s__uncultured_bacterium_g_Blautia, s__uncultured_bacterium_g_Allobaculum, etc.) were screened to be differential gut microbiota biomarkers for olive oil and showed attenuate metabolic syndrome activity against high-fructose/fat diet-induced rats (43).
Table 2.
Gut microbiota modulation of olive oil during its health-promoting effects.
| Health-promoting effect | Experimental model | Oil dosage and treatment time | Gut microbiota modulation | Gut microbiota marker | References |
|---|---|---|---|---|---|
| Attenuate metabolic syndrome | High-fructose/fat diet-induced rats | 10%, 12 weeks | Family level: Eubacteriaceae, Bifidobacteriaceae, Anaeroplasmataceae, Erysipelotrichaceae, and Clostridiaceae_1 (+); Corynebacteriaceae, Enterococcaceae, Aerococcaceae, Staphylococcaceae and Coriobacteriaceae (−) | c__Gammaproteobacteria, s__uncultured_bacterium_g_Blautia, o__Enterobacteriales, f__Enterobacteriaceae, s__uncultured_bacterium_g_Allobaculum and g__Allobaculum | (43) |
| Genus level: Olsenella, Bifidobacterium, [Eubacterium]_fissicatena_group, Ruminococcaceae_UCG-014 and Allobaculum (+); Bilophila, Aerococcus, Staphylococcus, uncultured_bacterium_f_Coriobacteriaceae, Faecalitalea, Enterococcus, Corynebacterium_1 and [Ruminococcus]_torques_group (−) | |||||
| Attenuate metabolic syndrome | Normal mice | 20%, 12 weeks | Phylum level: Proteobacteria (+) | NA | (44) |
| Genus level: Parasutterella, Marispirillum and Marinilabilia (+); Prevotella, Anaerophaga, Fusicatenibacter and Christensenella (−) | |||||
| Attenuate metabolic syndrome | Normal mice | 20%, 12 weeks | Family level: Erysipelotrichaceae and Sutterellaceae (+); Prevotellaceae and Christensenellaceae (−) | NA | (45) |
| Genus level: Parasutterella and Marinilabilia (+); Anaerophaga and Fusicatenibacter (−) | |||||
| Anti-hypertension | Spontaneously hypertensive rats | 20%, 12 weeks | Lactobacillus sp., Clostridia XIVa and Universal (+) | NA | (46) |
| Anti-food allergy | Ovalbumin-sensitized mice | 1.0, 2.0 and 3.0 g/kg BW, 7 weeks | Phylum level: Actinobacteriota (+) | f__Bifidobacteriaceae, o__Bifidobacteriales, o__Micrococcales, c__Actinobacteria, f__Streptococcaceae, f__Staphylococcaceae, o__Staphylococcales, f__ Enterobacteriaceae, o__Enterobacterales, f__Pseudomonadaceae and o__Pseudomonadales | (47) |
| Genus level: Clostridiaceae (+); Burkholderiaceae (−) | |||||
| Anti-diabetes | NOD/LtJ mice | 2.5 mL/kg BW, 14 weeks | Phylum level: Bacteroidetes, Verrucomicrobia, Cyanobacteria, and Bacteroidetes/Firmicutes ratio (+); Firmicutes (−) | Bacteroides, Muribaculaceae, Alistipes, Lachnoclostridium, Tyzzerella, Ruminococcaceae_UCG_005, Eubacterium_xylanophilum_group, Intestinimonas, Angelakisella, Akkermansia and Gastranaerophilales | (48) |
| Genus level: Bacteroides, Muribaculum, Akkermansia, Ruminococcaceae_UCG_005, Intestinimonas and Angelakisella (+); Lachnospira and Eubacterium_xylanophilum_group (−) | |||||
| Anti-obese | Overweight/obese subjects | 40 g/die, 3 months | Lactic acid bacteria (+) | NA | (19) |
| Anti-HIV | HIV-infected patients | 50 mL, 12 weeks | Genus level: Gardnerella (+); Mogibacterium, Dethiosulfovibrionaceae and Coprococcus (−) | g__Gardnerella | (49) |
| Immunoregulation | Normal mice | 157.8 g, 10 weeks | Phylum level: Deferibacteres (+); Firmicutes (−) | NA | (50) |
| Genus level: Mucispirillum, Lachnospiraceae, Bacteroides, Allobaculum and Coriobacteriaceae spp. (+); S24-7 spp. (−) |
Figure 2.
Olive oil modulates gut microbiota during its health-promoting effects.
Regarding the other above-mentioned health-promoting effects, olive oil boosted the abundances of Actinobacteria, Bacteroidetes, Verrucomicrobia, Cyanobacteria, and Deferibacteres as well as Bacteroidetes/Firmicutes ratio, and declined that of Firmicutes at the phylum level (47, 48, 50). At the genus level, olive oil raised the amounts of 12 bacteria (Clostridiaceae, Bacteroides, Muribaculum, Akkermansia, Ruminococcaceae_UCG_005, etc.), and reduced those of seven bacteria (Burkholderiaceae, Lachnospira, Eubacterium_xylanophilum_group, Mogibacterium, Dethiosulfovibrionaceae, etc.), while exerted anti-food allergy, anti-diabetes, anti-HIV, and immunoregulation effects (47–50). On the other hand, olive oil supplement added the abundances of Lactobacillus sp., Clostridia XIVa, and Universal as exerted anti-hypertension activity (46), and aggrandized the number of Lactic acid bacteria while exhibited anti-obese action (19). Moreover, for olive oil revealed anti-food allergy effect, 11 types of bacteria (f__Bifidobacteriaceae, o__Bifidobacteriales, o__Micrococcales, c__Actinobacteria, f__Streptococcaceae, etc.) were identified to be differential gut microbiota biomarkers (47). Olive oil exhibited anti-diabetes activity, and 11 types of bacteria (Bacteroides, Muribaculaceae, Alistipes, Lachnoclostridium, Tyzzerella, etc.) were characterized as differential gut microbiota biomarkers (48). As to olive oil showed anti-HIV action, g__Gardnerella was the differential gut microbiota biomarker (49).
Some similarities in gut microbiota modulation could be seen while olive oil exhibited different health-promoting effects in animal experiments. At the phylum level, the abundance of Firmicutes was reduced and that of Bacteroides was enhanced, while olive oil exerted anti-diabetes and immunoregulation effects (48, 50).
In short, olive oil could modulate gut microbiota during their health-promoting effects. Moreover, differential gut microbiota biomarkers have been gained for exerting attenuate metabolic syndrome, anti-food allergy, anti-diabetes, and anti-HIV actions. Furthermore, some similarities in gut microbiota modulation have been discovered as this oil exhibited anti-diabetes and immunoregulation effects.
3.3. Flaxseed oil modulation
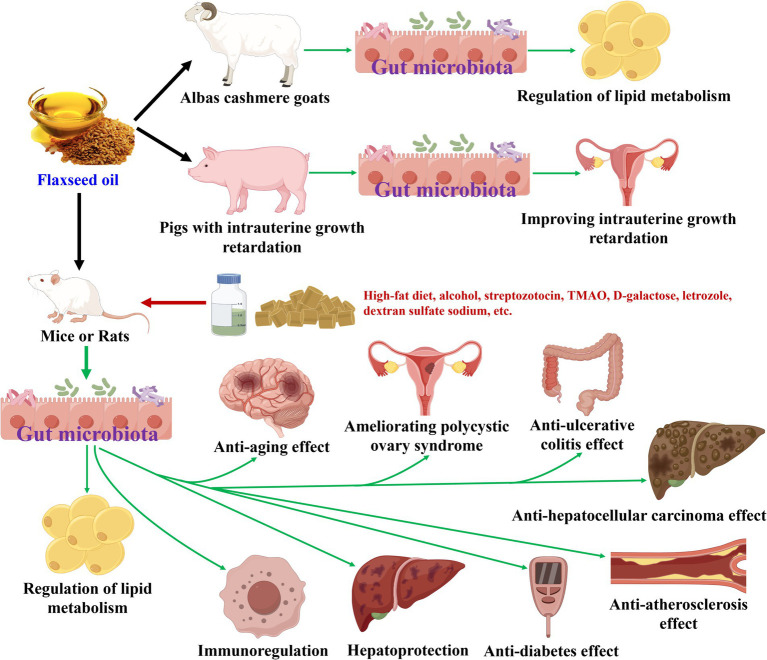
Flaxseed oil has been discovered to modulate gut microbiota during its health-promoting effects (18, 50–63), as displayed in Table 3 and Figure 3. In terms of regulation of lipid metabolism effect, flaxseed oil treatment increased the abundances of Actinobacteria, Proteobacteria, and Spirochaeta, and decreased those of Firmicutes, Saccharibacteria, and Verrucomicrobia at the phylum level in Albas cashmere goats (52, 53). At the genus level, flaxseed oil elevated the amounts of 17 bacteria (Uc_Ruminococcaceae, Uc_Lachnospiraceae, Oscillospira, Ruminococcus, Ruminococcus_2, etc.), and declined those of 10 bacteria (Sporosarcina, Mogibacteriaceae, Clostridium, unclassified_f_Peptostreptococcaceae, Clostridium_sensu_stricto_1, etc.) (51–53). Moreover, six types of bacteria (Ruminococcus, Anaerotruncus, Ruminococcaceae, Lachnospiraceae, Dehalobacterium, etc.) have been screened to be differential gut microbiota biomarkers for flaxseed oil and showed lipid metabolism regulation against high-fat diet-induced mice (51). While Lachnospiraceae_NK3A20_group has been identified as a differential gut microbiota biomarker for flaxseed oil-regulated lipid metabolism in Albas cashmere goats (53). Regarding anti-diabetes action, flaxseed oil supplement enhanced Bacteroidetes, and reduced Firmicutes and Firmicutes/Bacteroidetes ratio at phylum level in streptozotocin-nicotinamide-induced rats (55). At the genus level, flaxseed oil upregulated Alistipes and downregulated Blautia. While the study of Xia et al. (56) has revealed that flaxseed oil treatment raised the abundance of six bacteria (Bacteroidetes, Muribaculaceae, Streptococcaceae, Lactococcus, Streptococcus, etc.). To anti-atherosclerosis activity, flaxseed oil lessened the number of Eubacterium and Firmicutes/Bacteroidetes ratio at the phylum level in TMAO and high-fat diet-induced ApoE−/− mice (57, 58). At the genus level, flaxseed oil expanded the abundances of Alistipes and Odoribacter and diminished the amounts of seven bacteria (Intestinimonas, Bilophila, Anaerotruncus, Oscillibacter, Lachnoclostridium, etc.).
Table 3.
Gut microbiota modulation of flaxseed oil during its health-promoting effects.
| Health-promoting effect | Experimental model | Oil dosage and treatment time | Gut microbiota modulation | Gut microbiota marker | References |
|---|---|---|---|---|---|
| Immunoregulation | Normal mice | 157.8 g, 10 weeks | Phylum level: Firmicutes (−) | NA | (50) |
| Genus level: Allobaculum and Coriobacteriaceae spp. (+); Clostridi ales spp. (−) | |||||
| Regulation of lipid metabolism | High-fat diet-induced mice | 30, 60 and 90%, 8 weeks | Genus level: unclassified_Ruminococcaceae, unclassified_Lachnospiraceae, Oscillospira and Ruminococcus (+); unclassified_S24-7, Sporosarcina, Mogibacteriaceae and Clostridium (−) | Ruminococcus, Anaerotruncus, Ruminococcaceae, Lachnospiraceae, Dehalobacterium, and Eubacteriaceae | (51) |
| Regulation of lipid metabolism | Albas cashmere goats | 36%, 90 d | Phylum level: Actinobacteria (+) | NA | (52) |
| Genus level: Ruminococcus_2, Ruminococcaceae_UCG-014, Christensenellaceae_R-7_group, [Eubacterium]_coprostanoligenes_group, Turicibacter, Family_XIII_AD3011_group, Lactobacillus, Aeriscardovia, Bifidobacterium, Olsenella and Ureaplasma (+); unclassified_f_Peptostreptococcaceae, Clostridium_sensu_stricto_1, Lachnospiraceae_NK3A20_group, Acetitomaculum, Streptococcus, [Ruminococcus]_gauvreauii_group and Mycoplasma (−) | |||||
| Regulation of lipid metabolism | Albas cashmere goats | 2.0% or 2.5%, 90 d | Phylum level: Proteobacteria and Spirochaeta (+); Firmicutes, Saccharibacteria and Verrucomicrobia (−) | Lachnospiraceae_NK3A20_group | (53) |
| Genus level: Rikenellaceae_RC9_gut_group and Lachnospiraceae_NK3A20_group (+) | |||||
| Hepatoprotection | Alcohol-induced liver injury in mice | 39.6 g/L, 6 weeks | Phylum level: Proteobacteria (−) | NA | (59) |
| Genus level: Parabacteroides (+) | |||||
| Anti-diabetes | Streptozotocin-nicotinamide-induced rats | 10%, 5 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | NA | (55) |
| Genus level: Alistipes (+); Blautia (−) | |||||
| Anti-diabetes | Streptozotocin-induced mice | 7.0 and 10.5%, 6 weeks | Bacteroidetes, Muribaculaceae, Streptococcaceae, Lactococcus, Streptococcus and uncultured_bacterium_f_Muribaculaceae (+) | NA | (56) |
| Anti-atherosclerosis | TMAO-induced ApoE−/− mice | 102.1 g, 12 weeks | Phylum level: Eubacterium (−) | NA | (57) |
| Genus level: Alistipes and Odoribacter (+) | |||||
| Anti-atherosclerosis | High-fat diet-induced ApoE−/− mice | 10%, 12 weeks | Phylum level: Firmicutes/Bacteroidetes ratio (−) | NA | (58) |
| Genus level: Intestinimonas, Bilophila, Anaerotruncus, Oscillibacter, Lachnoclostridium, Enterorhabdus and Negativibacillus (−) | |||||
| Anti-aging | D-galactose-induced rats | 40 g/kg, 8 weeks | Genus level: uncultured_bacterium_f_Lachnospiraceae and Ruminococcaceae_UCG-005 (+); uncultured_bacterium_f_Desulfovibrionaceae, Akkermansia, Romboutsia, Prevotella_9 and [Eubacterium]_oxidoreducens_group (−) | p_Firmicutes, o_Clostridiales, c_Clostridia, f_Lachnospiraceae, s_uncultured_bacterium_f_Lachnospiracea, g_uncultured_bacterium_f_Lachnospiraceae, s_uncultured_bacterium_g_Lachnospiraceae_NK4A136_group, s_uncultured_bacterium_g_Lactobacillus, f_Lactobacillaceae, g_Lactobacillus and o_Lactobacillales | (60) |
| Ameliorating polycystic ovary syndrome | Letrozole-induced rats | 1 mL/kg, 8 weeks | Phylum level: Actinobacteria, Proteobacteria, and Firmicutes/Bacteroidetes ratio (−) | NA | (61) |
| Genus level: Lactobacillus, Allobaculum, Butyrivibrio, Desulfovibrio, Bifidobacterium, Faecalibacterium and Parabacteroides (+); Bacteroides and Streptococcus (−) | |||||
| Improving intrauterine growth retardation | Pigs with intrauterine growth retardation | 4%, 3 weeks | Phylum level: Actinobacteria and Melainabacteria (+); Spirochaetes (−) | NA | (62) |
| Genus level: Bifidobacterium and Blautia (+) | |||||
| Anti-ulcerative colitis | Dextran sulfate sodium-induced colitis in rats | 400, 800 and 1,600 mg/kg BW, 6 weeks | Phylum level: Bacteroidetes, Proteobacteria, and Verrucomicrobia (+) | NA | (63) |
| Genus level: Lactobacillus, Lachnospiraceae_NK4A136_group, Lachnoclostridium, Phascolarctobacterium and Prevotellaceae_UCG-001 (+); Romboutsia and Ruminococcaceae_UCG-005 (−) | |||||
| Anti-hepatocellular carcinoma | Orthotopic hepatocellular carcinoma mice | NA, 30 d | Phylum level: Firmicutes (+); Proteobacteria and Actinobacteriota (−) | NA | (18) |
| Genus level: norank_f_norank_o_Clostridia UCG-014, norank_f_norank_Clostridia_vadinBB60_group and Turicibacter (+); Escherichia-shigella, Enterorhabdus and Lactococcus (−) | |||||
| Improving lipid metabolism and gut barrier homeostasis | Normal rats | 5 g/kg BW, 8 weeks | Family level: Lactobacillaceae and Ruminococcaceae (−) | NA | (54) |
| Species level: uncultured_bacterium lahnospiraceae (+); Lactobacillus (−) |
Figure 3.
Flaxseed oil modulates gut microbiota during its health-promoting effects.
For aforementioned other effects, flaxseed oil aggrandized the abundances of Actinobacteria, Melainabacteria, Bacteroidetes, Proteobacteria, Verrucomicrobia, and/or Firmicutes and lowered those of Firmicutes, Proteobacteria, Actinobacteria and/or Spirochaetes as well as Firmicutes/Bacteroidetes ratio, at the phylum level (18, 50, 59, 61–63). At genus level, flaxseed oil increased the amounts of 19 bacteria (Allobaculum, Coriobacteriaceae spp., Parabacteroides, uncultured_bacterium_f_Lachnospiraceae, Ruminococcaceae_UCG-005, etc.) and decreased those of 12 bacteria (Clostridiales spp., uncultured_bacterium_f_Desulfovibrionaceae, Akkermansia, Romboutsia, Prevotella_9, etc.). Moreover, for the anti-aging action of flaxseed oil on D-galactose-induced rats, 11 types of bacteria (p_Firmicutes, o_Clostridiales, c_Clostridia, f_Lachnospiraceae, s_uncultured_bacterium_f_Lachnospiracea, etc.) have been identified to be differential gut microbiota biomarkers (60).
Some similarities and differences in gut microbiota modulation could be observed, while flaxseed oil showed different health-promoting effects in animal experiments. At the phylum level, the abundance of Firmicutes was downregulated while flaxseed oil exerted immunoregulation (50), regulation of lipid metabolism (53), and anti-diabetes (55) effects. Meanwhile, Firmicutes/Bacteroidetes ratio was diminished as this oil exhibited anti-diabetes (55), anti-atherosclerosis (58), and ameliorating polycystic ovary syndrome (61) activities. While that of Firmicutes was upregulated as flaxseed oil revealed anti-hepatocellular carcinoma action (18). The amount of Bacteroidetes was added while flaxseed oil displayed anti-diabetes (55) and anti-ulcerative colitis (63) effects. The number of Actinobacteria was increased while flaxseed oil reflected regulation of lipid metabolism (52) and improving intrauterine growth retardation (62) effects, whereas it was decreased as this oil showed ameliorating polycystic ovary syndrome action (61). The abundance of Proteobacteria was raised while flaxseed oil exerted regulation of lipid metabolism (53) and anti-ulcerative colitis (63) effects, while it was shrunk as flaxseed oil exhibited hepatoprotection (59), ameliorating polycystic ovary syndrome (61) and anti-hepatocellular carcinoma (18) actions. The amount of Verrucomicrobia was reduced while flaxseed oil revealed regulation of lipid metabolism effect (53), while it was elevated as this oil emerged anti-ulcerative colitis action (63). At the genus level, the number of Allobaculum was augmented, while flaxseed oil showed immunoregulation (50) and ameliorating polycystic ovary syndrome (61) effects. The amount of Lachnoclostridium was downregulated while flaxseed oil revealed anti-atherosclerosis effect (58), and that was upregulated as the oil showed anti-ulcerative colitis action (63). The number of Alistipes was enhanced as flaxseed oil displayed anti-diabetes (55) and anti-atherosclerosis (57) activities. The abundance of Enterorhabdus was declined while flaxseed oil exerted anti-atherosclerosis (58) and anti-hepatocellular carcinoma (18) actions. The amount of Romboutsia was decreased as flaxseed oil exhibited anti-aging (60) and anti-ulcerative colitis (63) activities. The abundance of Ruminococcaceae_UCG-005 was upregulated while flaxseed oil showed anti-aging effect (60) and was downregulated as this oil exhibited anti-ulcerative colitis activity (63). The quantity of Lactobacillus was enhanced while flaxseed oil exerted ameliorating polycystic ovary syndrome (61) and anti-ulcerative colitis (63) effects. The number of Bifidobacterium was enhanced, while flaxseed oil revealed regulation of lipid metabolism (52), ameliorating polycystic ovary syndrome (61), and improving intrauterine growth retardation (62) activities. The abundance of Blautia was decreased while flaxseed oil exhibited anti-diabetes effect (55), while it was increased as the oil exerted improving intrauterine growth retardation action (62). The amount of Streptococcus was declined, while flaxseed oil showed regulation of lipid metabolism (52) and ameliorating polycystic ovary syndrome (61) activities. The number of Turicibacter was added while flaxseed oil displayed regulation of lipid metabolism (52) and anti-hepatocellular carcinoma (18) effects. The abundance of Enterorhabdus was lowered while flaxseed oil reflected anti-atherosclerosis (58) and anti-hepatocellular carcinoma (18) actions.
In a word, flaxseed oil could modulate gut microbiota during their many health-promoting effects. Moreover, differential gut microbiota biomarkers have been acquired for their revealed lipid metabolism regulation and anti-aging effect. Furthermore, some differences and similarities in gut microbiota modulation have been found while this oil exhibited different health-promoting effects.
3.4. Other oil modulations
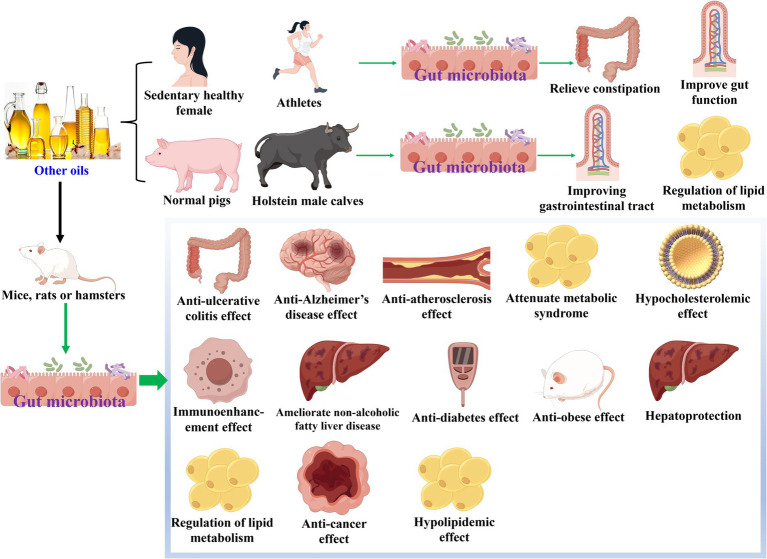
There were other edible plant oils which could modulate gut microbiota during their health-promoting effects, as shown in Table 4 and Figure 4. In terms of walnut oil, it increased the abundances of S24-7, Lachnospiraceae and Ruminococcaceae, and decreased that of Moraxellaceae at family level while exerted anti-Alzheimer’s disease effect on scopolamine-induced mice (64). At the phylum level, walnut oil elevated the amounts of Firmicutes, Actinobacteria, and/or Bacteroidetes, and declined those of Proteobacteria and/or Epsilonbacteraeota while revealed anti-ulcerative colitis action in dextran sulfate sodium-induced colitis mice (65) and anti-Alzheimer’s disease activity in scopolamine-induced mice (64). At the genus level, walnut oil enhanced the quantities of seven bacteria (Lactobacillus, Lachnospiraceae_NK4A136_group, Faecalibaculum, Bifidobacterium, [Ruminococcus]_torques_group, etc.), and reduced those of six bacteria (Helicobacter, Bacteroides, Clostridium_sensu_stricto_1, Staphylococcus, Desulfovibrio, etc.) (65). Moreover, Ruminococcaceae and Mogibacteriaceae were chosen to be differential gut microbiota biomarkers for walnut oil, which exhibited anti-Alzheimer’s disease effect against scopolamine-induced mice (64).
Table 4.
Gut microbiota modulation of other oils during their health-promoting effects.
| Oil | Health-promoting effect | Experimental model | Oil dosage and treatment time | Gut microbiota modulation | Gut microbiota marker | References |
|---|---|---|---|---|---|---|
| Walnut oil | Antioxidant, anti-inflammatory and immunoregulation | Normal mice | 2.5, 5 and 10 mL/kg, 4 weeks | Genus level: Lactobacillus (+); Helicobacter (−) | NA | (101) |
| Walnut oil | Anti-ulcerative colitis | Dextran sulfate sodium-induced colitis mice | 2.5 mL/kg, 4 weeks | Phylum level: Firmicutes and Actinobacteria (+); Proteobacteria and Epsilonbacteraeota (−) | NA | (65) |
| Genus level: Lactobacillus, Lachnospiraceae_NK4A136_group, Faecalibaculum, Bifidobacterium, [Ruminococcus]_torques_group, Akkermansia and Roseburia (+); Bacteroides, Helicobacter, Clostridium_sensu_stricto_1, Staphylococcus, Desulfovibrio and Streptococcus (−) | ||||||
| Walnut oil | Anti-Alzheimer’s disease | Scopolamine-induced mice | 1.63, 3.25 and 16.25 g/kg, 8 weeks | Phylum level: Firmicutes and Bacteroidetes (+); Proteobacteria (−) | Ruminococcaceae and Mogibacteriaceae | (64) |
| Family level: S24-7, Lachnospiraceae and Ruminococcaceae (+); Moraxellaceae (−) | ||||||
| Soybean oil | Anti-atherosclerosis | Normal mice | 80 and 160 mg, 4 weeks | Phylum level: Bacteroidetes, Deferribacteres and Proteobacteria (+); Firmicutes, Tenericutes and TM7 (−) | Prevotella, unclassified S24-7, Mucispirillum, Dehalobacterium, Ruminococcus, unclassified Peptococcaceae, Coprobacillus, Bilophila, Desulfovibrio, Anaeroplasma and Proteobacteria | (66) |
| Soybean oil | Attenuate metabolic syndrome | High-fat diet-induced mice | 25%, 2 or 8 weeks | Akkermansia muciniphila, Turicibacter, Thermicanus, Clostridium saccharogumia, and Lactobacillus pontis (+); Coprobacillus (−) | NA | (67) |
| Soybean oil | Increase insulin sensitivity and prevent fatty liver | Normal mice | 7% or 21%, 3 months | Akkermansia muciniphila and Faecalibacterium prausnitzii (+) | NA | (68) |
| Sea buckthorn seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | 50 and 100%, 6 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | NA | (69) |
| Genus level: norank_f_Bacteroidales_S24-7_group, Allobaculum and norank_o_Mollicutes_RF9 (+); Acietatifactor, Lactobacillus, and norank_f_Ruminococcaceae (−) | ||||||
| Sea buckthorn pulp oil | Immunoenhancement effect | Cyclophosphamide-induced mice | 5 and 10 mL/kg, 3 weeks | Genus level: Alistipes, Bacteroides, Anaerotruncus, Lactobacillus, ASF356, Roseburia (+); Mucispirillum, Anaeroplasma, Pelagibacterium, Brevundimonas, Ochrobactrum, Acinetobacter, Ruminiclostridium, Blautia, Ruminiclostridium, Oscillibacter and Faecalibaculum (−) | Tannerellaceae and Parabacteroides | (70) |
| Sea buckthorn seed and pulp oil | Ameliorate non-alcoholic fatty liver disease | High-fat diet-induced mice | 23.6%, 12 weeks | Oscillibacter and Roseburia (+); Lactobacillus (−) | NA | (71) |
| Coconut oil | Improve diabetes | Alloxan-induced rats | 20%, 16 weeks | Phylum level: Firmicutes, Actinobacteria, and Firmicutes/Bacteroidetes ratio (+); Bacteroidetes and Spirochaetes (−) | NA | (72) |
| Genus level: Lactobacillus and Allobaculum (+); Treponema and Turicibacter (−) | ||||||
| Coconut oil | Improving gastrointestinal tract | Normal pigs | 3 g/kg, 2 weeks | Alloprevotella, Bifidobacteriales and Lactobacilli (+); Corynebacterium, Pseudomonadales, Psychrobacter, and Mitsuokella (−) | NA | (73) |
| Coconut oil | Anti-obese | Obese rats | 0.01 and 0.02%, 4 weeks | Phylum level: Bacteroidetes (+); Firmicutes (−) | NA | (74) |
| Coconut oil | Anti-obese | Obese rats | NA, 8 weeks | Enterobacteriaceae, Escherichia and Enterococcus (+) | NA | (75) |
| Coconut oil | Anti-obese | High-sugar diet-induced rats | 1,000 mg/kg, 3 weeks | E. coli (−) | NA | (17) |
| Coconut oil | Regulation of lipid metabolism | Holstein male calves | NA, 6 weeks | Phylum level: Actinobacteria (+) | NA | (76) |
| Genus level: Erysipelotrichaceae_UCG-006 (+) | ||||||
| Perilla oil | Anti-diabetes | High-fat diet-induced KKAy mice | 0.67, 1.33 and 2.00 g/kg BW, 6 weeks | Genus level: Alistipes, Alloprevotella, Parabacteroides and Rikenella (+); unidentified_Ruminococcaceae, Lachnoclostridium unidentified_Lachnospiraceae, Oscillibacter, Blautia, Desulfovibrio, Angelakisella and Bilophila (−) | NA | (79) |
| Perilla oil | Anti-non-alcoholic fatty liver disease | High-fat diet-induced rats | 5.5%, 16 weeks | Phylum level: Bacteroidetes, Spirochaetes and Verrucomicrobia (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | NA | (78) |
| Genus level: Roseburia, Prevotella, Bacteroides and Akkermansia (+); Ruminococcus, Oscillospira and Clostridium (−) | ||||||
| Perilla oil | Relieve constipation | Sedentary healthy female | 9 g, 10 months | Family level: Clostridiaceae (+) | NA | (21) |
| Perilla oil | Improve gut function | Athletes | 3 g and 9 g, 8 weeks | Phylum level: Bacteroidetes (+); Firmicutes, Proteobacteria and Firmicutes/Bacteroidetes ratio (−) | NA | (77) |
| Family level: Lachnospiraceae (+) | ||||||
| Peony seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | 50 and 100%, 6 weeks | Phylum level: Firmicutes/Bacteroidetes ratio (−) | NA | (80) |
| Genus level: unclassified_f_Ruminococcaceae and Ruminococcus_2 (+); unclassified_f__Erysipelotrichaceae, Christensenellaceae_R-7_group, norank_o__Mollicutes_RF9, Peptococcus, norank_f__Eubacteriaceae and unclassified_f__Coriobacteriaceae | ||||||
| Peony seed oil | Alleviate hyperlipidemia and hyperglycemia | High-fat diet-induced mice | 0.5, 1.0 and 1.5 mL, 4 weeks | Phylum level: Proteobacteria and Deferribacteres (−) | Bacteroides, Turicibacter and Allobaculum | (81) |
| Genus level: Lactobacillus, Prevotella and Parabacteroides (+); Ruminococcaceae_Ruminococcu, Mucispirillum, Oscillospira and Coprococcus (−) | ||||||
| Decaisnea insignis seed oil | Hepatoprotection | Alcohol-induced mice | 3, 6 and 12 g/kg, 12 weeks | Phylum level: Saccharibacteria (+) | Lactobacillus_gasseri | (82) |
| Genus level: Lactobacillus, Conexibacter, Pepeococcus, and Ruminoccoceae_UCG_004 (+) | ||||||
| Decaisnea insignis seed oil | Hepatoprotection | L-carnitine-induced mice | 6 g/kg, 12 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Proteobacteria (−) | NA | (83) |
| Genus level: Akkermansia, Lactobacillaceae, Bacteroides, and Rikenellaceae (+); Helicobacter and Erysipelotrichaceae (−) | ||||||
| Sacha inchi oil | Hypolipidemic effect | High-fat diet-induced rats | 0.5, 1.0 and 1.5 mL/kg, 8 weeks | Phylum level: Firmicutes (+); Bacteroidetes (−) | Roseburia, Turicibacter, Butyrivibrio, Unidentified Enterobacteriaceae, Escherichia, and Bacteroides | (84) |
| Genus level: Alistipes (+); Unidentified Enterobacteriaceae, Bacteroides and Lachnoclostridium (−) | ||||||
| Sacha inchi oil | Regulation of lipid metabolism | High-fat diet-induced mice | 100 and 200 mg/kg, 45 d | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | Deferribacteraceae, Deferribacterales, and Deferribacteres | (85) |
| Schizochytrium sp. L. oil | Rhodospirillales and Alphaproteobacteria | |||||
| Millet bran oil | Attenuate metabolic syndrome | High-fat diet-induced mice | 2 g/kg, 12 weeks | Phylum level: Verrucomicrobia and Saccharibacteria (+) | Akkernansia, PrevotellaceaeUCG_001, Erysipelatoclostridium, Ruminococcaceae UCG_009, unclassified_f__Lachnospiraceae, Butyricimonas, Sulfuricurvum, and Arcobacter | (86) |
| Riceberry bran oil | Anti-cancer | Diethylnitrosamine and 1,2-dimethylhydrazine-induced rats | 100 mg/kg, 10 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | NA | (87) |
| Genus level: Ruminococcaceae UCG-013, Ruminococcaceae UCG-014, Adlercreutzia, Enterorhabdus, Papillibacter and Lachnospiraceae NK4A136 groups (+); Eubacterium coprostanoligenes, Ruminoclostridium 6 and Bacteroides (−) | ||||||
| Torreya grandis oil | Anti-obese | High-fat diet-induced mice | 250, 550 and 850 mg/kg, 8 weeks | Phylum level: Firmicutes and Actinobacteria (+); Bacteroidetes and Proteobacteria (−) | NA | (88) |
| Family level: Erysipelotrichaceae, Coriobacteriaceae, Lactobacillaceae, and Bifidobacteriaceae (+); Porphyromonadaceae (−) | ||||||
| Torreya grandis oil | Anti-Alzheimer’s disease | Scopolamine-induced mice | 1,000 and 3,000 mg/kg, 30 d | Phylum level: Firmicutes/Bacteroidetes ratio (+); Verrucomicrobiota (−) | NA | (89) |
| Species level: Allobaculum, Bifidobacterium, Olsenella, Parasutterella, unclassified Ruminococcaceae, the siraeum group of Eubacterium, Anaerotignum and Anaerovorax (+) | ||||||
| Safflower oil | Improve glucose intolerance | High fat/high sucrose diet-induced mice | NA, 40 weeks | Phylum level: Deferribacteres (+); Bacteroidetes, Proteobacteria and Actinobacteria (−) | NA | (90) |
| Genus level: Blautia (+); Barnesiella (−) | ||||||
| Peanut oil | Attenuate metabolic syndrome | High-fat/high sucrose diet-induced rats | 10%, 12 weeks | Phylum level: Firmicutes/Bacteroidetes ratio (+) | g__Faecalibaculum, s__uncultured_bacterium_g_Faecalibaculum, p__Proteobacteria, g__Ruminococcaceae_UCG_014, s__uncult ured_bacterium_g_Ruminococcaceae_UCG_014, g__Klebsiella, s__uncultured_bacterium_g_Clostridium_sensu_stricto_1, f__Clostridiaceae_1, g__Clostridium_sensu_stricto_1 and f__Peptostreptococcaceae | (43) |
| Genus level: Olsenella, Peptoclostridium, Ruminococcaceae_UCG-009, Weissella, Bifidobacterium, [Eubacterium]_fissicatena_group, [Eubacterium]_coprostanoligenes_group, Ruminococcaceae_NK4A214_group, Clostridium_sensu_stricto_1, Ruminococcaceae_UCG-014 and Faecalibaculum (+); Bilophila, Leuconostoc, [Eubacterium]_nodatum_group, Lactococcus, uncultured_bacterium_f_Coriobacteriaceae, Streptococcus, Rothia, [Ruminococcus]_torques_group, Bacteroides, Lachnoclostridium and Blautia (−) | ||||||
| Kiwifruit seed oil | Anti-obese | High-fat diet-induced mice | 1.0 and 3.0 mL/kg, 12 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | Pseudoflavonifractor, Flavonifractor, Intestinimonas, Romboutsia and Olsenlla | (91) |
| Genus level: Bacteroides, Barnesiella, Intestinimonas, Tannerella, Coprobacter, Alistipes, Odoribacter, Alloprevotella, Parabacteroides, Ruminococcus_2, Acetobacteroides, Macellibacteroides, Stomatobaculum and Clostridium XVII (+); Bilophila, Clostridium IV, Acetatifactor, Helicobacter, Clostridium XIVa, Lachnospiracea incertae sedis, Akkermansia, Mucispirillum, Anaerotruncus, Eisenbergiella, Hydrogenoanaerobacteruim, Lactobacillus, Staphylococcus, Peptococcus, Marvinbryantia and Acidaminobacter (−) | ||||||
| Okra seed oil | Hepatoprotection | Ethanol-induced mice | 400 and 800 mg/kg, 8 weeks | Phylum level: Bacteroidetes (+); Proteobacteria and Firmicutes/Bacteroidetes ratio (−) | NA | (92) |
| Genus level: Lactobacilli (+) | ||||||
| Hawthorn seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | 4.75 and 9.5%, 6 weeks | Genus level: Faecalibaculum, Ruminococcus_2 and norank_f__Clostridiales_vadinBB60_group (+); unclassifed_f_Christensenellaceae, Ruminococcaceae_NK4A214_group, norank_o_Gastranaerophilales and Peptococcus (−) |
NA | (96) |
| Wild melon seeds oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | 4.75 and 9.5%, 6 weeks | Phylum level: Bacteroidetes (+); Firmicutes (−) | NA | (93) |
| Genus level: Bifidobacterium, Ruminococcus_2, nonrank_f_Eubacteriaceae and unclassified_p_Firmicutes (+); Bilophila, Blautia, Lachnoclostridium, Lachnospiraceae_UCG_006, Ruminiclostridium_9, [Eubacterium]_coprostanoligenes_group and norank_f_Ruminococcaceae (−) | ||||||
| Tomato seed oil | Anti-hyperlipidemia | High-fat diet-induced mice | 5.9 and 11.8%, 12 weeks | Phylum level: Bacteroidetes (+); Firmicutes and Firmicutes/Bacteroidetes ratio (−) | norank_o__Gastranaerophilale, Phascolarctobacterium and Lactobacillus | (94) |
| Genus level: Alistipes, Phascolarctobacterium, Lactobacillus, and Anaerotruncus (+); Rikenella (−) | ||||||
| Almond oil | Anti-diabetes | Streptozotocin-induced rats | 2, 4 and 8 g/kg BW, 4 weeks | Phylum level: Bacteroidetes (+); Firmicutes (−) | NA | (95) |
| Genus level: Bacteroides, Lactobacillus and Lachnospiraceae_NK4A136_group (+); Ruminococcaceae_UCG-014, Clostridium_sensu_stricto_1 and Fusicatenibacter (−) |
Figure 4.
Other oils modulate gut microbiota during their health-promoting effects.
Regarding soybean oil, it raised the abundances of Bacteroidetes, Deferribacteres, and Proteobacteria, and shrunk those of Firmicutes, Tenericutes, and TM7 at the phylum level while revealed anti-atherosclerosis action to normal mice (66). Moreover, 11 kinds of bacteria (Prevotella, unclassified S24-7, Mucispirillum, Dehalobacterium, Ruminococcus, etc.) were identified as the differential gut microbiota biomarkers. On the other hand, the amounts of six bacteria (Akkermansia muciniphila, Turicibacter, Thermicanus, Clostridium saccharogumia, Lactobacillus pontis, etc.) were upregulated, and that of Coprobacillus was downregulated, while soybean oil exhibited attenuate metabolic syndrome effect (67) against high-fat diet-induced mice and increase insulin sensitivity and prevent fatty liver activities in normal mice (68). To sea buckthorn seed/pulp oil, it increased the number of Bacteroidetes and decreased that of Firmicutes as well as Firmicutes/Bacteroidetes ratio at the phylum level, while exhibited hypocholesterolemic effect on high-cholesterol diet-induced hamsters (69). At the genus level, it enhanced the amounts of nine bacteria (norank_f_Bacteroidales_S24-7_group, Allobaculum, norank_o_Mollicutes_RF9, Alistipes, Bacteroides, etc.), and lowered those of 13 bacteria (Acietatifactor, Lactobacillus, norank_f_Ruminococcaceae, Mucispirillum, Anaeroplasma, etc.), while exerted hypocholesterolemic (69) and immunoenhancement (70) effects. Moreover, Tannerellaceae and Parabacteroides were characterized as differential gut microbiota biomarkers while sea buckthorn pulp oil exhibited immunoenhancement effect toward cyclophosphamide-induced mice (70). Otherwise, sea buckthorn seed and pulp oil enlarged the abundances of Oscillibacter and Roseburia, and shrunk that of Lactobacillus while exerted ameliorate non-alcoholic fatty liver disease effects in high-fat diet-induced mice (71).
Coconut oil could regulate gut microbiota during exerted improvements in diabetes (72), improving the gastrointestinal tract (73), anti-obese (17, 74, 75), and regulation of lipid metabolism (76) effects. At the phylum level, coconut oil increased the abundances of Firmicutes, Actinobacteria, and/or Bacteroidetes along with Firmicutes/Bacteroidetes ratio, and decreased those of Bacteroidetes, Spirochaetes, and/or Firmicutes (72, 74, 76). At the genus level, the amounts of Lactobacillus, Allobaculum, and Erysipelotrichaceae_UCG-006 were augmented, and those of Treponema and Turicibacter were declined, while coconut oil reflected improved diabetes (72) and regulation of lipid metabolism (76) activities. On the other hand, coconut oil upregulated the numbers of six kinds of bacteria (Alloprevotella, Bifidobacteriales, Lactobacilli, Enterobacteriaceae, Escherichia, etc.), and downregulated those of Corynebacterium, Pseudomonadales, Psychrobacter, Mitsuokella, and E. coli, while exhibited improving gastrointestinal tract (73) and anti-obese (17, 75) activities.
Perilla oil, respectively, increased the abundances of Clostridiaceae and Lachnospiraceae while exhibited relieved constipation toward sedentary healthy female (21) and exerted improved gut function to athletes (77) at the family level. At the phylum level, the amounts of Bacteroidetes, Spirochaetes, and Verrucomicrobia were raised, and those of Firmicutes and Proteobacteria as well as Firmicutes/Bacteroidetes ratio were lessened, while perilla oil exerted anti-non-alcoholic fatty liver disease (78) and improved gut function (77) actions. At the genus level, the numbers of eight bacteria (Alistipes, Alloprevotella, Parabacteroides, Rikenella, Roseburia, etc.) were enhanced, while those of 11 bacteria (unidentified_Ruminococcaceae, Lachnoclostridium, unidentified_Lachnospiraceae, Oscillibacter, Blautia, etc.) were lowered, as perilla oil displayed anti-diabetes (79) and anti-non-alcoholic fatty liver disease (78) activities.
Peony seed oil declined the amounts of Proteobacteria and Deferribacteres accompanied by Firmicutes/Bacteroidetes ratio at the phylum level, while exhibited hypocholesterolemic (80) and alleviated hyperlipidemia and hyperglycemia (81) effects. At the genus level, peony seed oil increased the abundances of unclassified_f_Ruminococcaceae, Ruminococcus_2, Lactobacillus, Prevotella and/or Parabacteroides, and decreased those of 10 bacteria (unclassified_f_Erysipelotrichaceae, Peptococcus, Christensenellaceae_R-7_group, norank_o_Mollicutes_RF9, norank_f_Eubacteriaceae, etc.) (80, 81). Moreover, Bacteroides, Turicibacter, and Allobaculum were screened to be differential gut microbiota biomarkers, while peony seed oil alleviated hyperlipidemia and hyperglycemia against high-fat diet-induced mice (81). Decaisnea insignis seed oil enhanced the amounts of Saccharibacteria and/or Bacteroidetes and declined those of Firmicutes and Proteobacteria at phylum level, while showed hepatoprotection against alcohol or L-carnitine-induced mice (82, 83). At the genus level, the abundances of eight bacteria (Lactobacillus, Conexibacter, Pepeococcus, Ruminoccoceae_UCG_004, Akkermansia, etc.) were elevated, while those of Helicobacter and Erysipelotrichaceae were lowered (82, 83). Moreover, Lactobacillus_gasseri was identified to be differential gut microbiota biomarker for Decaisnea insignis seed oil that exhibited hepatoprotection on alcohol-induced mice (82). Sacha inchi oil increased the amount of Firmicutes, and decreased that of Bacteroidetes at the phylum level, while exerted hypolipidemic effect in high-fat diet-induced rats (84). At the genus level, it enhanced the abundance of Alistipes, and shrunk those of Unidentified Enterobacteriaceae, Bacteroides, and Lachnoclostridium (84). Moreover, six bacteria (Roseburia, Turicibacter, Butyrivibrio, Unidentified Enterobacteriaceae, Escherichia, etc.) were chosen to be differential gut microbiota biomarkers. While, sacha inchi oil upregulated the number of Bacteroidetes, and downregulated that of Firmicutes as well as Firmicutes/Bacteroidetes ratio at the phylum level, as exhibited regulation of lipid metabolism effect against high-fat diet-induced mice (85). Moreover, Deferribacteraceae, Deferribacterales, and Deferribacteres were identified to be differential gut microbiota biomarkers. Millet/riceberry bran oil increased the amounts of Verrucomicrobia, Saccharibacteria, and Bacteroidetes, and decreased that of Firmicutes along with Firmicutes/Bacteroidetes ratio at the phylum level, while exhibited attenuate metabolic syndrome effect (86) or anti-cancer activity (87). At genus level, the numbers of six bacteria (Ruminococcaceae UCG-013, Ruminococcaceae UCG-014, Adlercreutzia, Enterorhabdus, Papillibacter, etc.) were upregulated, and those of Eubacterium coprostanoligenes, Ruminoclostridium 6 and Bacteroides were downregulated, while riceberry bran oil exerted anti-cancer action on diethylnitrosamine and 1,2-dimethylhydrazine-induced rats (87). Moreover, eight bacteria (Akkernansia, unclassified_f__Lachnospiraceae, Prevotellaceae UCG_001, Erysipelatoclostridium, Ruminococcaceae UCG_009, etc.) were screened to be differential gut microbiota biomarkers, as millet bran oil showed attenuate metabolic syndrome effect (86). Torreya grandis oil augmented the abundances of Firmicutes and Actinobacteria along with Firmicutes/Bacteroidetes ratio, and declined those of Bacteroidetes, Proteobacteria, and Verrucomicrobiota at the phylum level, while exhibited anti-obese (88) and anti-Alzheimer’s disease (89) effects. At the family level, Torreya grandis oil aggrandized the amounts of Erysipelotrichaceae, Coriobacteriaceae, Lactobacillaceae, and Bifidobacteriaceae, and reduced that of Porphyromonadaceae, while exerted anti-obese effect on high-fat diet-induced mice (88). At the species level, Torreya grandis oil raised the numbers of eight bacteria (Allobaculum, Bifidobacterium, Olsenella, Parasutterella, unclassified Ruminococcaceae, etc.), while showed anti-Alzheimer’s disease action against scopolamine-induced mice (89).
Schizochytrium sp. L. oil (85), safflower oil (90), peanut oil (43), kiwifruit seed oil (91), okra seed oil (92), wild melon seed oil (93), tomato seed oil (94) or almond oil (95) could also modulate gut microbiota during their health-promoting effects. At the phylum level, the abundances of Bacteroidetes and/or Deferribacteres as well as Firmicutes/Bacteroidetes ratio were increased, and those of Firmicutes, Bacteroidetes, Proteobacteria, and/or Actinobacteria along with Firmicutes/Bacteroidetes ratio were decreased (43, 85, 90–95). At the genus level, the abundances of 33 bacteria (Blautia, Olsenella, Peptoclostridium, Ruminococcaceae_UCG-009, [Eubacterium]_fissicatena_group, etc.) were enhanced, while these oils displayed the health-promoting effects (43, 90–96). On the contrary, those of 39 bacteria (Barnesiella, Bilophila, Leuconostoc, [Eubacterium]_nodatum_group, Lactococcus, etc.) were reduced. Moreover, for Schizochytrium sp. L. oil exerted regulation of lipid metabolism effect on high-fat diet-induced mice, Rhodospirillales and Alphaproteobacteria were identified to be differential gut microbiota biomarkers (85). In terms of peanut oil exhibited attenuate metabolic syndrome action against high-fat/high sucrose diet-induced rats, 10 types of bacteria (g__Faecalibaculum, s__uncultured_bacterium_g_Faecalibaculum, p__Proteobacteria, f_Clostridiaceae_1, g__Ruminococcaceae_UCG_014, etc.) were screened to be differential gut microbiota biomarkers (43). To kiwifruit seed oil showed anti-obese action on high-fat diet-induced mice, Pseudoflavonifractor, Flavonifractor, Intestinimonas, Romboutsia and Olsenlla were characterized to be differential gut microbiota biomarkers (91). Regarding tomato seed oil showed anti-hyperlipidemia effect against high-fat diet-induced mice, norank_o_Gastranaerophilale, Phascolarctobacterium, and Lactobacillus were chosen to be differential gut microbiota biomarkers (94).
Overall, there were other oils which could modulate gut microbiota during their health-promoting effects. Moreover, differential gut microbiota biomarkers have been acquired for some of them to show health-promoting effects.
3.5. Influences of dosage and treatment time of edible plant oils
Different dosages of camellia oil, olive oil, and flaxseed oil have been demonstrated to exhibit different behaviors in modulating gut microbiota during their health-promoting effects (16, 51, 56, 63, 68). In terms of camellia oil, 2 and 4 mL/kg BW of it significantly enriched the abundances of Faecalibacterium, Muribaculaceae, and Coriobacteriaceae_UCG_002 in exercise mice, while 6 mL/kg BW of it obviously enhanced the amounts of Desulfobacteria and Faecalibaculum (16). Regarding olive oil, Akkermansia muciniphila was present at the dose of 7%, whereas Mucispirillum schaedleri was the most abundant at that of 21%, as it increased insulin sensitivity and prevented fatty liver in normal mice (68). For flaxseed oil, 400 mg/kg BW of it increased the quantities of Lactobacillus and Lachnospiraceae_NK4A136_group, while 1,600 mg/kg BW of it added the number of Prevotellaceae_UCG-001 during its anti-ulcerative colitis effect in dextran sulfate sodium-induced rats (63).
Moreover, peony seed oil (80, 81), perilla oil (77, 79), Torreya grandis oil (88, 89), and other oils (69, 91, 93–95) have also been proven to generate different modulations on gut microbiota because of different doses during their health-promoting effects. For example, 1.5 mL of peony seed oil upregulated the proportions of Lactobacillus and Prevotella, while 1.0 mL of it elevated the presence of Parabacteroides and reduced the prevalence of Ruminococcaceae_Ruminococcu and Mucispirillum, as alleviated hyperlipidemia and hyperglycemia in high-fat diet-induced mice (81). For perilla oil exhibited anti-diabetes activity against high-fat diet-induced KKAy mice, Dubosiella and Turicibacter were the most abundant genus in low-dose (0.67 g/kg BW) group relative to middle-dose (1.33 g/kg BW) and high-dose (2.00 g/kg BW) groups, while Lactobacillus was the most abundant genus in the high-dose group compared to the low-dose and middle-dose groups (79). 250, 550, and 850 mg/kg of Torreya grandis oil decreased Porphyromonadaceae to 15.57, 10.96, and 6.97%, increased Erysipelotrichaceae to 20.32, 36.55, and 38.75%, and enhanced Coriobacteriaceae to 7.65, 1.94, and 3.07% during their anti-obese effects in high-fat diet-induced mice (88). 50% of sea buckthorn seed oil boosted the number of norank_f_Bacteroidales_S24-7_group, whereas 100% of it increased the amounts of Allobaculum and norank_o_Mollicutes_RF9 in high-cholesterol diet-induced hamsters (69). 5.9% of tomato seed oil significantly changed the abundances of Anaerotruncus and Alistipes, while 11.8% of it obviously altered the amounts of Lactobacillus, Rikenella, and Enterorhabdus in high-fat diet-induced mice (94). The abundances of Pseudoflavonifractor, Flavonifractor, Intestinimonas, Romboutsia, and Olsenlla were markedly increased in the high-dose (3.0 mL/kg) kiwifruit seed oil group as compared to the low-dose (1.0 mL/kg) group, in high-fat diet-induced mice (91). 8 g/kg BW of almond oil notably changed the amounts of Ruminococcaceae_UCG-014, Lactobacillus, and Lachnospiraceae_NK4A136_group, 4 g/kg BW of it significantly altered the quantity of Bacteroides, and 2 g/kg BW of it obviously changed the abundances of Clostridium_sensu_stricto_1 and Fusicatenibacter in streptozotocin-induced rats (95). 9.5% of wild melon seed oil showed significant differences in Clostridiales_vadinBB60_group and Streptococcaceae in comparison with 4.75% of it during its hypocholesterolemic effect in high-cholesterol diet-induced hamsters (93).
On the other hand, treatment time has been indicated to influence the modulations of edible plant oils on gut microbiota in three animal studies (67, 90, 97). The study taken by Hidalgo et al. (97) has shown that 6 weeks of olive oil intervention could not significantly alter the gut microbiota of normal ICR mice, whereas 12 weeks of that showed significant changes. The investigation of Danneskiold-Samsøe et al. (90) has indicated that significant differences were found in the abundances of Bilophila, Olsenella, Clostridium XIVa, Parasutterella, and Pseudoflavonifactor in the cecum as well as Allobaculum and Parasutterella in the colon at different treatment time points (2, 5, 10, and 40 weeks) of safflower oil in normal C57BL/6 J mice. Another study by Patrone et al. (67) has revealed that lower proportions of Thermicanus, Akkermansia muciniphila, Lactobacillus pontis, Eubacterium biforme, Turicibacter, but higher Proteus, Ruminococcus flavefaciens, Clostridium perfringen, Allobaculum and Deltaproteobacteria of high-fat diet-induced mice were found in coconut oil group as compared with soybean oil group at 2 weeks. While at 8 weeks, Lactobacillus reuteri, F16, Anaerofustis, Allobaculum, and Deltaproteobacteria were significantly higher, whereas Akkermansia muciniphila was lower in coconut oil group compared to soybean oil group.
3.6. Comparisons between edible plant oils and edible animal oils
Comparisons between different edible plant oils on gut microbiota modulations have been made in some studies. Compared with camellia oil, different modulations of corn oil, olive oil, soybean oil, perilla oil, and sunflower oil on the gut microbiota of mice or rats were seen while exhibited hepatoprotection, anti-ulcerative colitis, anti-obese, and anti-Alzheimer’s disease effects (31, 32, 36, 38, 42). For example, Lee et al. (32) have found that Prevotella was higher and Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria were lower in camellia oil-treated ulcerative rats compared to soybean oil-treated ulcerative rats. Meanwhile, Actinobacteria and Firmicutes were higher and Bacteroidetes and Proteobacteria were lower in camellia oil-treated ulcerative rats compared to olive oil-treated ulcerative rats. Relative to olive oil, distinctions in gut microbiota modulation of mice, rats, or grouper have also been discovered in soybean oil, coconut oil, corn oil, and peanut oil as exerted increase insulin sensitivity and prevent fatty liver, attenuate metabolic syndrome, and improve gut health activities (43, 68, 98). For instance, as exhibited increase insulin sensitivity and prevent fatty liver effect on normal mice, 7% soybean oil treatment displayed a significant increase in Akkermansia muciniphila, whereas 7% olive oil consumption showed significant increments in 10 species (primarily Bacteroides acidifaciens, and Faecalibacterium prausnitzii), and 7% coconut oil administration had significant enhancements in eight species (particularly Mucispirillum schaedleri) (68). In comparison with flaxseed oil, different gut microbiota modulations of corn oil, olive oil, and soybean oil were generated in pigs, rats, and mice during their hepatoprotection, immunoregulation, anti-diabetes, and improving intrauterine growth retardation actions (50, 55, 59, 62). For example, as exerted anti-diabetes activity against streptozotocin-nicotinamide-induced rats, Blautia was higher and Alistipes was lower in corn oil group, as compared to flaxseed oil group (55). Additionally, distinctions of gut microbiota modulation to mice or suckling calves were found among soybean oil, peanut oil, coconut oil, palm oil, Schizochytrium sp. L. oil, sacha inchi oil, sea buckthorn pulp oil, sea buckthorn seed oil, walnut oil, and/or sunflower oil (65, 67, 71, 76, 85). For instance, significant differences of Lactobacillus, Oscillibacter, and/or Roseburia were observed among soybean oil, peanut oil, sea buckthorn seed oil, and sea buckthorn pulp oil treatments, during their ameliorate non-alcoholic fatty liver disease actions (71).
On the other hand, some studies have compared the gut microbiota modulations between edible plant oils and edible animal oils (including fish oil and lard oil) in vivo experiments (7, 57, 71, 78, 85, 98–100). In terms of comparisons with fish oil, the differential bacteria of Schizochytrium sp. L. oil group were Rhodospirillales and Alphaproteobacteria, whereas those of sacha inchi oil group were Deferribacteraceae, Deferribacterales, and Deferribacteres in high-fat diet-induced mice (85). Perilla oil has been reported to have similar gut microbiota modulations with fish oil in high-fat diet-induced rats (78). However, flaxseed oil was weaker than fish oil in modulating the gut microbiota of TMAO-induced ApoE−/− mice (57). And, soybean oil, fish oil, and lard oil showed differential influences on the gut microbiota of middle-aged rats (100). Corn oil, olive oil, and fish oil had significantly different modulations on the amounts of Photobacterium, Romboutsia, Epulopiscium, Clostridium_sensu_stricto_1, Staphylococcus and/or Leuconostoc in grouper (98). Compared with lard oil, significant changes in Blautia were produced by peanut oil, sea buckthorn seed oil, and sea buckthorn pulp oil, in high-fat diet-induced mice (71). Soybean oil, olive oil, and lard oil treatments exhibited obviously different modulations on the gut microbiota of mice at phylum, class, order, and family levels (7). Corn oil and lard oil revealed notably different alterations in the gut microbiota of hamsters at phylum, family, and genus levels (99).
4. Correlations between gut microbiota and biochemical indexes affected by edible plant oils
Significant correlations between modulated gut microbiota and changed biochemical indexes were found while edible plant oils exhibited health-promoting effects, as summarized in Table 5.
Table 5.
Correlations between modulated gut microbiota and changed biochemical indexes by edible plant oils.
| Oil | Health-promoting effect | Experimental model | Correlated gut microbiota | Correlated biochemical indexes | References |
|---|---|---|---|---|---|
| Camellia oil | Mild cognitive impairment protection | Aluminum chloride-induced rats | Ruminococcaceae_UCG-014, Bacteroides pectinophilus_group, Blautia, Lachnoclostridium and Prevotellaceae_UCG-001 | IL-6, TNF-α, IL-1β, BUN, GOT, SOD and IL-4 | (36) |
| Camellia oil | Anti-Alzheimer’s disease | Aluminum chloride-induced rats | [Eubacterium]_coprostanoligenes_group, Romboutsia, Clostridia_UCG-014, [Eubacterium]_xylanophilum_group, Monoglobus, Lachnoclostridium, Lactobacillus, Corynebacterium, Blautia, Lachnospiraceae_ND3007_group, f_Butyricicoccaceae_Unclassified, f_ Lachnospiraceae_Unclassified, Bacteroides, [Bacteroides]_pectinophilus_group, Roseburia, UCG-005, Prevotellaceae_NK3B31_group, Muribaculaceae, Christensensenellaceae_R-7_group, f_Oscillospiraceae_Unclassified, f_Ruminococcaceae_Unclassified, Atopostipes, Jeotgalicoccis and Staphylococcus | Iba1, p-p65, APP, BACE1, AB1-42, MDA, IL-6, escape_time_trial_12, escape_time_work, IL-1β, TNF-α, LC3B, Atg5, GPx, IL-4, IL-10, brain_weight, SOD, Beclin1, CAT, time_spent_in target_quadrant and HDL | (8) |
| Camellia oil | Hypolipidemic effect | High-fat diet-induced mice | Alistipes and Aerococcus | mTOR and RpS6KB1 | (39) |
| Camellia oil | Anti-fatigue | Rotarod test and Treadmill test in mice | Enterorhabdus, Parvibacter, Akkermansia, Dubosiella, Clostridia_UCG-014, Bifidobacterium, Ruminococcus, [Eubacterium]_coprostanoligenes_group, Ileibacterium, Allobaculum, Desulfovibrio, Oscillospiraceae, Lactobacillus, Muribaculaceae, Alloprevotella, UCG-010, Alistipes, Erysipelotrichaceae and Bacteroides | Rotarod time, running time, BUN, glycogen, ATP, GSH-Px, CAT, MDA, Keap1, Nrf2, NQO1, HO-1, GCLC, GCLM, SOD, myostain, FNDC5, Myh7, Myh2, Tnni1, Myh1, Myh4, ZO-1, Claudin-1 and Occludin | (16) |
| Camellia oil | Hepatoprotection | Alcohol-induced liver injury in mice | Christensenellaceae_R-7_group, Escherichia_shigella, Eubacterium_nodatum_group, Coriobacteriaceae_UCG-002, Parvibacter, norank_f_Eggerthellaceae, Eubacterium_brachy_group, Harryflintia, Staphylococcus, Enterococcus, NK4A214_group, Faecalibaculum, GCA-900066575, UCG-005, norank_f_norank_o_Rhodospirillales, A2, Alistipes, Erysipelatoclostridium, Blautia, Romboutsia, Parabacteroides, Eubacterium_fissicatena_group and norank_f_norank_o_Clostridia_UCG-014 | Liver TG, ALT, MDA, and GSH-Px | (42) |
| Olive oil | Attenuate metabolic syndrome | Normal mice | Desulfovibrio, Ruminiclostridium, Fusicatenibacter, Parasutterella, Olivibacter, Marispirillum, Spiroplasma, Marinilabilia, Desulfotomaculum and Helicobacter | Food intake, water intake, diuresis, body weight, systolic blood pressure, leptin, Insulin, triglycerides, T-CHO, and HDL/LDL | (45) |
| Olive oil | Attenuate metabolic syndrome | Normal mice | Prevotella, Marvinbryantia, Desulfovibrio, Anaerophaga, Fusicatenibacter, Parasutterella, Eubacterium, Erysipelotrichaceae, Olivibacter, Marispirillum and Enterobacter | Water intake, HDL/LDL, T-CHO, blood pressure, leptin, insulin, ghrelin, and diuresis | (44) |
| Olive oil | Anti-hypertension | Spontaneously hypertensive rats | Clostridia XIVa | Systolic blood pressure | (46) |
| Olive oil | Anti-diabetes | NOD/LtJ mice | Verrucomicrobia, Cyanobacteria, Firmicutes, Bacteroides, Akkermansia, Intestinimonas, Lachnospira, Eubacterium_xylanophilum_group, Muribaculaceae, Alistipes, Lachnoclostridium, Ruminococcaceae_UCG_005 and Gastranaerophilales | Islet number, C-Peptide, Random blood glucose, Th1/Th2, serum_TNF-α, insulitis, AUC area of OGTT, colon_IL-6, and colon_TNF-α | (48) |
| Flaxseed oil | Ameliorating polycystic ovary syndrome | Letrozole-induced rats | Lactobacillus, Allobaculum, Actinobacteria, Bacteroides, Butyrivibrio, Desulfovibrio, Ruminiclostridium and Bifidobacterium | LPS, FSH, FSH/LH, E2, PROG, T, SHBG, IL-1β, IL-6, IL-10, IL-17A, TNF-α, and MCP-1 | (61) |
| Flaxseed oil | Anti-hepatocellular carcinoma | Orthotopic hepatocellular carcinoma mice | Escherichia-shigella, Enterorhabdus, norank_f_norank_o_Clostridia UCG-014, Turicibacter, norank_f_norank_Clostridia_vadinBB60_group, Ruminococcus, Monoglobus, Eubacterium_brachy_group, unclassified_c_Bacteroidia and Lactococcus | CD4_Tcells, CD8_Tcells, CD8_PD1_Tcells, Treges, TNF-α, IFN-γ, IL-10, TGF-β1, ZO-1, and Claudin-4 | (18) |
| Flaxseed oil | Immunoregulation | Normal mice | Bacteroidetes | Triglyceride | (50) |
| Flaxseed oil | Anti-diabetes | Streptozotocin-nicotinamide-induced rats | Firmicutes, Bacteroidetes, Blautia and Alistipes | LPS, IL-1β, TNF-α, IL-6, and IL-17A | (55) |
| Flaxseed oil | Anti-atherosclerosis | High-fat diet-induced ApoE−/− mice | Intestinimonas, Alistipes, Muribaculum, Candidatus_Saccharimonas, Oscillibacter, Blautia, Parasutterella, Lachnoclostridium, Bilophila, Enterorhabdus, Anaerotruncus and Negativibacillus | LPS, IL-1β, TNF-α, IL-6, IL-10, IL-17A, and MCP-1 | (58) |
| Flaxseed oil | Regulation of lipid metabolism | Albas cashmere goats | [Ruminococcus]_gauvreauii_group, Streptococcus, Turicibacter, Acetitomaculum, Bifidobacterium, Christensenellaceae_R-7_group, Mycoplasma, Olsenella, Ruminococcaceae_UCG-014, Ruminococcus_2, Ureaplasma, Lactobacillus, Lachnospiraceae_NK3A20_group, Intestinibacter, Family_XIII_AD3011_group, Clostridium_sensu_stricto_1, unclassified_f__Bifidobacteriaceae, [Eubacterium]_coprostanoligenes_group and Aeriscardovia | Final body weight, total body weight gain, omental fat, kidney fat, mesenteric fat, glucose, β-hydroxybutyric acid, non-esterified fatty acid, triglyceride, cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, acetyl-CoA carboxylase, fatty acid synthetase, hormone-sensitive lipase, lipoprotein lipase, and malic dehydrogenase | (52) |
| Flaxseed oil | Anti-aging | D-galactose-induced rats | uncultured_bacterium_f_Muribaculaceae, Bacteroides, uncultured_bacterium_f_Desulfovibrionaceae, Ruminococcaceae_UCG-014, [Eubacterium]_oxidoreducens_group and Christensenellaceae_R-7_group | GSH-Px, T-AOC, CAT, Prkcq, Lat, Ptprc, Lcp2, Lck, zap70, Pik3cd, and Nfatc2 | (60) |
| Soybean oil | Increase insulin sensitivity and prevent fatty liver | Normal mice | Ruminococcus bromii, Ruminococcus flavefaciens, Clostridium cocleatum, Bacteroides acidifaciens, Lactobacillus reuteri and Akkermansia muciniphila | AUC, p-IRS, p-AKT, and LPS | (68) |
| Soybean oil | Attenuate metabolic syndrome | High-fat diet-induced mice | Anaerotruncus, Lactobacillus_plantarum, Syntrophomonas, Akkermansia_muciniphila, Clostridium_saccharogumia, Staphylococcus_aureus, YS2, RF32, Allobaculum, Anaerofustis, F16, Enterococcaceae, Actinomycetaceae and Agrobacterium | Crypt_depth, triglycerides, body weight, total and daily weight gain, mucosal lesion, leucocyte infiltration, Lep, ovarian fat, and cholesterol | (67) |
| Soybean oil | Anti-atherosclerosis | Normal mice | unclassified Bacteroidales, Prevotella, unclassified S24.7, Mucispirillum, Dehalobacterium, Ruminococcus, unclassified Lachnospiraceae, unclassified Peptococcaceae, Coprobacillus, Sutterella, Bilophila, Desulfovibrio and Anaeroplasma | Triglycerides, cholesterol, linoleic acid, lipid peroxides, and ROS | (66) |
| Walnut oil | Anti-Alzheimer’s disease | Scopolamine-induced mice | Firmicutes, Bacteroidetes, Proteobacteria, Cyanobacteria, and Verrucomicrobia | Slc6a4, Gng13, Kcnj12, Pik3r3, Kcnk3, Tph2, Kcnj4, Kcnq5, Plcb1, Cacnb1, Rgs2, Mylk3, Adcy5, Pde8b, Gng7, Chrm4, Htr2a, Kcnk2, Oxt, Gng10, Cacna1h, Kcna4, Htr6, Htr4, Agtr1a, Kcnq3, Chrm3, Cacng3, Camk2a, Cacnb3, Cacng8, Alox12b, Htr3a and Ptgs2 | (64) |
| Peony seed oil | Alleviate hyperlipidemia and hyperglycemia | High-fat diet-induced mice | Lactobacillus, Prevotellaceae_Prevotella, Bifidobacterium, Helicobacter, Prevotella, Parabacteroides, Coprococcus and Paraprevotella | Glu, TC, TG, HDL, and LDL-C | (81) |
| Peony seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | Anaeroplasma, Peptococcus, Butyricimonas, Candidatus_Saccharimonas, Christensenellaceae_R-7_group, norank_f_Clostridiales_vadinBB60_group, norank_f_Eubacteriaceae, norank_f_Lachnospiraceae, norank_f_Peptococcaceae, norank_o_Gastranaerophilales, norank_o_Mollicutes_RF9, Rikenellaceae_RC9_gut_group, Ruminiclostridium, Ruminiclostridium_1, Ruminiclostridium_9, Ruminococcaceae_NK4A214_group, unclassified_f_Coriobacteriaceae, unclassified_f_Christensenellaceae, unclassifed_f_Erysipelotrichaceae and unclassified_o_Bacteroidales | TC, TG, LPS, protein_LXRa, SREBP2, HMG-CoA-R | (80) |
| Sea buckthorn seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | Acietatifactor, Anaerovorax, Lactobacillus, [Eubacterium]_coprostanoligenes_group, unclassified_f_Ruminococcaceae, Parasutterella, Ruminococcaceae_UCG-014, norank_f_Rumincoccaceae, norank_f_Bacteroidales_S24-7_group and unclassified_k_norank | TC, MTP, ACAT2, and ABCG8 | (69) |
| Tomato seed oil | Anti-hyperlipidemia | High-fat diet-induced mice | norank_f_Peptococcaceae, norank_f_Erysipelotrichaceae, norank_f_Clostridiales_vadinBB60_group, [Eubacterium]_fissicatena_group, Rikenella, Lactobacillus, Lachnoclostridium, Roseburia, Odoribacter, Faecalibaculum, Bifidobacterium and Allobaculum | body weight, fat (perirenal + epididymal), TC, TG, LDL-C, HDL-C, and LDL-C/HDL-C | (94) |
| Almond oil | Anti-diabetes | Streptozotocin-induced rats | Clostridium_sensu_stricto_1, Fusicatenibacter, Ruminococcaceae_UCG-014, Lachnospiraceae_NK4A136_group and Bacteroides | FBG, insulin, body weight, SOD, CAT, MDA, TNF-α, IL-1β, Keap1, Nrf2, and HO-1 | (95) |
| Wild melon seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | Bilophila, Blautia, Escherichia-shigella, Lachnoclostridium, Lachnospiraceae_UCG-006, Lactococcus, Ruminiclostridium, Ruminiclostridium_9, Ruminococcaceae_UCG-004, [Eubacterium]_coprostanoligenes_group, norank_f_Eubacteriaceae, norank_f_Lachnospiraceae, norank_f_Ruminococcaceae, unclassified_f_Lachnospiraceae and unclassified_p_Firmicutes | TC | (93) |
| Torreya grandis oil | Anti-obese | High-fat diet-induced mice | Acetivibrio, Lactococcus, Eubacterium, Peptococcus, Clostridium, Sporobacter, Provencibacterium, Roseburia, Bacteroides, Lachnoclostridium, Akkermansia, Erysipelatoclostridium, Anaeroplasma, Lactobacillus, Faecalibaculum, Bifidobacterium, Allobaculum, Saccharofermentans, Paraeggerthella, Alistipes, Rikenella, Parabacteroides and Barnesiella | TC, TG, HDL-C, and LDL-C | (88) |
| Torreya grandis oil | Anti-Alzheimer’s disease | Scopolamine-induced mice | Anaeroplasma, Streptococcus, Allobaculum, Olsenella and Parasutterella | Escape latency, percentage of spontaneity, DI, and SOD | (89) |
| Hawthorn seed oil | Hypocholesterolemic effect | High-cholesterol diet-induced hamsters | Anaeroplasma, unclassified_f_Christensenellaceae, Candidatus_Saccharimonas, Peptococcus, norank_f_Peptococcaceae, Butyricimonas and norank_o_Gastranaerophilales | TC and LPS | (96) |
| Safflower oil | Anti-obese | Obese people | Adlercreutzia, Parabacteroides, Alistipes, Streptococcus, Anaerococcus, Blautia, Coprococcus, Hespellia, Parasporobacterium, Pseudobutyrivibrio, Roseburia, Faecalibacterium, Oscillibacter, Sporobacter, Ruminococcaceae, Firmicutes and Coprobacillus | TC, TG, LDL-C, and HDL-C | (20) |
4.1. Affected by camellia oil
For camellia oil exerted mild cognitive impairment protection on aluminum chloride-induced rats (36), Bacteroides pectinophilus_group was positively correlated with TNF-α, IL-1β, and IL-6. Blautia was positively correlated with BUN, TNF-α, and IL-6. Lachnoclostridium and Prevotellaceae_UCG-001 were positively correlated with GOT and TNF-α, respectively. Ruminococcaceae_UCG-014 was negatively correlated with SOD and IL-4. For camellia oil exhibited anti-Alzheimer’s disease effect against aluminum chloride-induced rats (8), eight bacteria (Romboutsia, Clostridia_UCG-014, [Eubacterium]_coprostanoligenes_group, [Eubacterium]_xylanophilum_group, Monoglobus, etc.) had significantly positive correlations with 11 changed biochemical indexes (Iba1, p-p65, APP, BACE1, AB1-42, etc.), and had obviously negative relations with another 11 changed biochemical indexes (LC3B, Atg5, GPx, IL-4, IL-10, etc.). While 16 kinds of bacteria (Blautia, Lachnospiraceae_ND3007_group, f_Butyricicoccaceae_Unclassified, f_ Lachnospiraceae_Unclassified, Bacteroides, etc.) showed opposite correlations with these biochemical indexes.
Camellia oil revealed hypolipidemic effect on high-fat diet-induced mice (39), Alistipes was positively correlated with mTOR and RpS6KB1, and Aerococcus was negatively correlated with RpS6KB1. As this oil showed anti-fatigue action toward mice received with Rotarod test and Treadmill test (16), 19 bacteria (Enterorhabdus, Parvibacter, Akkermansia, Dubosiella, Clostridia_UCG-014, etc.) had significant correlations with 25 changed biochemical indexes (Rotarod time, Running time, BUN, glycogen, ATP, etc.) in serum, liver, muscle, and/or colon. Among them, Muribaculaceae was one of the differential gut microbiota biomarkers for camellia oil. Regarding this oil exhibited hepatoprotection against alcohol-induced liver injury in mice (42), Enterococcus and NK4A214_group were negatively correlated with liver TG, while Faecalibaculum was positively correlated with liver TG. Staphylococcus, Enterococcus, Romboutsia, and Eubacterium_fissicatena_group were negatively correlated with GSH-Px, whereas Harryflintia and Parvibacter were positively correlated with GSH-Px. Alistipes was negatively correlated with MDA, while Eubacterium_brachy_group and Parvibacter were positively correlated with MDA. norank_f_Eggerthellaceae, Parvibacter, and Coriobacteriaceae_UCG-002 were negatively correlated with ALT, whereas 14 bacteria (GCA-900066575, Staphylococcus, UCG-005, norank_f_norank_o_Rhodospirillales, Eubacterium_nodatum_group, etc.) were positively correlated with ALT.
In a word, significant correlations between modulated gut microbiota and changed biochemical indexes occurred during camellia oil exhibited mild cognitive impairment protection, anti-Alzheimer’s disease, hypolipidemic, anti-fatigue, and hepatoprotection effects.
4.2. Affected by olive oil
For olive oil exerted attenuate metabolic syndrome effect on normal mice (45), Desulfovibrio was positively correlated with food intake, water intake, diuresis, and T-CHO. Ruminiclostridium was positively correlated with systolic blood pressure and HDL/LDL and was negatively correlated with water intake. Fusicatenibacter was positively correlated with water intake and diuresis and was negatively correlated with body weight, T-CHO, and HDL/LDL. Parasutterella was negatively correlated with insulin and HDL/LDL. Olivibacter was positively correlated with triglycerides and was negatively correlated with food intake. Marispirillum was negatively correlated with leptin, and Spiroplasma was negatively correlated with food intake and HDL/LDL. Marinilabilia was positively correlated with food intake, water intake, and diuresis. Desulfotomaculum was negatively correlated with food intake and T-CHO. Helicobacter was positively correlated with water intake and diuresis and was negatively correlated with leptin. In another study, as olive oil exhibited attenuate metabolic syndrome activity in normal mice (44), Prevotella was negatively correlated with T-CHO, while Anaerophaga was positively correlated with T-CHO. Marvinbryantia and Eubacterium were positively correlated with leptin, while Marispirillum was negatively correlated with leptin. Desulfovibrio was positively correlated with water intake, blood pressure, insulin, and diuresis. Fusicatenibacter was negatively correlated with HDL/LDL and T-CHO. Parasutterella was positively correlated with T-CHO and was negatively correlated with HDL/LDL. Erysipelotrichaceae was positively correlated with T-CHO and was negatively correlated with ghrelin. Olivibacter was positively correlated with T-CHO and ghrelin. Enterobacter was negatively correlated with water intake. Regarding olive oil showed anti-hypertension effect against spontaneously hypertensive rats, Clostridia XIVa was negatively correlated with systolic blood pressure (46). For olive oil revealed anti-diabetes action toward NOD/LtJ mice (48), Lachnospira and Eubacterium_xylanophilum_group were positively correlated with Random blood glucose, Th1/Th2, insulities, and AUC area of OGTT. Muribaculaceae was negatively correlated with serum_TNF-α. Verrucomicrobia and Akkermansia were negatively correlated with colon_TNF-α. Cyanobacteria and Gastranaerophilales were negatively correlated with insulitis, AUC area of OGTT, and colon_TNF-α. Alistipes was positively correlated with C-peptide and was negatively correlated with AUC area of OGTT. Intestinimonas was negatively correlated with insulitis. Ruminococcaceae_UCG_005 was positively correlated with islet number and negatively correlated with Random blood glucose and AUC area of OGTT. Lachnoclostridium was negatively correlated with insulitis and AUC area of OGTT. Bacteroides was negatively correlated with colon_IL-6. Among them, Muribaculaceae, Akkermansia, Gastranaerophilales, Alistipes, Intestinimonas, Ruminococcaceae_UCG_005, Lachnoclostridium, and Bacteroides were differential gut microbiota biomarkers for olive oil.
Overall, notable correlations between modulated gut microbiota and changed biochemical indexes existed during olive oil exerted attenuate metabolic syndrome, anti-hypertension, and anti-diabetes actions.
4.3. Affected by flaxseed oil
For flaxseed oil exhibited ameliorating polycystic ovary syndrome effect on letrozole-induced rats (61), Firmicutes/Bacteroidetes ratio was positively correlated with E2, PROG, T, IL-1β, TNF-α, and MCP-1 in plasma and with IL-6, IL-17A, and MCP-1 in the ovary and was negatively correlated with IL-10 in the ovary. Actinobacteria was positively correlated with LPS, FSH/LH, E2, PROG, T, IL-6, and TNF-α in plasma and with IL-1β, IL-6, IL-17A, and TNF-α in the ovary and was negatively correlated with FSH and IL-10 in plasma. Lactobacillus was positively correlated with FSH and E2 in plasma and was negatively correlated with LPS, FSH/LH, T, and IL-6 in plasma and with IL-1β, TNF-α, and MCP-1 in ovary. Bacteroides was positively correlated with LPS, FSH/LH, IL-1β, IL-6, and TNF-α in plasma and with IL-1β, IL-6, IL-17A, and TNF-α in the ovary. Allobaculum was positively correlated with PROG in plasma and was negatively correlated with IL-17A and MCP-1 in plasma and with MCP-1 in ovary. Butyrivibrio was positively correlated with FSH, E2, PROG, SHBG, and IL-10 in plasma, and was negatively correlated with LPS, FSH/LH, T, IL-1β, IL-6, and MCP-1 in plasma and with IL-1β and MCP-1 in the ovary. Desulfovibrio was positively correlated with E2, PROG, SHBG, and IL-10 in plasma and with IL-10 in the ovary, and was negatively correlated with LPS, FSH/LH, T, IL-1β, IL-6, IL-17A, TNF-α, and MCP-1 in plasma and IL-17A, TNF-α and with MCP-1 in the ovary. Ruminiclostridium was positively correlated with FSH, PROG, and IL-10 in plasma, and was negatively correlated with T in plasma. Bifidobacterium was positively correlated with FSH, E2, and IL-10 in plasma and with IL-10 and IL-17A in the ovary, and was negatively correlated with LPS, FSH/LH, T, IL-1β, IL-6, TNF-α, and MCP-1 in plasma and with IL-6 in the ovary.
As flaxseed oil exerted anti-hepatocellular activity against orthotopic hepatocellular carcinoma mice (18), Escherichia-shigella was negatively correlated with CD8_Tcells and Claudin-4. Enterorhabdus was positively correlated with IFN-γ and was negatively correlated with CD8_PD1_Tcells and IL-10. norank_f_norank_o_Clostridia UCG-014 was positively correlated with CD4_Tcells, CD8_Tcells and IFN-γ, and was negatively correlated with CD8_PD1_Tcells and IL-10. Turicibacter was positively correlated with TNF-α and was negatively correlated with IL-10. norank_f_norank_Clostridia_vadinBB60_group was positively correlated with CD4_Tcells, CD8_Tcells and IFN-γ, and was negatively correlated with CD8_PD1_Tcells and IL-10. Ruminococcus was positively correlated with CD4_Tcells and TNF-α and was negatively correlated with IL-10. Monoglobus was positively correlated with CD4_Tcells, CD8_Tcells, IFN-γ, and ZO-1, and was negatively correlated with CD8_PD1_Tcells and TGF-β1. Eubacterium_brachy_group was positively correlated with IL-10 and TGF-β1 and was negatively correlated with CD8_Tcells. unclassified_c_Bacteroidia was positively correlated with CD4_Tcells, CD8_Tcells and IFN-γ, and was negatively correlated with TGF-β1. Lactococcus was negatively correlated with ZO-1 and Claudin-4.
Regarding flaxseed oil showed immunoregulation in normal mice (50), Bacteroidetes was negatively correlated with plasma triglyceride concentration. For flaxseed oil revealed anti-diabetes effect toward streptozotocin-nicotinamide-induced rats (55), Firmicutes was positively correlated with LPS, IL-1β, TNF-α, IL-6, and IL-17A, Bacteroidetes was negatively correlated with LPS, IL-1β, TNF-α, and IL-17A, Blautia was positively correlated with LPS, IL-1β, TNF-α, and IL-6, and Alistipes was negatively correlated with LPS and TNF-α. As flaxseed oil displayed anti-atherosclerosis action against high-fat diet-induced ApoE−/− mice (58), Intestinimonas was positively correlated with LPS and IL-1β in plasma and with IL-6 and IL-1β in the aorta. Oscillibacter was positively correlated with IL-17A in plasma and with TNF-α, IL-6, IL-1β, and IL-17A in the aorta, and was negatively correlated with IL-10. Parasutterella was positively correlated with IL-6. Lachnoclostridium was positively correlated with LPS in plasma and with TNF-α in the aorta. Bilophila was positively correlated with LPS and IL-1β in plasma and with TNF-α, IL-6, and IL-1β in the aorta. Enterorhabdus was positively correlated with TNF-α in the aorta. Anaerotruncus was positively correlated with IL-1β in plasma and with TNF-α in the aorta. Negativibacillus was positively correlated with LPS and IL-1β in plasma.
For flaxseed oil reflected regulation of lipid metabolism activity on Albas cashmere goats (52), 19 bacteria ([Ruminococcus]_gauvreauii_group, Streptococcus, Turicibacter, Acetitomaculum, Bifidobacterium, etc.) exhibited significant correlations with 17 changed biochemical indexes (final body weight, total body weight gain, omental fat, kidney fat, mesenteric fat, etc.).
As flaxseed oil exhibited anti-aging effect against D-galactose-induced rats (60), uncultured_bacterium_f_Muribaculaceae was negatively correlated with Nfatc2. Christensenellaceae_R-7_group was negatively correlated with Prkcq, Ptprc, Lcp2, Lck, zap70, and Pik3cd. Bacteroides was positively correlated with T-AOC in serum, while [Eubacterium]_oxidoreducens_group was negatively correlated with T-AOC in serum. uncultured_bacterium_f_Desulfovibrionaceae was negatively correlated with GSH-Px in the liver. Ruminococcaceae_UCG-014 was positively correlated with T-AOC in the liver, and [Eubacterium]_oxidoreducens_group was negatively correlated with T-AOC in the liver. [Eubacterium]_oxidoreducens_group was negatively correlated with CAT in the liver.
In short, obvious correlations between modulated gut microbiota and changed biochemical indexes appeared during flaxseed oil displayed ameliorating polycystic ovary syndrome, anti-hepatocellular, immunoregulation, anti-diabetes, anti-atherosclerosis, regulation of lipid metabolism, and anti-aging effect activities.
4.4. Affected by other oils
For soybean oil exhibited increase insulin sensitivity and prevented fatty liver effect on normal mice (68), Ruminococcus bromii, Ruminococcus flavefaciens, and Clostridium cocleatum were positively correlated with p-IRS and p-AKT, and were negatively correlated with AUC. Bacteroides acidifaciens was positively correlated with LPS, and Lactobacillus reuteri and Akkermansia muciniphila were negatively correlated with LPS. As soybean oil exerted attenuate metabolic syndrome action against high-fat diet-induced mice (67), Anaerotruncus was positively correlated with body weight and ovarian fat. Lactobacillus_plantarum, Akkermansia_muciniphila, and Clostridium_saccharogumia were positively correlated with Lep. Syntrophomonas was positively correlated with mucosal lesion, leucocyte infiltration, and Lep. Staphylococcus aureus was positively correlated with total and daily weight gain, body weight, and ovarian fat. YS2 was positively correlated with cholesterol and ovarian fat and was negatively correlated with Crypt_depth. RF32 and Enterococcaceae were negatively and positively correlated with Crypt_depth, respectively. Allobaculum was positively correlated with Lep and cholesterol. Anaerofustis was positively correlated with triglycerides and cholesterol; F16 was positively correlated with cholesterol. Actinomycetaceae was negatively correlated with cholesterol and body weight, and Agrobacterium was negatively correlated with total and daily weight gain. Regarding soybean oil showed anti-atherosclerosis activity in normal mice (66), 13 bacteria (unclassified Bacteroidales, Prevotella, unclassified S24.7, Mucispirillum, Dehalobacterium, etc.) had significant correlations with triglycerides, cholesterol, linoleic acid, lipid peroxides and/or ROS in serum, peritoneal macrophages, or aorta. Among them, Prevotella, unclassified S24.7, Mucispirillum, Dehalobacterium, Ruminococcus, unclassified Peptococcaceae, Coprobacillus, Bilophila, Desulfovibrio, and Anaeroplasma were differential gut microbiota biomarkers for soybean oil.
For peony seed oil exhibited hypocholesterolemic effect against high-cholesterol diet-induced hamsters (80), eight bacteria (Butyricimonas, norank_f_Peptococcaceae, norank_o_Gastranaerophilales, unclassified_f_Coriobacteriaceae, Peptococcus, etc.) were positively correlated with TC, while Anaeroplasma, Candidatus_Saccharimonas, Rikenellaceae_RC9_gut_group, and Ruminiclostridium_1 were negatively correlated with TC. Six bacteria (norank_f_Eubacteriaceae, norank_o_Mollicutes_RF9, Candidatus_Saccharimonas, norank_f_Clostridiales_vadinBB60_group, Rikenellaceae_RC9_gut_group, etc.) were negatively correlated with TG. Seven bacteria (Butyricimonas, norank_f_Peptococcaceae, norank_o_Gastranaerophilales, Papillibacter, Peptococcus, etc.) were positively correlated with LPS, while Anaeroplasma and Candidatus_Saccharimonas were negatively correlated with LPS. Six bacteria (Christensenellaceae_R-7_group, norank_f_Lachnospiraceae, norank_o_Mollicutes_RF9, Ruminiclostridium, Ruminococcaceae_NK4A214_group, etc.) were negatively correlated with protein_LXRa. unclassifed_f_Erysipelotrichaceae was positively correlated with SREBP2. norank_f_Peptococcaceae, Peptococcus, Ruminococcaceae_NK4A214_group and unclassified_f_Christensenellaceae were positively correlated with HMG-CoA-R. As sea buckthorn seed oil exerted hypocholesterolemic effect on high-cholesterol diet-induced hamsters (69), Acietatifactor, Anaerovorax, Lactobacillus, [Eubacterium]_coprostanoligenes_group and unclassified_f_Ruminococcaceae were positively correlated with TC, while Parasutterella and Ruminococcaceae_UCG-014 were negatively correlated with TC. Norank_f_Rumincoccaceae and unclassified_k_norank were positively correlated with MTP, while norank_f_Bacteroidales_S24-7_group was negatively correlated with MTP. Lactobacillus was positively correlated with ACAT2, and unclassified_k_norank was positively correlated with ABCG8. Regarding hawthorn seed oil reflected hypocholesterolemic effect on high-cholesterol diet-induced hamsters (96), unclassified_f_Christensenellaceae, Peptococcus, norank_f_Peptococcaceae, Butyricimona, and norank_o_Gastranaerophilales were positively correlated with TC, and Anaeroplasma and Candidatus_Saccharimonas were negatively correlated with TC. Unclassified_f_Christensenellaceae and norank_o_Gastranaerophilales were positively correlated with LPS, while Anaeroplasma and Candidatus_Saccharimonas were negatively correlated with LPS. For wild melon seed oil reflected hypocholesterolemic effect toward high-cholesterol diet-induced hamsters (93), 13 bacteria (Bilophila, Blautia, Escherichia-shigella, Lachnoclostridium, Lachnospiraceae_UCG-006, etc.) were positively correlated with TC, whereas norank_f_Eubacteriaceae and unclassified_p_Firmicutes were negatively correlated with TC.
Regarding walnut oil displayed anti-Alzheimer’s disease effect toward scopolamine-induced mice (64), Firmicutes, Bacteroidetes, Proteobacteria, Cyanobacteria, and Verrucomicrobia had significant correlations with differential genes enriched in the cortisol synthesis and secretion pathway, oxytocin signaling pathway, cholinergic signaling pathway, and serotonergic signaling pathway. Torreya grandis oil revealed anti-Alzheimer’s disease action against scopolamine-induced mice (89), and Anaeroplasma was positively correlated with escape latency. Streptococcus was negatively correlated with percentage of spontaneity. Allobaculum and Olsenella were positively correlated with DI. Parasutterella was positively correlated with SOD.
For Torreya grandis oil showed anti-obese activity on high-fat diet-induced mice (88), 23 bacteria (Acetivibrio, Lactococcus, Eubacterium, Peptococcus, Clostridium, etc.) had significant correlations with serum TC, TG, HDL-C, and LDL-C. As safflower oil exhibited anti-obese effect on obese people (20), 17 types of bacteria (Adlercreutzia, Parabacteroides, Alistipes, Streptococcus, Anaerococcus, etc.) exhibited notable correlations with serum TC, TG, LDL-C, and HDL-C.
Regarding tomato seed oil showed anti-hyperlipidemia action against high-fat diet-induced mice (94), Rikenella and Odoribacter were positively correlated with body weight, while Bifidobacterium and Allobaculum were negatively correlated with body weight. Odoribacter was positively correlated with fat (perirenal + epididymal). norank_f_Clostridiales_vadinBB60_group and Lactobacillus were negatively correlated with TC. Rikenella was positively correlated with TG, while norank_f_Clostridiales_vadinBB60_group was negatively correlated with TG. Bifidobacterium was negatively correlated with LDL-C. Roseburia, Lachnoclostridium, and Faecalibaculum were negatively correlated with HDL-C. Faecalibaculum was positively correlated with LDL-C/HDL-C, whereas Lactobacillus was negatively correlated with LDL-C/HDL-C. Among them, Lactobacillus was one of the differential gut microbiota biomarkers for tomato seed oil. For peony seed oil reflected alleviated hyperlipidemia and hyperglycemia effect on high-fat diet-induced mice (81), Bifidobacterium was positively correlated with Glu, while Prevotellaceae_Prevotella, Prevotella, Parabacteroides, and Paraprevotella were negatively correlated with Glu. Lactobacillus and Prevotella were negatively correlated with TC. Bifidobacterium was positively correlated with TG, Helicobacter was positively correlated with HDL, and Coprococcus was positively correlated with LDL-C. As almond oil displayed anti-diabetes activity in streptozotocin-induced rats (95), Clostridium_sensu_stricto_1 was positively correlated with FBG, MDA, TNF-α, IL-1β, and Keap1 and was negatively correlated with insulin, body weight, SOD, CAT, Nrf2, and HO-1. Ruminococcaceae_UCG-014 was negatively correlated with body weight, SOD, Nrf2, and HO-1. Fusicatenibacter was positively correlated with FBG, MDA, TNF-α, IL-1β, and Keap1, and was negatively correlated with insulin, body weight, SOD, CAT, and Nrf2. Lachnospiraceae_NK4A136_group was positively correlated with SOD and Nrf2 and was negatively correlated with FBG and IL-1β. Bacteroides was positively correlated with insulin, SOD, CAT, Nrf2, and HO-1 and was negatively correlated with FBG and IL-1β.
Overall, remarkable correlations between modulated gut microbiota and changed biochemical indexes were also observed during other oils reflected increase insulin sensitivity and prevent fatty liver, attenuate metabolic syndrome, anti-atherosclerosis, hypocholesterolemic, anti-Alzheimer’s disease, anti-obese, anti-hyperlipidemia, alleviate hyperlipidemia and hyperglycemia, and anti-diabetes effects.
5. Gut microbiota-derived metabolites altered by edible plant oils
5.1. Alterations on SCFAs
Camellia oil increased the levels of propionic acid and butyric acid in feces while exerted Alzheimer’s disease effect on aluminum chloride-induced rats (8). Meanwhile, this oil elevated the amounts of total SCFAs, acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid in colon contents, while exhibited anti-ulcerative colitis action against dextran sulfate sodium-induced mice (33). The oil reduced the contents of acetic acid, butyric acid, isobutyric acid, isovaleric acid and pentanoic acid in colonic contents, as showed hypolipidemic activity toward high-fat diet-induced mice (39).
Olive oil and flaxseed oil enhanced propionic acid production in plasma while displayed immunoregulation in normal mice (50). Moreover, Flaxseed oil supplementation increased the levels of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and/or isovaleric acid in feces, while exhibited ameliorating polycystic ovary syndrome (61), anti-hepatocellular carcinoma (18), anti-atherosclerosis (57, 58) and anti-diabetes (55) effects.
Sea buckthorn pulp oil increased the concentrations of acetic acid, butyric acid, and total SCFAs in feces, while exerted immunoenhancement effect on cyclophosphamide-induced mice (70). Sea buckthorn seed oil elevated the levels of acetic acid, propionic acid, butyric acid, and total SCFAs in feces, as exhibited hypocholesterolemic effect against high-cholesterol diet-induced hamsters (69). Coconut oil enhanced the production of propionic acid and butyric acid in feces, while showed anti-obese effect on obese rats (75). Riceberry bran oil aggrandized the levels of butyric acid and valeric acid and reduced that of acetic acid in feces, while showed anti-cancer activity in diethylnitrosamine and 1,2-dimethylhydrazine-induced rats (87). Walnut oil raised the amounts of total SCFAs, acetic acid, propionic acid, and butyric acid in feces, as revealed anti-ulcerative colitis activity against dextran sulfate sodium-induced colitis mice (65). Walnut oil increased the levels of propionic acid, butyric acid, and valeric acid in plasma, while displayed anti-Alzheimer’s disease effect in scopolamine-induced mice (64). Decaisnea insignis seed oil enhanced the concentrations of total SCFAs, acetic acid, propionic acid, and butyric acid in feces, as revealed hepatoprotection in L-carnitine-induced mice (83). Wild melon seed oil increased the levels of total SCFAs, acetic acid, propionic acid, and butyric acid in feces, as showed hypocholesterolemic effect on high-cholesterol diet-induced hamsters (93). Torreya grandis oil elevated the butyric acid concentration in serum, while exerted anti-Alzheimer’s disease action against scopolamine-induced mice (89). Hawthorn seed oil added the contents of total SCFAs, acetic acid, propionic acid, and butyric acid in feces, as revealed hypocholesterolemic effect on high-cholesterol diet-induced hamsters (96). Okra seed oil decreased concentrations of acetic acid, isobutyric acid, and isovaleric acid, and increased that of butyric acid in cecal contents of ethanol-induced mice (92).
5.2. Alterations on other gut microbiota-derived metabolites
Flaxseed oil intervention modulated the microbial bile acids (BAs) metabolism in feces while exhibited anti-atherosclerosis action on high-fat diet-induced ApoE−/− mice, showing increases of lithocholic acid, allocholic acid, glycocholic acid, and taurocholic acid and decreases of allolithocholic acid, isolithocholic acid, 7-ketodeoxycholic acid, β-ursodeoxycholic acid, chenodeoxycholic acid, and hyodeoxycholic acid (58). Flaxseed oil caused a higher conjugated BA concentration and conjugated/free BA ratio in cecal contents in high-fat diet-induced mice (51). Thereinto, the concentrations of several unconjugated BAs, including chenodeoxycholic acid and β-murocholic acid, were higher. Moreover, the concentrations of taurine-conjugated BAs, and in particular taurocholic acid and tauro-ursodesoxycholic acid were increased. Furthermore, the amounts of cholic acid and tauro-β-murocholic acid were elevated. Sacha inchi oil declined primary BAs including cholic acid, glycocholic acid, taurochenodeoxycholic acid, and taurocholic acid in the feces of high-fat diet-induced rats (84). Meanwhile, it significantly modulated the levels of glycerolipids and glycerophospholipids in the liver.
Sea buckthorn seed oil changed the compositions of hepatic fatty acids and fecal fatty acids of high-cholesterol diet-induced hamsters (69). Tomato seed oil reduced saturated fatty acids, monounsaturated fatty acids, and total fatty acid concentrations, and added polyunsaturated fatty acids in the liver and/or feces of high-fat diet-induced mice (94). Okra seed oil increased the levels of pentadecanoic acid, palmitic acid, heptadecanoic acid, linoleic acid, oleic acid, etc., and decreased those of myristic acid and trans-9-octadecenoic acid in serum, while showed hepatoprotection on ethanol-induced mice (92). Coconut oil changed the fatty acid composition of the liver and longissimus dorsi while exhibited regulation of lipid metabolism effect in Holstein male calves (76). Torreya grandis oil supplement generated alterations in fatty acid composition in livers of high-fat diet-induced mice, revealing increments of eicosadienoic acid, sciadonic acid, n-6 PUFA, MUFA, and PUFA, and reductions of arachidonic acid and SFA (88).
About 117 metabolites and 392 metabolites in feces were, respectively, upregulated and downregulated by camellia oil treatment, while it revealed gastroprotection on ethanol-induced mice (41). These metabolites were amino acid and its metabolites, terpenoids, heterocyclic compounds, and lignans compounds, which were significantly enriched in four key metabolic pathways, including biosynthesis of cofactors, metabolic pathway, purine metabolism, and ammonia acid biosynthesis. 159 significantly different metabolites (lipids and lipid-like molecules) in serum metabolites of mice were identified between olive oil-treated group and diabetic group (48). These metabolites were significantly enriched in 18 metabolic pathways. Decaisnea insignis seed oil led to increases in concentrations of squalene, hesperidin, 2-aminophenol, 5-hydroxyindole-3-acetic acid, abietic acid, etc., and decreases in levels of androsterone, triacetin, farnesal, indole-3-acetamide, thymidine-5′-monophosphate dreg pored, etc., in the cecal contents of alcohol-induced mice (82). Moreover, five correlated KEGG pathways were enriched by these metabolites, including steroid hormone biosynthesis, steroid biosynthesis, pyrimidine metabolism, tryptophan metabolism, and tryptophan metabolism pathways. Peony seed oil notably changed 30 metabolites in feces of high-fat diet-induced mice, including organic acids and derivatives, phenylpropanoids and polyketides, benzenoids, organoheterocyclic compounds, lipids and lipid-like molecules, etc. (81).
5.3. Correlations between altered metabolites and modulated gut microbiota
5.3.1. SCFAs and gut microbiota
For camellia oil exhibited anti-Alzheimer’s disease effect on aluminum chloride-induced rats (8), the acetic acid in feces was positively correlated with Prevotellaceae_NK3B31_group, and negatively correlated with f__Ruminococcaceae_Unclassified, Atopostipes, Jeotgalicoccus and Staphylococcus. Meanwhile, the butyric acid in feces was positively correlated with Clostridia_UCG-014 and Lachnoclostridium, and negatively correlated with Blautia, Lachnospiraceae_ND3007_group, f__Butyricicoccaceae_Unclassified, UCG-005, and Christensenellaceae_R-7_group. Additionally, the propionic acid in feces was negatively correlated with Roseburia.
Regarding flaxseed oil exerted anti-hepatocellular carcinoma effect against orthotopic hepatocellular carcinoma mice (18), the acetic acid in feces was negatively correlated with Eubacterium brachy_group. Meanwhile, the butyric acid, isobutyric acid, propionic acid, and isovaleric acid in feces were negatively correlated with Lactococcus. In addition, the valeric acid in feces was negatively correlated with Eubacterium brachy_group, Lactococcus, and Parabacteroides. As flaxseed oil exhibited anti-atherosclerosis action on TMAO-induced ApoE−/− mice (57), the acetic acid in feces was positively correlated with Alistipes, Bifidobacterium, Odoribacter, Parasutterella, and norank Bacteroidales S24-7, and negatively correlated with seven bacteria (Blautia, Clostridium_sensu_stricto_1, Lactococcus, Romboutsia, Roseburia, etc.). The propionic acid in feces was positively correlated with six bacteria (Alistipes, Bifidobacterium, Coriobacteriaceae UCG-002, Odoribacter, Parasutterella, etc.), and negatively correlated with 15 bacteria (Anaerotruncus, Bilophila, Blautia, Clostridium_sensu_stricto_1, Lachnoclostridium, etc.). The butyric acid in feces was positively correlated with Alistipes, Desulfovibrio, Lactobacillus, and Odoribacter, and negatively correlated with six bacteria (Akkermansia, Bilophila, Blautia, Coriobacteriaceae UCG-002, Faecalibaculum, etc.). The valeric acid in feces was positively correlated with six bacteria (Alistipes, Desulfovibrio, Enterorhabdus, Lactobacillus, Odoribacter, etc.), and negatively correlated with Akkermansia, Blautia, Clostridium_sensu_stricto_1, Faecalibaculum, and Roseburia. The total SCFAs in feces were positively correlated with Alistipes, Bifidobacterium, Odoribacter, Parasutterella, and norank Bacteroidales S24-7, and negatively correlated with 12 bacteria (Anaerotruncus, Blautia, Clostridium_sensu_stricto_1, Lachnoclostridium, Lactococcus, etc.). On the other hand, for flaxseed oil revealed anti-atherosclerosis action on high-fat diet-induced ApoE−/− mice (58), the acetic acid and propionic acid in feces were negatively correlated with seven bacteria (Intestinimonas, Oscillibacter, Lachnoclostridium, Bilophila, Enterorhabdus, etc.). The isobutyric acid in feces was negatively correlated with Intestinimonas, Oscillibacter, Blautia, and Bilophila. The isovaleric acid in feces was negatively correlated with Intestinimonas, Blautia, Bilophila, Enterorhabdus, and Negativibacillus. The valeric acid in feces was negatively correlated with eight bacteria (Intestinimonas, Oscillibacter, Blautia, Lachnoclostridium, Bilophila, etc.). For flaxseed oil displayed ameliorating polycystic ovary syndrome activity in letrozole-induced rats (61), the acetic acid in feces was positively correlated with Lactobacillus, Butyrivibrio, Desulfovibrio, and Bifidobacterium, and negatively correlated with Firmicutes/Bacteroidetes ratio. The propionic acid in feces was positively correlated with Lactobacillus, Butyrivibrio, and Desulfovibrio, and negatively correlated with Firmicutes/Bacteroidetes ratio. The butyric acid in feces was positively correlated with Lactobacillus, Allobaculum, Butyrivibrio, and Desulfovibrio, and negatively correlated with Actinobacteria and Bacteroides. The valeric acid in feces was positively correlated with Butyrivibrio, and negatively correlated with Actinobacteria and Bacteroides.
For sea buckthorn seed oil exerted hypocholesterolemic effect against high-cholesterol diet-induced hamsters (69), the acetic acid, propionic acid, valeric acid, and total SCFAs in feces were positively correlated with Parasutterella and Ruminococcaceae_UCG-014, and negatively correlated with Acietatifactor, Lactobacillus, and [Eubacterium]_coprostanoligenes_group. Meanwhile, the acetic acid was negatively correlated with Anaerovorax and Butyricimonas, the propionic acid was negatively correlated with unclassified_f_Ruminococcaceae and Ruminococcaceae_UCG-009, the valeric acid was negatively correlated with unclassified_f_Ruminococcaceae, Coriobacteriaceae_UCG-002, Butyricimonas, and Anaerovorax, and the total SCFAs was negatively correlated with unclassified_f_Ruminococcaceae, Coriobacteriaceae_UCG-002, and Butyricimonas. Moreover, the butyric acid in feces was positively correlated with Parasutterella, and negatively correlated with unclassified_f_Ruminococcaceae, [Eubacterium]_coprostanoligenes_group, Lactobacillus, Butyricimonas, and Acietatifactor. As tomato seed oil exhibited anti-hyperlipidemia activity in high-fat diet-induced mice (94), the acetic acid in feces was positively correlated with Allobaculum, Bifidobacterium, norank_f_Erysipelotrichaceae and unclassified_f_Erysipelotrichaceae, and negatively correlated with Anaerotruncus, Lachnospiraceae_NK4A136_group, Odoribacter, and [Eubacterium]_fissicatena_group. The propionic acid was positively correlated with unclassified_f_Erysipelotrichaceae, and negatively correlated with Lactobacillus, norank_f_Clostridiales_vadinBB60_group, and norank_f_Peptococcaceae. The butyric acid was positively correlated with Ruminiclostridium_5, and negatively correlated with norank_o_Gastranaerophilales. The valeric acid was positively correlated with Bifidobacterium, Pseudomonas, and norank_f_Peptococcaceae, and negatively correlated with [Eubacterium]_fissicatena_group. The total SCFAs were positively correlated with Allobaculum, Bifidobacterium, norank_f_Erysipelotrichaceae, and unclassified_f_Erysipelotrichaceae, and negatively correlated with Anaerotruncus. Among them, Lactobacillus and norank_o_Gastranaerophilales were two differential gut microbiota biomarkers for tomato seed oil. Regarding Decaisnea insignis seed oil displayed hepatoprotection on L-carnitine-induced mice (83), the acetic acid in feces was positively correlated with Alistipes. The propionic acid was negatively correlated with eight bacteria (norank_f_Erysipelotrichaceae, Turicibacter, [Eubacterium]_fissicatena_group, Clostridium_sensu_stricto_1, Faecalibaculum, etc.). The butyric acid was positively correlated with 13 bacteria (unclassified_o_Bacteroidales, norank_o_Mollicutes_RF9, Ruminococcus_1, [Eubacterium]_xylanophilum_group, [Eubacterium]_fissicatena_group, etc.), and negatively correlated with eight bacteria (Alistipes, Lachnospiraceae_NK4A136_group, Odoribacter, Rikenella, Ruminiclostridium, etc.). The isovaleric acid was negatively correlated with norank_f_Ruminococcaceae, Helicobacter, and Clostridium_sensu_stricto_1. The valeric acid was positively correlated with Ruminiclostridium and Prevotellacee_NK3B31_group. For wild melon seeds oil showed hypocholesterolemic effect against high-cholesterol diet-induced hamsters (93), the acetic acid, propionic acid, butyric acid, valeric acid, and total SCFAs in feces were positively correlated with unclassified_p_Firmicutes, nonrank_f_Eubacteriaceae, norank_f__Clostridiales_vadinBB60_group and Ruminococcus_2, and negatively correlated with norank_f_Ruminococcaceae and Escherichia-shigella. In addition, the acetic acid was positively correlated with norank_o_Mollicutes_RF9, Rikenellaceae_RC9_gut_group and Bifidobacterium, and negatively correlated with norank_f_Lachnospiraceae and Ruminiclostridium. The propionic acid was positively correlated with Parasutterella and Bifidobacterium, and negatively correlated with norank_f_Lachnospiraceae. The butyric acid was positively correlated with Parasutterella and Bifidobacterium, and negatively correlated with Coriobacteriaceae_UCG-002 and Blautia. The valeric acid was positively correlated with norank_o_Mollicutes_RF9, Parasutterella, and Candidatus_Saccharimonas. The total SCFAs were positively correlated with Parasutterella and Bifidobacterium and negatively correlated with norank_f_Lachnospiraceae and Blautia. For Torreya grandis oil exhibited anti-Alzheimer’s disease activity on scopolamine-induced mice, the acetic acid in serum was positively correlated with Allobaculum, Parasutterella, and Bifidobacterium (89). For peony seed oil revealed hypocholesterolemic effect toward high-cholesterol diet-induced hamsters (80), the acetic acid and propionic acid were positively correlated with unclassifed_f_Erysipelotrichaceae and Bifidobacterium, and negatively correlated with Ruminiclostridium_9 and norank_o_Gastranaerophilales. In addition, the acetic acid was positively correlated with Anaeroplasm, and negatively correlated with Papillibacter. The butyric acid and valeric acid were positively correlated with Ruminococcus_2 and Bifidobacterium, and the valeric acid was negatively correlated with norank_o_Gastranaerophilales and Butyricimonas. The total SCFAs were positively correlated with unclassifed_f_Erysipelotrichaceae and Bifidobacterium, and negatively correlated with norank_o_Gastranaerophilales and Butyricimonas. For hawthorn seed oil showed hypocholesterolemic effect on high-cholesterol diet-induced hamsters (96), the acetic acid, propionic acid, butyric acid, valeric acid, and total SCFAs in feces were positively correlated with Ruminococcus_2, and negatively correlated with eight bacteria (Ruminiclostridium_9, norank_f_Ruminococcaceae, Desulfovibrio, Butyricimonas, Oscillibacter, etc.). In addition, the acetic acid was negatively correlated with Lachnospiraceae_NK4A136_group, Blautia, and Ruminiclostridium_5. The propionic acid was positively correlated with Faecalibaculum and Anaeroplasm, and negatively correlated with eight bacteria (Lachnospiraceae_NK4A136_group, Peptococcus, norank_o_Gastranaerophilales, Ruminiclostridium, Ruminococcaceae_NK4A214_group, etc.). The butyric acid was positively correlated with Alistipes and Faecalibaculum, and negatively correlated with norank_o_Gastranaerophilales, Ruminococcaceae_NK4A214_group, Peptococcus, Roseburia, and unclassified_f_Christensenellaceae. The valeric acid was positively correlated with Faecalibaculum, and negatively correlated with norank_o_Gastranaerophilales, Ruminococcaceae_NK4A214_group, Peptococcus, Roseburia, and unclassified_f_Christensenellaceae. The total SCFAs were positively correlated with Faecalibaculum and Anaeroplasm, and negatively correlated with seven bacteria (Lachnospiraceae_NK4A136_group, norank_o_Gastranaerophilales, Ruminiclostridium, Peptococcus, Blautia, etc.).
5.3.2. Gut microbiota-derived metabolites and gut microbiota
For camellia oil exhibited gastroprotection on ethanol-induced mice (41), close correlations were found between metabolites and nine bacterial taxa (Rhodobacter, Bosea, Bacteroides, Dorea, Streptococcus, etc.) in feces. Among them, 40 metabolites (5,6-dimethylbenzimidazole, vincamine, gingerenone, amino acid, etc.) were negatively associated with Rhodobacter, Bosea, Bacteroides, and Dorea. Among them, Bosea and Dorea were two of differential gut microbiota biomarkers for camellia oil. Regarding the anti-diabetes effect of olive oil on NOD/LtJ mice (48), the majority of upregulated serum lipid metabolites were positively correlated with seven types of bacteria (Verrucomicrobia, Akkermansia, Cyanobacteria, Bacteroides, Gastranaerophilales, etc.), and negatively correlated with Lachnospira and Eubacterium_xylanophilum_group. Conversely, most decreased serum lipid metabolites were positively correlated with Lachnospira and Eubacterium_xylanophilum_group, and negatively correlated with Lachnoclostridium, Cyanobacteria, Gastranaerophilales, Bacteroides, and Ruminococcaceae_UCG-005. Among them, Akkermansia, Bacteroides, Gastranaerophilales, Lachnoclostridium, Ruminococcaceae_UCG-005, and Eubacterium_xylanophilum_group were differential gut microbiota biomarkers for olive oil. As flaxseed oil exerted anti-atherosclerosis effect against high-fat diet-induced ApoE−/− mice (55), the BAs included LCA, ACA, GCA, and TCA were negatively correlated with seven bacteria (Intestinimonas, Bilophila, Anaerotruncus, Oscillibacter, Negativibacillus, etc.), and alloLCA, isoLCA, 7-ketolca, β-UDCA, CDCA, HDCA, and α-MCA were positively correlated with these bacteria. For flaxseed oil showed regulation of lipid metabolism activity on high-fat diet-induced mice (51), 12-DHCA, TDCA, and DCA were closely correlated with the most bacterial genera, and the most metabolites were positively correlated with Coprobacillus, Adelercreutzia, and Ruminococcus. Among them, Ruminococcus was one of the differential gut microbiota biomarkers for flaxseed oil. For flaxseed oil revealed regulation of lipid metabolism action on Albas cashmere goats (53), significant correlations were observed between blood fatty acid composition and duodenal, ileal, jejunal, cecal, and colonic differential bacteria. Especially, three fatty acids, including c18:3n3, C20:4n6, and c20:5n3, were significantly correlated with differential bacteria ([Eubacterium]_coprostanoligenes_group, Acetitomaculum, Anaerofustis, Brevibacillus, Denitrobacterium, etc.) in duodenum, jejunum, ileum, cecum, and colon.
For sea buckthorn seed oil exerted hypocholesterolemic effect toward high-cholesterol diet-induced hamsters (69), total neutral sterols, coprostanol, coprostanone, cholesterol, dihydrocholesterol, total acidic sterols, lithocholic acid, deoxycholic acid, chenodeoxycholic acid, and cholic acid were significantly correlated with 19 key genera bacteria (norank_f_Bacteroidales_S24-7_group, Acietatifactor, Allobaculum, norank_o_Mollicutes_RF9, Lactobacillus, etc.). At the same time, peony seed oil exhibited hypocholesterolemic effect on high-cholesterol diet-induced hamsters (80), these metabolites were closely related to 27 specific genera bacteria (Allobaculum, Anaeroplasm, Bifidobacterium, Butyricimonas, unclassified_f_Ruminococcaceae, etc.). For hawthorn seed oil showed hypocholesterolemic effect on high-cholesterol diet-induced hamsters (96), these metabolites were obviously correlated with 30 specific genera bacteria (Faecalibaculum, Ruminococcus_2, Peptococcus, unclassifed_f_Christensenellaceae, norank_o_Gastranaerophilales, etc.). For wild melon seeds oil exhibited hypocholesterolemic effect against high-cholesterol diet-induced hamsters (93), these metabolites were notably correlated with 26 specific genera of bacteria (Bifidobacterium, Ruminococcus_2, nonrank_f_Eubacteriaceae, Bilophila, Blautia, etc.). On the other hand, for peony seed oil exerted alleviate hyperlipidemia and hyperglycemia action on high-fat diet-induced mice (81), 12 metabolites (adenosine, choline, β-asarone, glycitein, betaine, etc.) were notably correlated with 12 bacteria (Lactobacillus, Bifidobacterium, Parabacteroides, Coprococcus, Paraprevotella, etc.). For Decaisnea insignis seed oil displayed hepatoprotection against alcohol-induced mice (82), 25 metabolites (asparagine, L-kynurenine, chlorogenic acid, sarcosine, synephrine, etc.) were obviously correlated with 15 bacteria (Desulforibrio, [Eubacterium]_coprostandigenes_group, Akkermansia, Ruminoccaceae_UCG_004, Streptococcus, etc.). For sacha inchi oil reflected hypolipidemic effect on high-fat diet-induced rats (84), significant corrections were found between the primary bile acids (CA, GCA, TCDCA, and TCA) in the metabolisms of glycerolipid and glycerophospholipid and the differential bacteria (Roseburia, Turicibacter, Butyrivibrio, Escherichia, Bacteroides, etc.). For tomato seed oil showed anti-hyperlipidemia activity on high-fat diet-induced mice (94), fecal bile acids (SFA, MUFA, PUFA, and total FA) were closely related to Lactobacillus, Odoribacter, Rikenella, norank_f__Clostridiales_vadinBB60_group and/or [Eubacterium]_fissicatena_group. Among them, Lactobacillus was one of the differential gut microbiota biomarkers for tomato seed oil.
5.4. Correlations between altered metabolites and changed biochemical indexes
In terms of SCFAs, for flaxseed oil exerted anti-diabetes action on streptozotocin-nicotinamide-induced rats (55), the acetic acid was negatively correlated with TNF-α, IL-1β, IL-6, and IL-17A. Meanwhile, the propionic acid and butyric acid were negatively correlated with IL-1β, IL-6, and IL-17A. For flaxseed oil exhibited anti-atherosclerosis activity in high-fat diet-induced ApoE−/− mice (58), the acetic acid and propionic acid were negatively correlated with LPS, TNF-α, IL-1β, and IL-17A in plasma and with TNF-α, IL-1β, and IL-6 in the aorta. The isobutyric acid was positively correlated with IL-10 in the aorta, and negatively correlated with TNF-α and IL-1β in plasma and with TNF-α and IL-6 in the aorta. The isovaleric acid was negatively correlated with TNF-α and IL-1β in plasma and with IL-1β in the aorta. The valeric acid was positively correlated with IL-10 in the aorta, and negatively correlated with LPS, TNF-α, IL-1β, and IL-17A in plasma and with TNF-α, IL-1β, and IL-6 in the aorta.
Regarding other gut microbiota-derived metabolites, olive oil showed anti-diabetes effect on NOD/LtJ mice (48), the increased serum metabolites were positively correlated with islet number, function, and glycemic control, while were negatively correlated with inflammation and cell immunity. For instance, madecassic acid exhibited a positive correlation with islet number but a negative correlation with insulitis, Th1/Th2, MLN_IL-6, AUC area of OGTT and Random blood glucose_15W. Unsaturated fatty acids such as linoleic acid, erucic acid, nervonic acid, and eicosenoic acid were negatively correlated with insulitis and the AUC area of OGTT. Conversely, those decreased lipids, such as oleamide, methyllycaconitine, 5-aminovaleric acid betaine, and ginsenoside f3, were mainly positively correlated with colon_IL-6, insulitis, and AUC area of OGTT. For flaxseed oil exhibited anti-atherosclerosis activity on high-fat diet-induced ApoE−/− mice (58), alloLCA and isoLCA were positively correlated with LPS in plasma, and CDCA and HDCA were positively correlated with TNF-α in aorta. LCA was negatively correlated with IL-1β and IL-6 in the aorta. 7-ketoLCA was positively correlated with LPS in plasma and TNF-α in the aorta. β-UDCA was positively correlated with IL-1β and IL-17A in plasma and aorta. α-MCA was positively correlated with IL-17A in plasma and with IL-1β and IL-17A in the aorta. ACA was negatively correlated with LPS and TNF-α in plasma. GCA was negatively correlated with LPS, TNF-α, and IL-17A in plasma, and with TNF-α in the aorta. TCA was negatively correlated with LPS, TNF-α, and IL-1β in plasma, and with TNF-α and IL-1β in the aorta. For sacha inchi oil exerted hypolipidemic effect against high-fat diet-induced rats (84), CA was positively correlated with Cyp7a1 and Hsd3b7, and negatively correlated with Amacr, Slc27a5, Slc10a1, and FXR. GCA was negatively correlated with Slc27a5, Slc10a1, and Abcc2. TCDCA was negatively correlated with Amacr, Slc27a5, and Slc10a1. TCA was negatively correlated with Slc27a5 and Slc10a1. Meanwhile, lipids (TAG (16:0/16:0/22:4), DG (18:1/20:0), LysoPC (18:2), PC (18:0/20:4), PE (18,1/22:4), etc.) involved in the metabolisms of glycerolipid and glycerophospholipid were significantly correlated with differential genes (Pemt, Crls1, Lipc, Acadm, Scd2, etc.).
6. Conclusion and prospects
Many edible plant oils (especially camellia oil, olive oil, and flaxseed oil) could modulate gut microbiota during their health-promoting effects. Moreover, the modulated gut microbiota was significantly correlated with the changed biochemical indexes. Furthermore, the altered metabolites by edible plant oils were obviously correlated with the modulated gut microbiota and changed biochemical indexes. Overall, it could be speculated that edible plant oils modulate gut microbiota to alter SCFAs and other gut microbiota-derived metabolites, thereby changing biochemical indexes to exhibit health-promoting effects (Figure 5). This review provides theoretical basis for understanding and future research in health-promoting effects of edible plant oils from the perspective of gut microbiota modulation.
Figure 5.
The speculation of “edible plant oils→gut microbiota→metabolites→biochemical indexes→health-promoting effects”.
There are still some questions that remain to be solved: (i) the interactions between edible plant oils and gut microbiota have been rarely studied, and the key bioactive nutrients for edible plant oils in modulating gut microbiota during their health-promoting effects have not been well-ascertained; (ii) which gut microbiota and metabolites are exactly important for edible plant oils in exerting health-promoting effects, and how the screened differential gut microbiota biomarkers along with the altered metabolites mediate the health-promoting effects of edible plant oils, are unknown; (iii) the mechanisms of edible plant oils in modulating gut microbiota, and gut microbiota altering metabolites as well as metabolites changed biochemical indexes have not been investigated; (iv) cohort or clinical studies and the related specific mechanisms concerning gut microbiota modulation of edible plant oils have been rarely uncovered, and the relevant existing researches were absence of longitudinal data.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (XN202001); Jiangxi Provincial Natural Science Foundation (20232BAB215062 and 20202BABL216081); the Science and Technology Research Project of Jiangxi Provincial Education Department (GJJ190805); and University-Level Scientific Research Projects of Gannan Medical University (QD201913, QD202128, TD202230, and TD2022GR04).
Author contributions
QZ: Funding acquisition, Investigation, Visualization, Writing – original draft. A-QC: Investigation, Visualization, Writing – original draft. JH: Investigation, Visualization, Writing – original draft. MW: Investigation, Writing – original draft. J-HL: Project administration, Writing – review & editing. AW: Investigation, Writing – original draft. X-YW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zhou XR, Liu Q, Singh S. Engineering nutritionally improved edible plant oils. Annu Rev Food Sci Technol. (2023) 14:247–69. doi: 10.1146/annurev-food-052720-104852 [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Zhao WW, Lai Y, Zhang BH, Zhang DQ. Edible plant oil: global status, health issues, and perspectives. Front Plant Sci. (2020) 11:1315. doi: 10.3389/fpls.2020.01315, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen CT, Shen MY, Liu GY, Liu XF, Liang L, Li YD, et al. Edible vegetable oils from oil crops: preparation, refining, authenticity identification and application. Process Biochem. (2023) 124:168–79. doi: 10.1016/j.procbio.2022.11.017 [DOI] [Google Scholar]
- 4.Tian MK, Bai YC, Tian HY, Zhao XB. The chemical composition and health-promoting benefits of vegetable oils-a review. Molecules. (2023) 28:6393. doi: 10.3390/molecules28176393, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza B, Ninfali P. Antioxidants in extra virgin olive oil and table olives: connections between agriculture and processing for health choices. Antioxidants. (2020) 9:41. doi: 10.3390/antiox9010041, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Qiu FC, Chen L, Liu RJ, Chang M, Wang XG. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. (2021) 344:128660. doi: 10.1016/j.foodchem.2020.128660, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Liu HC, Zhu HJ, Xia H, Yang X, Yang LG, Wang SK, et al. Different effects of high-fat diets rich in different oils on lipids metabolism, oxidative stress and gut microbiota. Food Res Int. (2021) 141:110078. doi: 10.1016/j.foodres.2020.110078, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Wu YH, Chen SY, Yang JY, Chen YY, Yen GC. Gac (Momordica cochinchinensis Spreng.) oil supplementation mitigates cognitive decline in AlCl3-induced rats through the regulation of Alzheimer's disease markers and autophagy via gut-brain communication. J Funct Foods. (2023) 110:105860. doi: 10.1016/j.jff.2023.105860 [DOI] [Google Scholar]
- 9.Moral R, Escrich E. Influence of olive oil and its components on breast cancer: molecular mechanisms. Molecules. (2022) 27:477. doi: 10.3390/molecules27020477, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin PJ, Shen JJ, Wei JG, Chen YQ. A critical review of the bioactive ingredients and biological functions of camellia oleifera oil. Curr Res Food Sci. (2024) 8:100753. doi: 10.1016/j.crfs.2024.100753, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilal RM, Liu CJ, Zhao HH, Wang YZ, Farag MR, Alagawany M, et al. Olive oil: nutritional applications, beneficial health aspects and its prospective application in poultry production. Front Pharmacol. (2021) 12:723040. doi: 10.3389/fphar.2021.723040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Wen CT, Duan YQ, Deng QC, Peng DF, Zhang HH, et al. The composition, extraction, analysis, bioactivities, bioavailability and applications in food system of flaxseed (Linum usitatissimum L.) oil: a review. Trends Food Sci Technol. (2021) 118:252–60. doi: 10.1016/j.tifs.2021.09.025 [DOI] [Google Scholar]
- 13.Deen A, Visvanathan R, Wickramarachchi D, Marikkar N, Nammi S, Jayawardana BC, et al. Chemical composition and health benefits of coconut oil: an overview. J Sci Food Agr. (2021) 101:2182–93. doi: 10.1002/jsfa.10870, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Hsu CL, Schnabl B. The gut-liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. (2023) 21:719–33. doi: 10.1038/s41579-023-00904-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perler BK, Friedman ES, Wu GD. The role of the gut microbiota in the relationship between diet and human health. Annu Rev Physiol. (2023) 85:449–68. doi: 10.1146/annurev-physiol-031522-092054 [DOI] [PubMed] [Google Scholar]
- 16.Huang SY, Sun HY, Lin D, Huang XJ, Chen RR, Li ML, et al. Camellia oil exhibits anti-fatigue property by modulating antioxidant capacity, muscle fiber, and gut microbial composition in mice. J Food Sci. (2024) 89:2465–81. doi: 10.1111/1750-3841.16983, PMID: [DOI] [PubMed] [Google Scholar]
- 17.de Souza DM, Cavalcante HC, Dos Santos Lima M, Alves AF, da Veiga Dutra ML, D'Oliveira AB, et al. Intermittent fasting associated with coconut oil (Cocos nucifera L.) alters gut-liver axis parameters in diet-induced obese rats. Nutrition. (2024) 121:112370. doi: 10.1016/j.nut.2024.112370, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Li YW, Shen WK, Wang T, Liu YY, Ma JB, et al. Dietary supplementation of α-linolenic acid-rich flaxseed oil enhances anti-PD-1 protection against orthotopic hepatocellular carcinoma by reshaping gut homeostasis and improving anti-tumor immunity via gut-liver axis in mice. J Funct Foods. (2024) 116:106157. doi: 10.1016/j.jff.2024.106157 [DOI] [Google Scholar]
- 19.Luisi MLE, Lucarini L, Biffi B, Rafanelli E, Pietramellara G, Durante M, et al. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front Pharmacol. (2019) 10:1366. doi: 10.3389/fphar.2019.01366, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pu S, Khazanehei H, Jones PJ, Khafipour E. Interactions between obesity status and dietary intake of monounsaturated and polyunsaturated oils on human gut microbiome profiles in the canola oil multicenter intervention trial (COMIT). Front Microbiol. (2016) 7:1612. doi: 10.3389/fmicb.2016.01612, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura A, Sugita M. Perilla oil, an omega-3 unsaturated fatty acid-rich oil, enhances diversity of gut microbiota and may relieve constipation in sedentary healthy female: a nonrandomized placebo-controlled pilot study. Die Dermatol. (2023) 2:191–202. doi: 10.3390/dietetics2020015 [DOI] [Google Scholar]
- 22.Yang RN, Zhang LX, Li PW, Yu L, Mao J, Wang XP, et al. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends Food Sci Technol. (2018) 74:26–32. doi: 10.1016/j.tifs.2018.01.013 [DOI] [Google Scholar]
- 23.Ye Z, Xu YJ, Liu YF. Influences of dietary oils and fats, and the accompanied minor content of components on the gut microbiota and gut inflammation: a review. Trends Food Sci Technol. (2021) 113:255–76. doi: 10.1016/j.tifs.2021.05.001 [DOI] [Google Scholar]
- 24.Millman JF, Okamoto S, Teruya T, Uema T, Ikematsu S, Shimabukuro M, et al. Extra-virgin olive oil and the gut-brain axis: influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutr Rev. (2021) 79:1362–74. doi: 10.1093/nutrit/nuaa148, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavahian M, Khaneghah AM, Lorenzo JM, Munekata PE, Garcia-Mantrana I, Collado MC, et al. Health benefits of olive oil and its components: impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci Technol. (2019) 88:220–7. doi: 10.1016/j.tifs.2019.03.008 [DOI] [Google Scholar]
- 26.Gao L, Jin LH, Liu QN, Zhao KX, Lin L, Zheng JY, et al. Recent advances in the extraction, composition analysis and bioactivity of Camellia (Camellia oleifera Abel.) oil. Trends Food Sci Technol. (2023) 143:104211. doi: 10.1016/j.tifs.2023.104211 [DOI] [Google Scholar]
- 27.Zhang F, Zhu F, Chen BL, Su EZ, Chen YZ, Cao FL. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: a review. Food Res Int. (2022) 156:111159. doi: 10.1016/j.foodres.2022.111159, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Visioli F, Davalos A, de Las L, Hazas MC, Crespo MC, Tomé-Carneiro J. An overview of the pharmacology of olive oil and its active ingredients. Br J Pharmacol. (2020) 177:1316–30. doi: 10.1111/bph.14782, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza A, Knap N, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. (2018) 19:686. doi: 10.3390/ijms19030686, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang ZX, Ying RF, Lv BF, Yang LH, Xu Z, Yan LQ, et al. Flaxseed oil: extraction, health benefits and products. Qual Assur Saf Crop. (2021) 13:1–19. doi: 10.15586/qas.v13i1.783 [DOI] [Google Scholar]
- 31.Wu CC, Tung YT, Chen SY, Lee WT, Lin HT, Yen GC. Anti-inflammatory, antioxidant, and microbiota-modulating effects of camellia oil from Camellia brevistyla on acetic acid-induced colitis in rats. Antioxidants. (2020) 9:58. doi: 10.3390/antiox9010058, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee WT, Tung YT, Wu CC, Tu PS, Yen GC. Camellia oil (Camellia oleifera Abel.) modifies the composition of gut microbiota and alleviates acetic acid-induced colitis in rats. J Agric Food Chem. (2018) 66:7384–92. doi: 10.1021/acs.jafc.8b02166, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Jiang QH, Jiang CK, Lu HL, Zhou TY, Hu WJ, Tan CP, et al. Camellia oil alleviates DSS-induced colitis in mice by regulating the abundance of intestinal flora and suppressing the NF-κB signaling pathway. J Funct Foods. (2023) 108:105777. doi: 10.1016/j.jff.2023.105777 [DOI] [Google Scholar]
- 34.Weng MH, Chen SY, Li ZY, Yen GC. Camellia oil alleviates the progression of Alzheimer's disease in aluminum chloride-treated rats. Free Radic Biol Med. (2020) 152:411–21. doi: 10.1016/j.freeradbiomed.2020.04.004, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Guo PL, Zeng MN, Cao B, Liu M, Zhang YH, Jia JF, et al. Camellia oil improves Aβ25-35-induced memory impairment by regulating the composition of the gut microbiota and lipid metabolism in mice. J Funct Foods. (2022) 96:105214. doi: 10.1016/j.jff.2022.105214 [DOI] [Google Scholar]
- 36.Chen SY, Weng MH, Li ZY, Wang GY, Yen GC. Protective effects of camellia and olive oils against cognitive impairment via gut microbiota-brain communication in rats. Food Funct. (2022) 13:7168–80. doi: 10.1039/d1fo04418d, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Huang TY, Zhou WK, Ma XG, Jiang JH, Zhang FA, Zhou W, et al. Oral administration of camellia oil ameliorates obesity and modifies the gut microbiota composition in mice fed a high-fat diet. FEMS Microbiol Lett. (2021) 368:fnab063. doi: 10.1093/femsle/fnab063, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Waleed ASA, Huang TC, Deng LL, Han M, Zhao Y, Geng Z. Perilla, sunflower, and tea seed oils as potential dietary supplements with anti-obesity effects by modulating the gut microbiota composition in mice fed a high-fat diet. Eur J Nutr. (2023) 62:2509–25. doi: 10.1007/s00394-023-03155-3, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Gao J, Ma L, Yin J, Liu G, Ma J, Xia ST, et al. Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway. Food Funct. (2022) 13:4977–92. doi: 10.1039/d1fo04075h, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Huang TY, Jiang JH, Cao YJ, Huang JZ, Zhang FA, Cui GZ. Camellia oil (Camellia oleifera Abel.) treatment improves high-fat diet-induced atherosclerosis in apolipoprotein E (ApoE) −/− mice. Biosci Microbiota Food Health. (2023) 42:56–64. doi: 10.12938/bmfh.2022-005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu ZX, Zhang HM, Yang YZ, Ma XY, Yang C. Camellia oil (Camellia oleifera Abel.) alleviates gastric injury induced by ethanol associated with modulation of gut microbiota in mice. Oil Crop Sci. (2023) 8:61–71. doi: 10.1016/j.ocsci.2023.02.006 [DOI] [Google Scholar]
- 42.Guo R, Zhu JY, Chen L, Li JM, Ding QC, Han Q, et al. Dietary camellia seed oil attenuates liver injury in mice chronically exposed to alcohol. Front Nutr. (2022) 9:1026740. doi: 10.3389/fnut.2022.1026740, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao ZH, Shi AM, Wang Q, Zhou JR. High oleic acid peanut oil and extra virgin olive oil supplementation attenuate metabolic syndrome in rats by modulating the gut microbiota. Nutrients. (2019) 11:3005. doi: 10.3390/nu11123005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prieto I, Hidalgo M, Segarra AB, Martínez-Rodríguez AM, Cobo A, Ramírez M, et al. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS One. (2018) 13:e0190368. doi: 10.1371/journal.pone.0190368, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez N, Prieto I, Hidalgo M, Segarra AB, Martínez-Rodríguez AM, Cobo A, et al. Refined versus extra virgin olive oil high-fat diet impact on intestinal microbiota of mice and its relation to different physiological variables. Microorganisms. (2019) 7:61. doi: 10.3390/microorganisms7020061, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hidalgo M, Prieto I, Abriouel H, Villarejo AB, Ramírez-Sánchez M, Cobo A, et al. Changes in gut microbiota linked to a reduction in systolic blood pressure in spontaneously hypertensive rats fed an extra virgin olive oil-enriched diet. Plant Foods Hum Nutr. (2018) 73:1–6. doi: 10.1007/s11130-017-0650-1, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Li J, Guo YJ, Ma LY, Liu YX, Kuang HY, et al. Dietary olive oil enhances the oral tolerance of the food allergen ovalbumin in mice by regulating intestinal microecological homeostasis. J Food Biochem. (2022) 46:e14297. doi: 10.1111/jfbc.14297, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Shen YM, Lu SP, Wu J. EVOO supplement prevents type 1 diabetes by modulating gut microbiota and serum metabolites in NOD mice. Life Sci. (2023) 335:122274. doi: 10.1016/j.lfs.2023.122274, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Olalla J, García de Lomas JM, Chueca N, Pérez-Stachowski X, De Salazar A, Del Arco A, et al. Effect of daily consumption of extra virgin olive oil on the lipid profile and microbiota of HIV-infected patients over 50 years of age. Medicine (Baltimore). (2019) 98:e17528. doi: 10.1097/MD.0000000000017528, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millman J, Okamoto S, Kimura A, Uema T, Higa M, Yonamine M, et al. Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur J Nutr. (2020) 59:2411–25. doi: 10.1007/s00394-019-02088-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang C, Xu ZX, Huang QD, Wang X, Huang FH, Deng QC. Targeted microbiome metabolomics reveals flaxseed oil supplementation regulated the gut microbiota and farnesoid X receptor pathway in high-fat diet mice. Food Sci Hum Wellness. (2023) 12:2324–35. doi: 10.1016/j.fshw.2023.03.036 [DOI] [Google Scholar]
- 52.Liu SL, Wang X, Li YH, Shi BL, Guo XY, Zhao YL, et al. Flaxseed oil and heated flaxseed supplements have different effects on lipid deposition and ileal microbiota in albas cashmere goats. Animals. (2021) 11:790. doi: 10.3390/ani11030790, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo YM, Liu SL, Li YH, Guo XY, Zhao YL, Shi BL, et al. Intestinal microbiota community and blood fatty acid profiles of albas cashmere goats fed with flaxseed oil and whole flaxseed. Animals. (2023) 13:3531. doi: 10.3390/ani13223531, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Chen J, Zhou SM, Jiang YR, Wang Y, Li Y. The effect of flaxseed oil after deep frying on lipid metabolism and gut barrier homeostasis. Food Res Int. (2024) 175:113728. doi: 10.1016/j.foodres.2023.113728, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Zhu LL, Sha LP, Li K, Wang Z, Wang T, Li YW, et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. (2020) 19:20. doi: 10.1186/s12944-019-1167-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia H, Wang Y, Shi XL, Liao W, Wang SK, Sui J, et al. Beneficial effects of dietary flaxseed oil through inflammation pathways and gut microbiota in streptozotocin-induced diabetic mice. Food Secur. (2023) 12:3229. doi: 10.3390/foods12173229, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He ZY, Hao WJ, Kwek E, Lei L, Liu JH, Zhu HY, et al. Fish oil is more potent than flaxseed oil in modulating gut microbiota and reducing trimethylamine-N-oxide-exacerbated atherogenesis. J Agric Food Chem. (2019) 67:13635–47. doi: 10.1021/acs.jafc.9b06753, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Li YW, Yu Z, Liu YY, Wang T, Liu YJ, Bai ZX, et al. Dietary α-linolenic acid-rich flaxseed oil ameliorates high-fat diet-induced atherosclerosis via gut microbiota-inflammation-artery axis in ApoE−/− mice. Front Cardiovasc Med. (2022) 9:830781. doi: 10.3389/fcvm.2022.830781, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang XX, Wang H, Yin PP, Fan H, Sun LW, Liu YJ. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. (2017) 16:44. doi: 10.1186/s12944-017-0431-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun XY, Yu JT, Wang Y, Luo JM, Zhang GG, Peng XC. Flaxseed oil ameliorates aging in d-galactose induced rats via altering gut microbiota and mitigating oxidative damage. J Sci Food Agric. (2022) 102:6432–42. doi: 10.1002/jsfa.12010, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Wang T, Sha LP, Li YW, Zhu LL, Wang Z, Li K, et al. Dietary α-linolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones-microbiota-inflammation axis in rats. Front Endocrinol. (2020) 11:284. doi: 10.3389/fendo.2020.00284, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Che LQ, Zhou Q, Liu Y, Hu L, Peng X, Wu C, et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. (2019) 10:8149–60. doi: 10.1039/c9fo01877h, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Zhou Q, Ma L, Zhao WY, Zhao W, Han X, Niu JH, et al. Flaxseed oil alleviates dextran sulphate sodium-induced ulcerative colitis in rats. J Funct Foods. (2020) 64:103602. doi: 10.1016/j.jff.2019.103602 [DOI] [Google Scholar]
- 64.Zheng JY, Kang T, Jiang C, Lin LK, Gao L, Jin LH, et al. Gut microbiome and brain transcriptome analyses reveal the effect of walnut oil in preventing scopolamine-induced cognitive impairment. Food Funct. (2023) 14:9707–24. doi: 10.1039/d3fo01893h [DOI] [PubMed] [Google Scholar]
- 65.Miao FJ, Shan CL, Ma T, Geng SX, Ning DL. Walnut oil alleviates DSS-induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb Pathog. (2021) 154:104866. doi: 10.1016/j.micpath.2021.104866 [DOI] [PubMed] [Google Scholar]
- 66.Korach-Rechtman H, Rom O, Mazouz L, Freilich S, Jeries H, Hayek T, et al. Soybean oil modulates the gut microbiota associated with atherogenic biomarkers. Microorganisms. (2020) 8:486. doi: 10.3390/microorganisms8040486, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patrone V, Minuti A, Lizier M, Miragoli F, Lucchini F, Trevisi E, et al. Differential effects of coconut versus soy oil on gut microbiota composition and predicted metabolic function in adult mice. BMC Genomics. (2018) 19:808. doi: 10.1186/s12864-018-5202-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.López-Salazar V, Tapia MS, Tobón-Cornejo S, Díaz D, Alemán-Escondrillas G, Granados-Portillo O, et al. Consumption of soybean or olive oil at recommended concentrations increased the intestinal microbiota diversity and insulin sensitivity and prevented fatty liver compared to the effects of coconut oil. J Nutr Biochem. (2021) 94:108751. doi: 10.1016/j.jnutbio.2021.108751 [DOI] [PubMed] [Google Scholar]
- 69.Hao WJ, He ZY, Zhu HY, Liu JH, Kwek E, Zhao YM, et al. Sea buckthorn seed oil reduces blood cholesterol and modulates gut microbiota. Food Funct. (2019) 10:5669–81. doi: 10.1039/c9fo01232j, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Zhou HC, He SB, Zhang XF, Ling YH, Li XY, et al. The immunoenhancement effects of sea buckthorn pulp oil in cyclophosphamide-induced immunosuppressed mice. Food Funct. (2021) 12:7954–63. doi: 10.1039/d1fo01257f, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Zhou SM, Jiang YR. Sea buckthorn pulp and seed oils ameliorate lipid metabolism disorders and modulate gut microbiota in C57BL/6J mice on high-fat diet. Front Nutr. (2022) 9:1067813. doi: 10.3389/fnut.2022.1067813, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Djurasevic S, Bojic S, Nikolic B, Dimkic I, Todorovic Z, Djordjevic J, et al. Beneficial effect of virgin coconut oil on alloxan-induced diabetes and microbiota composition in rats. Plant Foods Hum Nutr. (2018) 73:295–301. doi: 10.1007/s11130-018-0689-7, PMID: [DOI] [PubMed] [Google Scholar]
- 73.Rolinec M, Medo J, Gábor M, Miluchová M, Bíro D, Šimko M, et al. The effect of coconut oil addition to feed of pigs on rectal microbial diversity and bacterial abundance. Animals. (2020) 10:1764. doi: 10.3390/ani10101764, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahnan AD, Agustinah W. Effect of virgin coconut oil supplementation on obese rats’ anthropometrical parameters and gut Bacteroidetes and Firmicutes change ratio. Cord. (2016) 32:14–4. doi: 10.37833/cord.v32i1.41 [DOI] [Google Scholar]
- 75.Araújo de Vasconcelos MH, Tavares RL, Dutra MLDV, Batista KS, D'Oliveira AB, Pinheiro RO, et al. Extra virgin coconut oil (Cocos nucifera L.) intake shows neurobehavioural and intestinal health effects in obesity-induced rats. Food Funct. (2023) 14:6455–69. doi: 10.1039/d3fo00850a, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Hu FM, Piao MY, Yang CT, Diao QY, Tu Y. Effects of coconut oil and palm oil on growth, rumen microbiota, and fatty acid profile of suckling calves. Microorganisms. (2023) 11:655. doi: 10.3390/microorganisms11030655, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawamura A, Nemoto K, Sugita M. Effect of 8-week intake of the n-3 fatty acid-rich perilla oil on the gut function and as a fuel source for female athletes: a randomised trial. Br J Nutr. (2022) 129:1–11. doi: 10.1017/S0007114522001805, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian Y, Wang HL, Yuan FH, Li N, Huang Q, He L, et al. Perilla oil has similar protective effects of fish oil on high-fat diet-induced nonalcoholic fatty liver disease and gut dysbiosis. Biomed Res Int. (2016) 2016:9462571. doi: 10.1155/2016/9462571, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang F, Zhu HJ, Hu MY, Wang J, Xia H, Yang X, et al. Perilla oil supplementation improves hypertriglyceridemia and gut dysbiosis in diabetic KKAy mice. Mol Nutr Food Res. (2018) 62:e1800299. doi: 10.1002/mnfr.201800299, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwek E, Zhu HY, Ding HF, He ZY, Hao WJ, Liu JH, et al. Peony seed oil decreases plasma cholesterol and favorably modulates gut microbiota in hypercholesterolemic hamsters. Eur J Nutr. (2022) 61:2341–56. doi: 10.1007/s00394-021-02785-9, PMID: [DOI] [PubMed] [Google Scholar]
- 81.Liang ZY, He YL, Wei DF, Fu PX, Li YY, Wang H, et al. Tree peony seed oil alleviates hyperlipidemia and hyperglycemia by modulating gut microbiota and metabolites in high-fat diet mice. Food Sci Nutr. (2024) 12:4421–34. doi: 10.1002/fsn3.4108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu XX, Zhao K, Yang XB, Zhao Y. Gut microbiota and metabolome response of Decaisnea insignis seed oil on metabolism disorder induced by excess alcohol consumption. J Agric Food Chem. (2019) 67:10667–77. doi: 10.1021/acs.jafc.9b04792, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Zhang XN, Wu Q, Zhao Y, Yang XB. Decaisnea insignis seed oil inhibits trimethylamine-N-oxide formation and remodels intestinal microbiota to alleviate liver dysfunction in L-carnitine feeding mice. J Agric Food Chem. (2019) 67:13082–92. doi: 10.1021/acs.jafc.9b05383, PMID: [DOI] [PubMed] [Google Scholar]
- 84.Li P, Huang JZ, Xiao N, Cai X, Yang YY, Deng JW, et al. Sacha inchi oil alleviates gut microbiota dysbiosis and improves hepatic lipid dysmetabolism in high-fat diet-fed rats. Food Funct. (2020) 11:5827–41. doi: 10.1039/d0fo01178a, PMID: [DOI] [PubMed] [Google Scholar]
- 85.Lai MY, Liu YL, Han XJ, Chen YH, Liu B, Zeng F. Three different oils improve HFD-induced lipid metabolism disorders via driving gut microbiota. Eur J Lipid Sci Technol. (2024) 126:2300129. doi: 10.1002/ejlt.202300129 [DOI] [Google Scholar]
- 86.Yin RY, Fu YX, Yousaf L, Xue Y, Hu JR, Hu XS, et al. Crude and refined millet bran oil alleviate lipid metabolism disorders, oxidative stress and affect the gut microbiota composition in high-fat diet-induced mice. Int J Food Sci Technol. (2022) 57:2600–10. doi: 10.1111/ijfs.15063 [DOI] [Google Scholar]
- 87.Phannasorn W, Pharapirom A, Thiennimitr P, Guo H, Ketnawa S, Wongpoomchai R. Enriched riceberry bran oil exerts chemopreventive properties through anti-inflammation and alteration of gut microbiota in carcinogen-induced liver and colon carcinogenesis in rats. Cancers. (2022) 14:4358. doi: 10.3390/cancers14184358, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Li Y, Wang R, Ji HF, Lu CY, Su XR. Chinese Torreya grandis cv. Merrillii seed oil affects obesity through accumulation of sciadonic acid and altering the composition of gut microbiota. Food Sci Hum Wellness. (2022) 11:58–67. doi: 10.1016/j.fshw.2021.07.007 [DOI] [Google Scholar]
- 89.Ma JC, Yuan T, Gao YQ, Zeng XM, Liu ZG, Gao JM. Torreya grandis oil attenuates cognitive impairment in scopolamine-induced mice. Food Funct. (2023) 14:10520–34. doi: 10.1039/d3fo03800a, PMID: [DOI] [PubMed] [Google Scholar]
- 90.Danneskiold-Samsøe NB, Andersen D, Radulescu ID, Normann-Hansen A, Brejnrod A, Kragh M, et al. A safflower oil based high-fat/high-sucrose diet modulates the gut microbiota and liver phospholipid profiles associated with early glucose intolerance in the absence of tissue inflammation. Mol Nutr Food Res. (2017) 61:1600528. doi: 10.1002/mnfr.201600528, PMID: [DOI] [PubMed] [Google Scholar]
- 91.Qu LL, Liu QQ, Zhang Q, Tuo XX, Fan DD, Deng JJ, et al. Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem Toxicol. (2019) 125:85–94. doi: 10.1016/j.fct.2018.12.046, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Lu YL, Yang XB, Zhao Y. Supplementation of okra seed oil ameliorates ethanol-induced liver injury and modulates gut microbiota dysbiosis in mice. Food Funct. (2019) 10:6385–98. doi: 10.1039/c9fo00189a, PMID: [DOI] [PubMed] [Google Scholar]
- 93.Hao WJ, Zhu HY, Chen JN, Kwek E, He ZY, Liu JH, et al. Wild melon seed oil reduces plasma cholesterol and modulates gut microbiota in hypercholesterolemic hamsters. J Agric Food Chem. (2020) 68:2071–81. doi: 10.1021/acs.jafc.9b07302 [DOI] [PubMed] [Google Scholar]
- 94.He WS, Li LL, Rui JX, Li JJ, Sun YY, Cui DD, et al. Tomato seed oil attenuates hyperlipidemia and modulates gut microbiota in C57BL/6J mice. Food Funct. (2020) 11:4275–90. doi: 10.1039/d0fo00133c, PMID: [DOI] [PubMed] [Google Scholar]
- 95.Liu R, Shu Y, Qi WH, Rao WL, Fu ZH, Shi ZX, et al. Protective effects of almond oil on streptozotocin-induced diabetic rats via regulating Nrf2/HO-1 pathway and gut microbiota. J Food Qual. (2021) 2021:1–14. doi: 10.1155/2021/5599219 [DOI] [Google Scholar]
- 96.Kwek E, Yan C, Ding HF, Hao WJ, He ZY, Liu JH, et al. Effects of hawthorn seed oil on plasma cholesterol and gut microbiota. Nutr Metab. (2022) 19:55. doi: 10.1186/s12986-022-00690-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hidalgo M, Prieto I, Abriouel H, Cobo A, Benomar N, Gálvez A, et al. Effect of virgin and refined olive oil consumption on gut microbiota. Comparison to butter. Food Res Int. (2014) 64:553–9. doi: 10.1016/j.foodres.2014.07.030, PMID: [DOI] [PubMed] [Google Scholar]
- 98.Yan XB, Huang WB, Suo XX, Pan SM, Li T, Liu H, et al. Integrated analysis of microbiome and host transcriptome reveals the damageprotective mechanism of corn oil and olive oil on the gut health of grouper (♀ Epinephelus fuscoguttatus × ♂ E. Lanceolatu). Int J Biol Macromol. (2023) 253:127550. doi: 10.1016/j.ijbiomac.2023.127550, PMID: [DOI] [PubMed] [Google Scholar]
- 99.Kwek E, Yan C, Ding HF, Hao WJ, He ZY, Ma KY, et al. Effects of thermally-oxidized frying oils (corn oil and lard) on gut microbiota in hamsters. Antioxidants. (2022) 11:1732. doi: 10.3390/antiox11091732, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li H, Zhu YY, Zhao F, Song SX, Li YQ, Xu XL, et al. Fish oil, lard and soybean oil differentially shape gut microbiota of middle-aged rats. Sci Rep. (2017) 7:826. doi: 10.1038/s41598-017-00969-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miao FJ, Shan CL, Shah SAH, Akhtar RW, Wang XJ, Ning DL. Effect of walnut (Juglans sigillata) oil on intestinal antioxidant, anti-inflammatory, immunity, and gut microbiota modulation in mice. J Food Biochem. (2021) 45:e13567. doi: 10.1111/jfbc.13567 [DOI] [PubMed] [Google Scholar]