Abstract
The opioid peptides [Leu]enkephalin and dynorphin-(1-13) were shown to enhance glycogen breakdown when added directly to hepatocytes. This was the result of a concerted effect on the enzymes of glycogen metabolism, with a stimulation of glycogen phosphorylase activity and a simultaneous decrease in glycogen synthase I activity. The latter only became significant when the enzyme was activated by incubating the cells in presence of 20 mM- or 40 mM-glucose. The effect of the opioid peptides was independent of an increase in cyclic AMP or any change in the activity ratio of the cyclic AMP-dependent protein kinase and was abolished by depleting the cells of Ca2+. Both [Leu]enkephalin and dynorphin-(1-13) produced a significant decrease in cyclic AMP formation, suggesting that in liver, as in neuronal tissue, they may act by inhibiting adenylate cyclase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. H., Green I. C., Titheradge M. A. The stimulation of glycogenolysis and gluconeogenesis in isolated hepatocytes by opioid peptides. Biochem J. 1983 Nov 15;216(2):507–510. doi: 10.1042/bj2160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- BORISON H. L., FISHBURN B. R., BHIDE N. K., McCARTHY L. E. Morphine-induced hyperglycemia in the cat. J Pharmacol Exp Ther. 1962 Nov;138:229–235. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréant B., Keppens S., De Wulf H. Heterologous desensitization of the cyclic AMP-independent glycogenolytic response in rat liver cells. Biochem J. 1981 Dec 15;200(3):509–514. doi: 10.1042/bj2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Chisholm A. B., Allan E. H., Titheradge M. A. Regulation of mitochondrial pyruvate carboxylation in isolated hepatocytes by acute insulin treatment. Biochem J. 1983 Aug 15;214(2):451–458. doi: 10.1042/bj2140451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. K., Campanile C. P., Garrison J. C. The hepatic angiotensin II receptor. II. Effect of guanine nucleotides and interaction with cyclic AMP production. J Biol Chem. 1982 May 10;257(9):4959–4965. [PubMed] [Google Scholar]
- Dave J. R., Rubinstein N., Eskay R. L. Evidence that beta-endorphin binds to specific receptors in rat peripheral tissues and stimulates the adenylate cyclase-adenosine 3',5'-monophosphate system. Endocrinology. 1985 Oct;117(4):1389–1396. doi: 10.1210/endo-117-4-1389. [DOI] [PubMed] [Google Scholar]
- Dey P. K., Feldberg W. Hyperglycaemia produced by drugs with analgesic properties introduced into the cerebral ventricles of cats. Br J Pharmacol. 1975 Jun;54(2):163–170. doi: 10.1111/j.1476-5381.1975.tb06925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Gupta K. P. Morphine hyperglycaemia. J Physiol. 1974 May;238(3):487–502. doi: 10.1113/jphysiol.1974.sp010539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green I. C., Ray K., Perrin D. Opioid peptide effects on insulin release and c-AMP in islets of Langerhans. Horm Metab Res. 1983 Mar;15(3):124–128. doi: 10.1055/s-2007-1018648. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Jard S., Cantau B., Jakobs K. H. Angiotensin II and alpha-adrenergic agonists inhibit rat liver adenylate cyclase. J Biol Chem. 1981 Mar 25;256(6):2603–2606. [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Vasopressin and angiotensin control the activity of liver phosphodiesterase. Biochem J. 1984 Aug 15;222(1):277–280. doi: 10.1042/bj2220277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Koski G., Tocque B., Simonds W. F. On the mechanism of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:153–159. [PubMed] [Google Scholar]
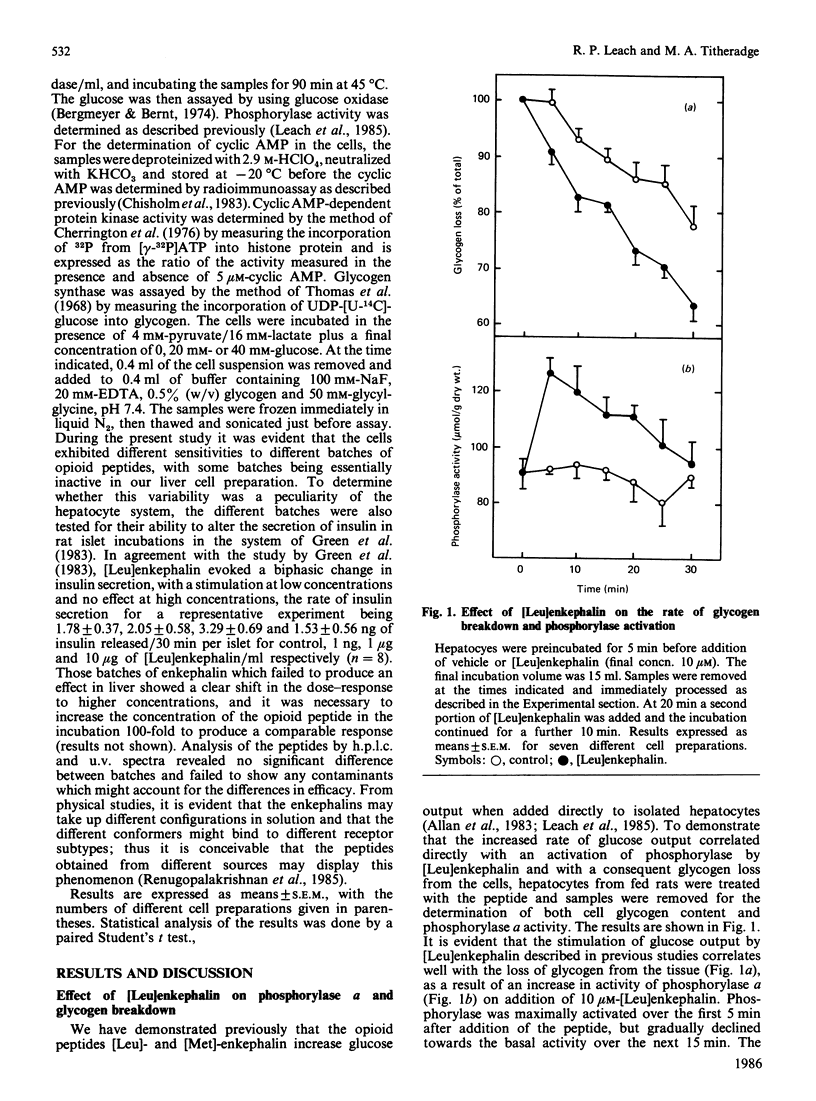
- Leach R. P., Allan E. H., Titheradge M. A. The stimulation of glycogenolysis in isolated hepatocytes by opioid peptides. Biochem J. 1985 Apr 1;227(1):191–197. doi: 10.1042/bj2270191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Fukushima T., Saito H., Saito S. In vivo and in vitro effects of beta-endorphin on glucose metabolism in the rat. Horm Metab Res. 1984 Jan;16(1):27–31. doi: 10.1055/s-2007-1014686. [DOI] [PubMed] [Google Scholar]
- Renugopalakrishnan V., Rapaka R. S., Collette T. W., Carreira L. A., Bhatnagar R. S. Conformational states of Leu5- and Met5-enkephalins in solution. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1029–1035. doi: 10.1016/0006-291x(85)90288-8. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Kirk C. J., Hems D. A. Role of extracellular calcium in the action of vasopressin on hepatic glycogenolysis. FEBS Lett. 1976 Oct 15;69(1):199–202. doi: 10.1016/0014-5793(76)80686-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wood C. L., Blum J. J. Effect of vasoactive intestinal polypeptide on glycogen metabolism in rat hepatocytes. Am J Physiol. 1982 Apr;242(4):E262–E272. doi: 10.1152/ajpendo.1982.242.4.E262. [DOI] [PubMed] [Google Scholar]



