Abstract
The ovine genome contains 15 to 20 copies of endogenous retroviruses (enJSRVs) highly related to the oncogenic jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus. enJSRVs are highly expressed in the endometrial lumenal epithelia (LE) and glandular epithelia (GE) of the ovine uterus. The effects of neonatal age, estrous cycle, pregnancy, and progesterone on expression of enJSRVs in the ovine uterus were determined. Expression of enJSRV RNAs was absent from the uterus of ewes at birth, but enJSRV RNAs were expressed specifically in the LE and developing GE from postnatal day (PND) 7 to PND 56. In adult ewes, enJSRV RNAs were detected only in the epithelia of the uterine endometrium, as well as epithelia of the oviduct, cervix, and vagina. In cyclic ewes, endometrial enJSRV RNA abundance was lowest on day 1, increased 12-fold between days 1 and 13, and then decreased to day 15. In pregnant ewes, levels of endometrial enJSRV RNAs were high on day 11, increased to day 13, and then decreased to day 19. In day 17 and 19 conceptuses, enJSRV RNAs were also detected in binucleate cells of the trophectoderm. Immunoreactive JSRV capsid and envelope proteins were detected in the endometrial LE and GE, as well as in the binucleate cells of the conceptus. In transfection assays utilizing ovine endometrial LE cells, progesterone increased transcriptional activity of several enJSRV long terminal repeats. Collectively, these results indicate that transcription of enJSRVs in the endometrial epithelia of the ovine uterus is increased by progesterone and might support a role for enJSRVs in conceptus-endometrium interactions during the peri-implantation period and early placental morphogenesis.
A distinctive feature of retroviruses is their presence as inherited elements in the germ line of most eukaryotes. These elements, known as endogenous retroviruses (ERVs), are transmitted through the germ line as stable Mendelian genes, yet they exhibit structural and sequence similarities to infectious exogenous retroviruses (9). It is assumed that ERVs were derived from integration events during the evolution of ancient exogenous retroviruses (e.g., transmitted horizontally) into the germ line of host animal species. In recent years, considerable effort has been directed toward understanding the biological significance of ERVs, particularly those present in the human germ line (8, 30, 31). Generally, endogenous proviruses are transcriptionally silent and are often defective, typically differing from the exogenous counterpart by deletions or point mutations that render them incapable of forming infectious virus (9, 11). However, several ERVs maintain at least some intact open reading frames with expression associated with either beneficial or detrimental effects to the host (9). Specific expression of some ERVs in the placenta has lead to various hypotheses that these elements play a role in mammalian reproduction (6, 7, 23, 33, 42, 43, 54, 55).
Sheep represent an interesting model with which to study the biology of ERVs and their interaction with host species. The ovine genome contains 15 to 20 copies of endogenous retroviruses (enJSRVs) (3, 24, 25, 36, 57) that are highly related to two oncogenic exogenous betaretroviruses, Jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus (ENTV) (14, 40). enJSRV RNAs are highly expressed in the epithelium of the uterus (38, 48), while the exogenous pathogenic viruses JSRV and ENTV appear to have a strict tropism for secretory cells of the respiratory tract (16, 36, 37). Expression of enJSRV RNAs in the ovine uterus was initially identified by differential display PCR and PCR-based subtraction hybridization experiments (48). In situ hybridization analyses discovered that enJSRV RNAs were restricted in expression to the endometrial lumenal epithelium (LE) and glandular epithelium (GE) (48). Indeed, the expression level of the enJSRVs in the uterine endometrial epithelia is very high relative to a number of other genes expressed in the same epithelia, as well as expression of enJSRVs in other sheep tissues (35, 38). The high level and specificity of temporal and spatial expression of enJSRVs in the endometrium of the ovine uterus might suggest physiological functions of these elements in regulation of conceptus-endometrium interactions, as well as placental morphogenesis, during the peri-implantation period. On the other hand, tropism for the genital tract of the exogenous viral ancestors of the current enJSRVs might also explain the specific expression of the endogenous loci in the epithelium of the uterus.
To further investigate the role of enJSRVs in sheep uterine biology, studies were conducted to determine effects of neonatal age, day of the estrous cycle and early pregnancy, and progesterone on expression of enJSRVs in the ovine uterus. Expression of enJSRV RNAs was detected only in epithelia of oviduct, uterus, cervix, and vagina. Changes in abundance of enJSRV RNAs in the endometrium during the estrous cycle and early pregnancy suggested that progesterone, acting via the progesterone receptor, increases transcription of enJSRV genes. Transient transfection assays established that progesterone increased transcriptional activity of several enJSRV long terminal repeats (LTRs). Immunoreactive JSRV capsid and envelope proteins were detected in the endometrial LE and GE as well as in binucleate cells of the conceptus trophectoderm that form syncytia with the epithelium of the uterus.
MATERIALS AND METHODS
Animals.
Mature ewes of primarily Rambouillet or Suffolk breeding were observed daily for estrus by using vasectomized rams. All ewes exhibited at least two estrous cycles of normal duration (∼16 to 18 days). Experimental and surgical procedures involving animals were approved by the Institutional Agricultural Animal Care and Use Committee of Texas A&M University.
Study 1: expression of enJSRVs mRNAs in the uterus of neonatal ewe lambs.
Rambouillet-Suffolk ewe lambs were assigned randomly at birth (postnatal day [PND] 0) to be necropsied on PND 1 (n = 6), 7 (n = 5), 14 (n = 5), 21 (n = 4), 28 (n = 5), 42 (n = 5), or 56 (n = 5) as described previously (51). The uterus was obtained and trimmed free of the broad ligament, oviduct, and cervix. Sections (∼1 cm) from the midportion of each uterine horn were fixed in fresh 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.2) and embedded in Paraplast Plus (Oxford Labware, St. Louis, Mo.).
Study 2: expression of enJSRV RNAs in reproductive tract tissues of adult ewes.
Adult, cyclic ewes (n = 6) were synchronized to estrus and bred with fertile rams as described previously (20). On day 9 postmating, ewes were necropsied, and the entire reproductive tract was excised. Sections of the oviduct (ampulla and isthmus), cervix, and vagina (anterior and posterior) were fixed in fresh 4% paraformaldehyde for histology. Each uterine horn was flushed with 10 ml of Dulbecco's modified Eagle's medium (DMEM)–F-12 medium (Sigma Chemical Co., St. Louis, Mo.) and examined for the presence of a hatched blastocyst to confirm pregnancy. The uterine horns were then separated, and sections from each uterine horn (near the utero-tubal junction, the midportion, and uterine body) were fixed in 4% paraformaldehyde and then processed and embedded in Paraplast Plus.
Study 3: expression of enJSRV RNAs and protein in uterus of cyclic and pregnant ewes.
At estrus, ewes were assigned randomly to cyclic or pregnant status. Ewes assigned to pregnant status were bred with intact rams at estrus (day 0) and then ovariohysterectomized (n = 4 ewes/day) on day 1, 3, 5, 7, 9, 11, 13, or 15 of the estrous cycle and day 9, 11, 13, 15, 17, or 19 of pregnancy (day 0 = estrus/mating) as described previously (45). Pregnancy was confirmed by the presence of an apparently normal conceptus in uterine flushings. At hysterectomy, several sections from the middle of each uterine horn were fixed in fresh 4% paraformaldehyde and then embedded in Paraplast Plus.
Several sections of each uterine horn were also embedded in Tissue-Tek Optimal Cutting Temperature (OCT) compound (Miles, Inc., Oneonta, N.Y.), frozen in liquid nitrogen vapor, and stored at −80°C. The remaining endometrium was physically dissected from myometrium, frozen in liquid nitrogen, and stored at −80°C for RNA extraction. In monovulatory pregnant ewes, uterine tissue samples were marked as contralateral or ipsilateral to the ovary bearing the corpus luteum. No contralateral uterine samples were used in RNA and protein analyses.
RNA isolation and slot blot analyses.
Total cellular RNA was isolated from endometrium by using Trizol (Gibco-BRL, Bethesda, Md.). The quantity of RNA was assessed spectrophotometrically, and the integrity of RNA was examined by gel electrophoresis in a denaturing 1% agarose gel.
Steady-state levels of enJSRV RNAs were assessed by slot blot hybridization as described previously (50). Denatured total endometrial RNA (20 μg) from each ewe was analyzed with a radiolabeled antisense ovine enJSRV cRNA probe generated from a partial ovine endometrial enJSRV cDNA (DD54) that was cloned by differential display (DD)-PCR as previously described (48). To correct for variation in total RNA loading, a duplicate RNA slot membrane was hybridized with radiolabeled antisense 18S rRNA and cRNA (pT718S; Ambion, Austin, Tex.). Following washing, nonspecific hybridization was removed by RNase A digestion (2). The radioactivity associated with each slot was quantified by electronic autoradiography with an Instant Imager (Packard Instrument Company, Meridian, Conn.) and expressed as total counts (TCs).
In situ hybridization analysis.
The enJSRV RNAs were localized in uterine tissue sections (5 μm) by in situ hybridization analysis as described previously (48). Deparaffinized, rehydrated, and deproteinated uterine tissue sections were hybridized with radiolabeled antisense or sense cRNA probes generated from linearized ovine endometrial enJSRV cDNA (DD54) (48) by in vitro transcription with [α-35S]UTP. After hybridization, washing, and RNase A digestion, slides were dipped in NTB-2 liquid photographic emulsion (Kodak, Rochester, N.Y.), stored at 4°C for 1 week, and developed in Kodak D-19 developer. Slides were then counterstained with Harris modified hematoxylin (Fisher Scientific, Fairlawn, N.J.), dehydrated through a graded series of alcohol to xylene, and protected with a coverslip.
Immunofluorescence analyses.
JSRV protein was localized in frozen uterine tissue sections by immunofluorescence by methods similar to those described previously (26). Briefly, uterine tissues embedded in OCT compound from study 3 were sectioned with a cryostat (8 μm) and mounted on Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, Pa.). Frozen sections were fixed in −20°C methanol for 10 min, permeabilized with 0.3% Tween 20 in 0.02 M PBS, and then blocked in antibody dilution buffer (2 parts 0.02 M PBS, 1.0% bovine serum albumin, 0.3% Tween 20 [pH 8.0], 1 part glycerol) containing 5% normal goat serum for 1 h at room temperature. Sections were rinsed in PBS and incubated overnight at 4°C with the primary antibodies towards the JSRV major capsid protein or the envelope protein described below. Antibodies towards the JSRV capsid protein (no. 1520) were kindly provided by J. M. Sharp (Moredun Research Institute, Midlothian, Scotland) and produced as described previously (37). Antiserum towards JSRV envelope (no. 1721 and 1723) was obtained by injecting rabbits with synthetic peptides derived from the envelope region of JSRV (M. Palmarini and H. Fan, unpublished results). Substitution of primary antibody with normal rabbit serum (Sigma-Aldrich, St. Louis, Mo.) at the same concentration was used as the negative control. Following three rinses in PBS for 10 min each, sections were incubated with fluorescein-conjugated goat anti-rabbit immunoglobulin G (IgG) (Zymed, San Francisco, Calif.) for 1 h at room temperature and again washed in PBS three times for 10 min each. Coverslips were placed over a layer of Prolong antifade mounting reagent (Molecular Probes, Eugene, Oreg.).
Transient transfection and luciferase assays.
Immortalized ovine endometrial LE cells described previously (27) were grown in DMEM with the addition of 10% charcoal-stripped fetal bovine serum. Transient transfections were performed on ovine LE cells (2 × 105 to 4 × 105) plated on six-well plates approximately 24 h prior to transfection. Each well of subcultured ovine LE cells was cotransfected with 300 ng of firefly luciferase (luc gene) reporter plasmid, 1,000 ng of a human progesterone receptor (PR) mammalian overexpression vector (53), and 50 ng of pRL-null (Promega, Madison, Wis.), a construct that expresses the renilla luciferase gene, by using Fugene (Roche, Basel, Switzerland). Luciferase reporter plasmids were described previously (36, 38). Briefly, pJS21-luc was constructed by placing the JSRV21 LTR in front of luc. For construction of penJS56A1-luc, penJS5F16-luc, and penJS59A1-luc, the LTRs of the endogenous proviral clones enJS56A1, enJS5F16, and enJS59A1 were placed in front of the luc gene. The ENTV-luc has the ENTV LTR placed in front of the luc gene. The ENTV LTR was kindly provided by Cristina Cousens (Moredun Research Institute). Transfected cells were then treated with vehicle as a control or with progesterone (0.02 M) for 48 h. Cells were then harvested, and luciferase activity was determined by using the dual luciferase reporter system (Promega) and a TD 20/20 luminometer (Turner Designs, Sunnyvale, Calif.). All assays were conducted in triplicate with at least two independent experiments. Firefly luciferase data were normalized by using pRL-null luciferase values. Data are expressed as fold increase in relative light units of progesterone-treated cells compared to values for untreated cells.
Photomicroscopy and digital imaging.
Images of representative fields of in situ hybridization and immunofluorescence slides were recorded with a Zeiss Axioplan2 microscope (Carl Zeiss, Thornwood, N.Y.) fitted with a Hamamatsu C-5810 chilled three-color charge-coupled device (CCD) camera (Hamamatsu Corporation, Bridgewater, N.J.). Digital images were captured and/or assembled with Adobe Photoshop 4.0 (Adobe Systems, Seattle, Wash.) and a MacIntosh PowerMac G3 computer (Apple Computer, Cupertino, Calif.). Black-and-white prints were generated electronically with a Kodak DS8650 color printer.
Statistical analyses.
Data from slot blot hybridization analyses were subjected to least-squares analysis of variance (LS-ANOVA) by the general linear models (GLM) procedures of Statistical Analysis System version 8.1 for Windows (SAS Institute, Cary, N.C.). Slot blot hybridization data for enJSRV RNAs (total counts) were corrected for differences in sample loading by using the 18S rRNA data as a covariate in LS-ANOVA. Data from study 3 were analyzed for effects of day and pregnancy status (cyclic or pregnant), as well as their interaction. Within pregnancy status, LS regression analyses were used to determine effects of day on endometrial mRNA levels. All tests of significance were performed by using the appropriate error terms according to the expectation of the mean squares for error (49). Data are presented as LS mean TCs with standard errors (SE).
RESULTS
Expression of enJSRV RNAs in the uterus of neonatal ewe lambs (study 1).
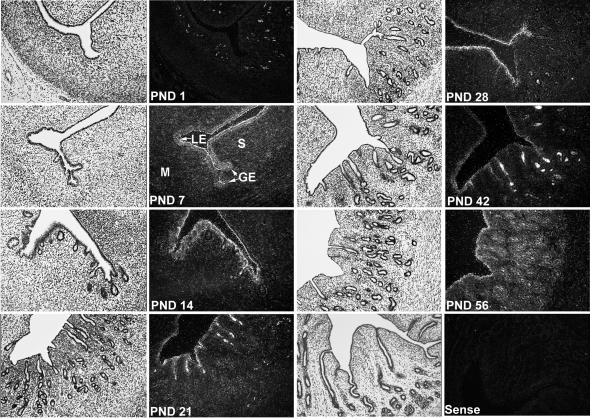
Previously, high levels of enJSRV RNAs were detected in the endometrial epithelium of uteri from adult ewes (48). In the present study, expression of enJSRV RNAs was studied in the uteri of neonatal ewe lambs between PND 1 and PND 56, because this represents a period of active uterine morphogenesis in which the endometrial GE differentiates from LE (51, 52). As illustrated in Fig. 1, the endometrial GE differentiates and buds into the underlying stroma from the LE between PNDs 1 and 7. Between PNDs 14 and 56, there is extensive coiling and branching morphogenesis of nascent endometrial glands. By PND 56, the uterine wall appears to be histoarchitecturally mature (52). During this period, PR is expressed by the LE and developing GE, but progesterone is absent or below detectable limits in the peripheral blood (52).
FIG. 1.
In situ hybridization analysis of enJSRV mRNA expression in the developing neonatal ovine uterus. Cross-sections of the uterine wall from neonatal ewes (PND 0 = birth) were hybridized with α-35S-labeled antisense or sense ovine enJSRV cRNA probes as described in Materials and Methods. Protected transcripts were visualized by liquid emulsion autoradiography for 1 week and imaged under bright-field or dark-field illumination. S, stroma; M, myometrium. Magnification, ×260.
In situ hybridization analyses revealed that enJSRV RNAs were absent or below detectable limits in all uterine cell types on PND 1 (Fig. 1). The white cells in the dark-field photomicrograph of the PND 1 uterine section are not positive for enJSRV RNAs, but rather are darkly pigmented melanocytes that appear white in dark-field photomicrographs. In contrast to PND 1, expression of enJSRV RNAs was detected in the LE and budding GE on PND 7 and was present in all epithelia to PND 56. Thus, enJSRVs are expressed in uterine epithelia in the absence of detectable progesterone in peripheral blood. We cannot specify which enJSRV loci are specifically expressed, because the DD54 probe most likely cross-reacts with most enJSRVs, given the high degree of homology of the known sequences of these elements (3, 4, 38).
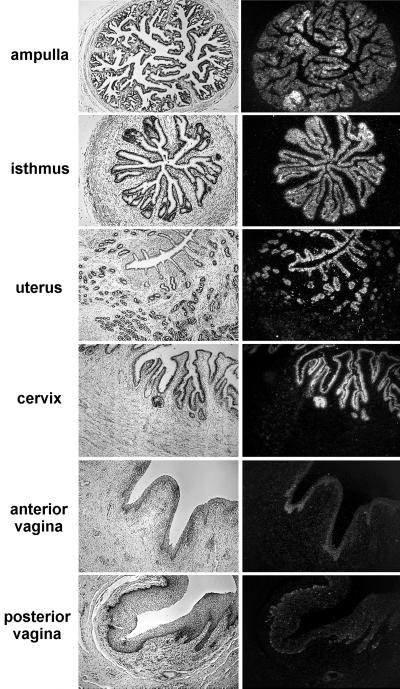
Expression of enJSRVs in tissues of the adult ovine reproductive tract (study 2).
In previous studies, enJSRV RNAs were detected in several tissues, including the uterus and lung (36, 48), but other tissues in the female reproductive tract were not studied. In this study, expression of enJSRV RNAs was surveyed in adult reproductive tract tissues of Müllerian duct origin from day 9 pregnant ewes (Fig. 2). As expected, abundant expression of enJSRV RNAs was detected in the endometrial LE and GE of all regions of the uterus. In addition, abundant expression of enJSRV RNAs was detected in the epithelia of the ampulla and isthmus regions of the oviduct, as well as in the cervix. Although expression of enJSRV RNAs was detected in the posterior and anterior regions of the vagina, expression was very low compared to that in other reproductive tract tissues.
FIG. 2.
In situ hybridization analysis of enJSRV mRNA expression in different tissues of the adult ovine female reproductive tract. Cross-sections of different regions of the female reproductive tract from day 9 pregnant ewes were hybridized with α-35S-labeled antisense or sense ovine enJSRV cRNA probes as described in Materials and Methods. Protected transcripts were visualized by liquid emulsion autoradiography for 1 week and imaged under bright-field or dark-field illumination. Magnification, ×260.
Expression of enJSRV RNAs and proteins in the uterus of cyclic and pregnant ewes (study 3).
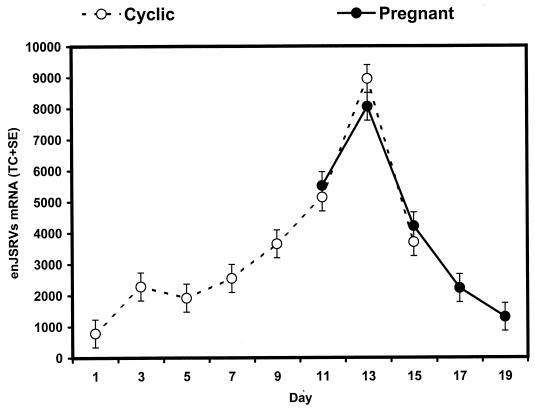
In order to determine the effects of day of the estrous cycle and early pregnancy on enJSRV expression in the endometrium, steady-state levels of enJSRV RNAs were determined in ovine endometrial total RNA by slot blot hybridization analysis with the DD54 partial enJSRV env cDNA as a probe (48). As previously reported, Northern blot analyses of ovine endometrial total RNA with antisense DD54 cRNA detected two RNA species 7.5 and 2.4 kb in size (48). In analogy with the exogenous JSRV, the 7.5-kb RNA transcript would represent the full-length enJSRV genome, whereas the 2.4-kb RNA transcript corresponds to the correctly spliced env mRNA (32).
As illustrated in Fig. 3, steady-state levels of enJSRV RNAs in the endometrium was affected (P < 0.01) by day of the estrous cycle or pregnancy, but not by pregnancy status (P > 0.10, day x status). In cyclic ewes, endometrial enJSRV RNAs increased 12-fold between days 1 and 13 and then decreased to day 15 (P < 0.01, cubic effect of day). The increase in endometrial enJSRV expression was highly correlated with changes in peripheral blood progesterone content (46). In pregnant ewes, endometrial enJSRV RNA expression was high on day 11, increased to day 13, and then decreased to day 19 (P < 0.01, cubic effect of day).
FIG. 3.
Effects of day of the estrous cycle or early pregnancy on expression of enJSRV mRNAs in the ovine endometrium. Slot blot hybridization analysis of enJSRV mRNAs in endometrium from cyclic and pregnant ewes was performed as described in Materials and Methods. Steady-state levels of enJSRV mRNAs are presented as LSM TCs with SE.
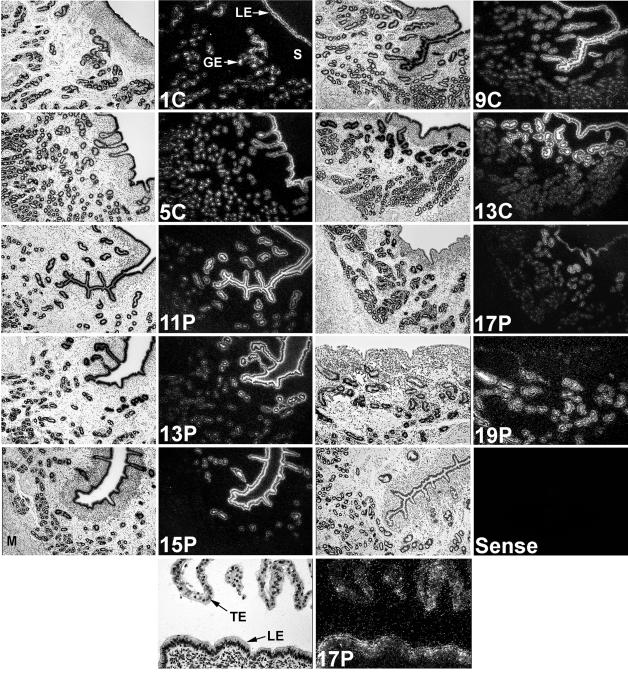
In situ hybridization analyses revealed that changes in the abundance of enJSRV RNAs in the endometrial LE were similar to changes in steady-state levels of enJSRV RNAs in the endometrium (Fig. 4). As expected, enJSRV RNAs were expressed predominantly in the LE and GE of the endometrium, whereas expression was not detected in the endometrial stroma or myometrium. In pregnant ewes, enJRSV RNAs were abundant in the LE on days 11 to 13, but declined to almost undetectable levels between days 15 and 19. Interestingly, the overall abundance of enJSRV RNAs was lower or absent in the LE that appeared to be in contact with the conceptus trophectoderm during this peri-implantation period of pregnancy. Expression of enJSRV RNAs was abundant in GE of the upper stratum compactum stroma, whereas expression in the GE in the stratum spongiosum near the myometrium was less abundant.
FIG. 4.
In situ hybridization analysis of enJSRV mRNA expression in the uteri of cyclic and pregnant ewes. Cross-sections of the uterine wall from cyclic (C) and pregnant (P) ewes were hybridized with [α-35S]-labeled antisense or sense ovine enJSRV cRNA probes as described in Materials and Methods. Protected transcripts were visualized by liquid emulsion autoradiography for 1 week and imaged under bright-field or dark-field illumination. S, stroma; M, myometrium; TE, trophectoderm. Magnification, ×260, except for the 17P panel at the bottom (×520).
In addition to the endometrial epithelia, expression of enJSRV RNAs was detected in selected, specific trophectodermal cell types of the developing placenta. Based on their morphology, these cells appear to be the binucleate cells of the conceptus trophectoderm. During synepitheliochorial placentation in ruminants, the binucleate cells fuse with the endometrial epithelium and produce placental lactogen (56).
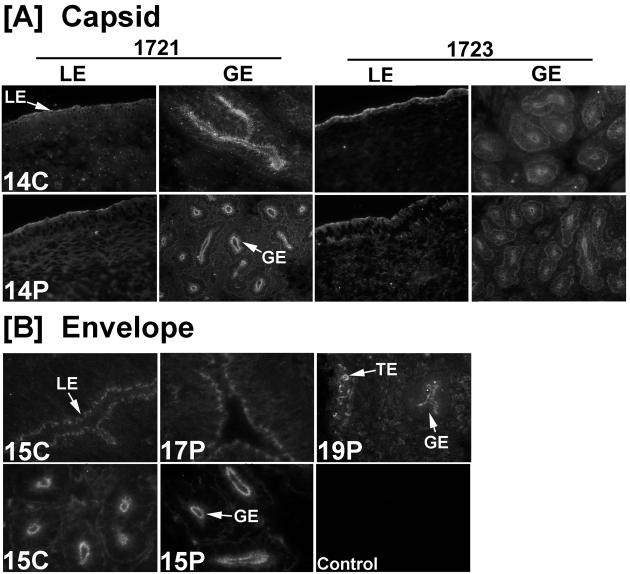
Immunofluorescence analyses of uterine sections from cyclic and pregnant ewes for JSRV envelope and capsid protein expression are presented in Fig. 5. As observed for enJSRV RNA expression, immunoreactive JSRV envelope and capsid proteins were detected specifically in the apical portion of the endometrial LE and GE, but not in the stroma or myometrium. The binucleate cells of the conceptus trophectoderm were also positive. Thus, the cell types in the ovine uterus expressing enJSRV RNAs also produce envelope and capsid proteins.
FIG. 5.
Immunoreactive JSRV capsid (A) and env protein (B) expression in the ovine endometrium. Immunofluorescence staining of frozen uterine sections from cyclic (C) and pregnant (P) ewes was conducted with specific antibodies or irrelevant IgG as a control as described in Materials and Methods. TE, trophectoderm. Magnification, ×230.
enJSRV LTRs are activated by progesterone.
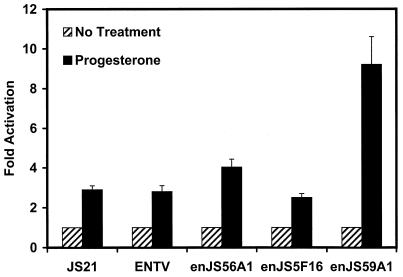
In cyclic and early pregnant ewes, expression of enJSRV genes in the endometrial epithelia is highly correlated with circulating levels of progesterone in peripheral blood and the presence of PR in uterine epithelia. Therefore, a plausible hypothesis is that one or more enJSRV LTRs, in which the retroviral promoter and enhancers are located, are regulated by progesterone. Although 15 to 20 copies of enJSRVs are present in the sheep genome, the expression observed in the uterus might derive from only one or a few loci. As illustrated in Fig. 6, transient transfection experiments were utilized to determine the responsiveness of LTRs from three endogenous loci (enJS56A1, enJS5F16, and enJS59A1) and exogenous JSRV21 (40) and ENTV (14) to progesterone. Progesterone stimulated (P < 0.01) the expression of the enJS59A1 LTR almost 10-fold and that of the enJS56A1 LTR about 4-fold. However, progesterone had minimal effects on the enJS5F16 LTR and on the LTRs from the exogenous viruses JSRV and ENTV. These results support the idea that increased expression of some enJSRV genes is mediated by increased transactivation of the LTR by liganded PR.
FIG. 6.
Effects of progesterone on activity of several enJSRV LTRs in transient transfection assays utilizing ovine endometrial LE cells. Results are expressed as fold increases relative to activity of the reporter constructs without the addition of progesterone. All assays were conducted in triplicate with at least two independent experiments. Results are presented as mean fold activation with SE.
The enJSRVs LTR used in this study are derived from proviral clones. Future studies of LTRs derived from transcriptionally active enJSRVs might be more appropriate in determining the response of these loci to hormones.
DISCUSSION
As a first effort to understand the significance of enJSRVs in sheep reproductive biology, the present studies assessed the influence of age, stage of the estrous cycle and pregnancy, and progesterone on enJSRV expression in the ovine uterus. Available results suggest that, during evolution, a function for these elements has been maintained that might be beneficial to the host. At the very least, enJSRV expression in the ovine female reproductive tract is apparently not detrimental to reproduction.
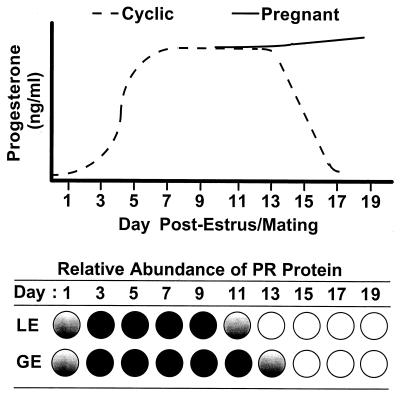
The overall pattern of enJSRV expression in the ovine endometrial epithelia and results of transient transfection experiments strongly suggest that enJSRV LTR(s) are responsive to ligand-activated PR. Levels of expression of enJSRV RNAs were low on day 1 of the estrous cycle and increased to maximal levels on day 13 in the endometrium of both cyclic and pregnant ewes. In situ hybridization analyses revealed that changes in the expression of enJSRV RNA occurred in both the LE and GE of the endometrium. Overall, these changes in steady-state enJSRV RNA levels closely paralleled the ontogeny of ovarian progesterone in the peripheral circulation as well as expression of the PR in endometrial epithelia (46). The initial increase in enJSRV RNAs between days 1 and 13 of the estrous cycle correlates with an increase in progesterone due to formation of the corpus luteum after ovulation. As illustrated in Fig. 7, PR expression is abundant in the LE and GE between days 5 and 11 of the estrous cycle in the ovine uterus. However, continuous exposure of the ovine uterus to progesterone for 8 to 10 days down-regulates expression of the PR (47). Expression of the PR declines in the LE to undetectable levels between days 11 and 13, whereas PR expression in the GE is progressively lost between days 13 and 19 of early pregnancy (46). Transient transfection experiments presented here indicate that the LTRs of several enJSRVs are transactivated by the liganded PR. However, the LTRs of the same enJSRVs do not require progesterone for basal expression. This contention is supported by the detection of enJSRV RNAs in endometrial epithelia of the developing uterus of neonatal ewes. Although the LE and GE express the PR throughout neonatal endometrial development, progesterone is below detectable limits in the peripheral circulation (52).
FIG. 7.
Schematic representation of progesterone levels in the peripheral blood relative to expression of the PR protein in the LE and GE in cyclic and early pregnant ewes. The relative amount of PR protein in the LE and GE is noted as absent (open circles), low abundance (partially [gradient] filled circles), and abundant (solid circles).
During early pregnancy, maximal expression of enJSRV genes was detected during the preimplantation period. The progressive decline in expression of enJSRVs in the epithelium likely results from the loss of PR expression in the LE and GE (46). In addition, loss of the LE itself occurs during synepitheliochorial placentation as the trophectoderm fuses with the LE and forms a syncytium beginning on day 16 of pregnancy. Of great interest for comparative physiology is the expression of enJSRVs in the binucleate cells of the conceptus trophectoderm. Binucleate trophectodermal cells fuse with the endometrial LE in both caruncular and intercaruncular areas to form a syncytium (56). Only the binucleate cells display invasive properties in the placenta of ruminants as well as other species. Indeed, retroviral particles have been observed in the placenta of many animal species, including ruminants (28). The presence of enJSRV gene expression in the developing placenta is strikingly similar to that observed for the human endogenous retrovirus, HERV-W (6). HERV-W is specifically expressed in the syncytiotrophoblast of the human placenta, which is formed by fusion of the trophoblast with the epithelium of the maternal uterus. HERV-W envelope protein, as for many retroviral envelope proteins, is able to induce formation of syncytia when expressed in vitro, thereby advancing the hypothesis that HERV-W is involved in human placental morphogenesis (7, 33). Similarly, ERV-3, another human retrovirus (10, 12), is also abundantly expressed in the syncytiotrophoblast, although the presence of a stop codon before the membrane anchor domain of the ERV-3 env gene would probably inhibit cell fusion mediated by the envelope protein (13). Collectively, these observations support the theory that an ancient retroviral infection had profound consequences for mammalian evolution (23). The presence of enJSRV RNAs as well as envelope and capsid protein expression might suggest involvement of this betaretrovirus in synepitheliochorial placentation in sheep.
Immunofluoresence analyses in the present study indicated that expression of enJSRV genes in the pregnant ovine uterus was not limited to RNA, because both JSRV envelope and capsid proteins were detected in the endometrial epithelia and trophectodermal binucleate cells. The expression of enJSRV genes in the newborn lamb may explain some aspects of the pathogenesis of ovine pulmonary adenocarcinoma (OPA) and enzootic nasal tumor (ENT) induced by the highly related exogenous viruses JSRV and ENTV. Sheep affected by OPA or ENT do not have a humoral immune response towards JSRV or ENTV (34, 44). Thus, it appears that the enJSRVs elements are recognized as self-antigens due to their basal expression, and sheep are tolerized towards infection by the exogenous JSRV. Indeed, preliminary experiments detected expression of enJSRV RNAs in the uterus, lungs, and gut of fetal sheep (T. E. Spencer, P. J. Griebel, and M. Palmarini, unpublished results). How the induction of tolerance to an exogenous retrovirus could be beneficial for its host is not readily apparent, although this might be the price of utilizing enJSRVs in ovine uterine function. In the present study, enJSRV expression was detected in all reproductive tract tissues of Müllerian duct origin. This is in accord with the hypothesis that ancestors of the modern JSRV and ENTV did not have a tropism for the respiratory apparatus, but rather for the genital tract, and were probably transmitted from sheep to sheep during the process of mating (38). Thus, these exogenous viruses might have been the cause of immunomediated disorders so that the induction of tolerance might have proved beneficial for sheep. These speculations would be very difficult to address experimentally.
In addition to trophectodermal binucleate cell biology, expression of enJSRVs in the endometrial epithelia may play another role in placental morphogenesis. Expression of enJSRVs in uterine epithelia between days 11 and 19 of pregnancy is highly correlated with tau interferon (IFN-τ) production by mononuclear cells of the trophectoderm (19, 22). In sheep, implantation is preceded by elongation of the conceptus from a tubular to filamentous form between days 12 and 15 (19), an event that involves trophoblast cell rearrangement and proliferation (22). Conceptus elongation is concomitant with production of the pregnancy recognition hormone, IFN-τ, which prevents transcriptional up-regulation of the estrogen receptor and oxytocin receptor genes in the endometrial LE (5, 46). The expression of IFN-τ genes is unusual, because they are not induced by virus (15, 29), only transcribed in the mononuclear cells of the trophectoderm (18, 22), and are sustained over several days (1) rather than limited to a few hours (17). Given the proliferation of the trophectoderm during the implantation period, the enJSRV envelope proteins might also stimulate cell division in these cells, analogous to transforming properties induced by the exogenous JSRV envelope (32, 41), although the enJSRV sequences known so far lack the putative phosphatidylinositol 3-kinase (PI-3K) docking site in the transmembrane region that has been found to be necessary for transformation in vitro (39).
In conclusion, the results of this study support the hypothesis that enJSRVs may play key roles in the reproductive physiology of sheep during the peri-implantation period. Sheep can be used as a model to understand the biological significance of endogenous retroviruses with respect to conceptus (embryo and associated extraembryonic placental membranes) development, implantation, and pregnancy recognition signaling.
ACKNOWLEDGMENTS
We are grateful to Mike Sharp (Moredun Research Institute, Midlothian, Scotland) for providing the rabbit antiserum towards the major capsid protein of JSRV, to Bert W. O'Malley and Ming-Jer Tsai (Baylor College of Medicine, Houston, Tex.) for provision of the human PR, and to Claudio Murgia for reporter assays.
This work was supported, in part, by NRI Competitive Grants Program/USDA grant 98–35203-6322 to T.E.S. and NIH grant P30 ES09106.
REFERENCES
- 1.Ashworth C J, Bazer F W. Changes in ovine conceptus and endometrial function following asynchronous embryo transfer or administration of progesterone. Biol Reprod. 1989;40:425–433. doi: 10.1095/biolreprod40.2.425. [DOI] [PubMed] [Google Scholar]
- 2.Ausbel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 2000. [Google Scholar]
- 3.Bai J, Bishop J V, Carlson J O, DeMartini J C. Sequence comparison of JSRV with endogenous proviruses: envelope genotypes and a novel ORF with similarity to a G-protein-coupled receptor. Virology. 1999;258:333–343. doi: 10.1006/viro.1999.9728. [DOI] [PubMed] [Google Scholar]
- 4.Bai J, Zhu R Y, Stedman K, Cousens C, Carlson J, Sharp J M, DeMartini J C. Unique long terminal repeat U3 sequences distinguish exogenous jaagsiekte sheep retroviruses associated with ovine pulmonary carcinoma from endogenous loci in the sheep genome. J Virol. 1996;70:3159–3168. doi: 10.1128/jvi.70.5.3159-3168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazer F W, Spencer T E, Ott T L. Interferon tau: a novel pregnancy recognition signal. Am J Reprod Immunol. 1997;37:412–420. doi: 10.1111/j.1600-0897.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 6.Blond J-L, Bèseme F, Duret L, Bouton O, Bedin F, Perron H, Mandrand B, Mallet F. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J Virol. 1999;73:1175–1185. doi: 10.1128/jvi.73.2.1175-1185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blond J L, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset F L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bock M, Stoye J P. Endogenous retroviruses and the human germline. Curr Opin Genet Dev. 2000;10:651–655. doi: 10.1016/s0959-437x(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 9.Boeke J D, Stoye J P. Retrotransposons, endogenous retroviruses and the evolution of retroelements. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 343–436. [PubMed] [Google Scholar]
- 10.Boyd M T, Bax C M, Bax B E, Bloxam D L, Weiss R A. The human endogenous retrovirus ERV-3 is upregulated in differentiating placental trophoblast cells. Virology. 1993;196:905–909. doi: 10.1006/viro.1993.1556. [DOI] [PubMed] [Google Scholar]
- 11.Coffin J M. Retroviridae: the viruses and their replication. In: Fields BN, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. New York, N.Y: Lippincott-Raven Publishers; 1996. pp. 763–843. [Google Scholar]
- 12.Cohen M, Kato N, Larsson E. ERV3 human endogenous provirus mRNAs are expressed in normal and malignant tissues and cells, but not in choriocarcinoma tumor cells. J Cell Biochem. 1988;36:121–128. doi: 10.1002/jcb.240360203. [DOI] [PubMed] [Google Scholar]
- 13.Cohen M, Powers M, O'Connell C, Kato N. The nucleotide sequence of the env gene from the human provirus ERV3 and isolation and characterization of an ERV3-specific cDNA. Virology. 1985;147:449–458. doi: 10.1016/0042-6822(85)90147-3. [DOI] [PubMed] [Google Scholar]
- 14.Cousens C, Minguijon E, Dalziel R G, Ortin A, Garcia M, Park J, Gonzalez L, Sharp J M, de las Heras M. Complete sequence of enzootic nasal tumor virus, a retrovirus associated with transmissible intranasal tumors of sheep. J Virol. 1999;73:3986–3993. doi: 10.1128/jvi.73.5.3986-3993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross J C, Roberts R M. Constitutive and trophoblast-specific expression of a class of bovine interferon genes. Proc Natl Acad Sci USA. 1991;88:3817–3821. doi: 10.1073/pnas.88.9.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De las Heras M, Sharp J M, Garcia de Jalon J A, Dewar P. Enzootic nasal tumour of goats: demonstration of a type D-related retrovirus in nasal fluids and tumours. J Gen Virol. 1991;72:2533–2535. doi: 10.1099/0022-1317-72-10-2533. [DOI] [PubMed] [Google Scholar]
- 17.De Maeyer E, De Maeyer-Guignard J. You cannot get away from interferon. J Interferon Res. 1987;7:467–470. doi: 10.1089/jir.1987.7.467. [DOI] [PubMed] [Google Scholar]
- 18.Farin C E, Imakawa K, Hansen T R, McDonnell J J, Murphy C N, Farin P W, Roberts R M. Expression of trophoblastic interferon genes in sheep and cattle. Biol Reprod. 1990;43:210–218. doi: 10.1095/biolreprod43.2.210. [DOI] [PubMed] [Google Scholar]
- 19.Godkin J D, Bazer F W, Moffatt J, Sessions F, Roberts R M. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J Reprod Fertil. 1982;65:141–150. doi: 10.1530/jrf.0.0650141. [DOI] [PubMed] [Google Scholar]
- 20.Gray C A, Bazer F W, Spencer T E. Effects of neonatal progestin exposure on female reproductive tract structure and function in the adult ewe. Biol Reprod. 2001;64:797–804. doi: 10.1095/biolreprod64.3.797. [DOI] [PubMed] [Google Scholar]
- 21.Guillomot M. Cellular interactions during implantation in domestic ruminants. J Reprod Fertil Suppl. 1995;49:39–51. [PubMed] [Google Scholar]
- 22.Guillomot M, Michel C, Gaye P, Charlier N, Trojan J, Martal J. Cellular localization of an embryonic interferon, ovine trophoblastin and its mRNA in sheep embryos during early pregnancy. Biol Cell. 1990;68:205–211. doi: 10.1016/0248-4900(90)90309-q. [DOI] [PubMed] [Google Scholar]
- 23.Harris J R. The evolution of placental mammals. FEBS Lett. 1991;295:3–4. doi: 10.1016/0014-5793(91)81370-n. [DOI] [PubMed] [Google Scholar]
- 24.Hecht S J, Carlson J O, DeMartini J C. Analysis of a type D retroviral capsid gene expressed in ovine pulmonary carcinoma and present in both affected and unaffected sheep genomes. Virology. 1994;202:480–484. doi: 10.1006/viro.1994.1366. [DOI] [PubMed] [Google Scholar]
- 25.Hecht S J, Stedman K E, Carlson J O, DeMartini J C. Distribution of endogenous type B and type D sheep retrovirus sequences in ungulates and other mammals. Proc Natl Acad Sci USA. 1996;93:3297–3302. doi: 10.1073/pnas.93.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson G A, Stewart M D, Gray C A, Choi Y, Burghardt R C, Yu-Lee L Y, Bazer F W, Spencer T E. Effects of the estrous cycle, pregnancy, and interferon tau on 2′,5′-oligoadenylate synthetase expression in the ovine uterus. Biol Reprod. 2001;64:1392–1399. doi: 10.1095/biolreprod64.5.1392. [DOI] [PubMed] [Google Scholar]
- 27.Johnson G A, Burghardt R C, Taylor K M, Fleming J G W, Bazer F W, Spencer T E. Development and characterization of immortalized ovine endometrial cell lines. Biol Reprod. 1999;61:1324–1330. doi: 10.1095/biolreprod61.5.1324. [DOI] [PubMed] [Google Scholar]
- 28.Kalter S S, Heberling R L, Helmke R J, Panigel M, Smith G C, Kraemer D C, Hellman A, Fowler A K, Strickland J E. A comparative study on the presence of C-type viral particles in placentas from primates and other animals. Bibl Haematol. 1975;40:391–401. doi: 10.1159/000397557. [DOI] [PubMed] [Google Scholar]
- 29.Leaman D W, Cross J C, Roberts R M. Multiple regulatory elements are required to direct trophoblast interferon gene expression in choriocarcinoma cells and trophectoderm. Mol Endocrinol. 1994;8:456–468. doi: 10.1210/mend.8.4.8052267. [DOI] [PubMed] [Google Scholar]
- 30.Lower R. The pathogenic potential of endogenous retroviruses: facts and fantasies. Trends Microbiol. 1999;7:350–356. doi: 10.1016/s0966-842x(99)01565-6. [DOI] [PubMed] [Google Scholar]
- 31.Lower R, Lower J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc Natl Acad Sci USA. 1996;93:5177–5184. doi: 10.1073/pnas.93.11.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda N, Palmarini M, Murgia C, Fan H. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc Natl Acad Sci USA. 2001;98:4449–4454. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mi S, Lee X, Li X, Veldman G M, Finnerty H, Racie L, LaVallie E, Tang X Y, Edouard P, Howes S, Keith J C, Jr, McCoy J M. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 34.Ortin A, Minguijon E, Dewar P, Garcia M, Ferrer L M, Palmarini M, Gonzalez L, Sharp J M, De las Heras M. Lack of a specific immune response against a recombinant capsid protein of jaagsiekte sheep retrovirus in sheep and goats naturally affected by enzootic nasal tumour or sheep pulmonary adenomatosis. Vet Immunol Immunopathol. 1998;61:229–237. doi: 10.1016/s0165-2427(97)00149-9. [DOI] [PubMed] [Google Scholar]
- 35.Palmarini M, Cousens C, Dalziel R G, Bai J, Stedman K, DeMartini J C, Sharp J M. The exogenous form of jaagsiekte retrovirus is specifically associated with a contagious lung cancer of sheep. J Virol. 1996;70:1618–1623. doi: 10.1128/jvi.70.3.1618-1623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmarini M, Datta S, Omid R, Murgia C, Fan H. The long terminal repeat of jaagsiekte sheep retrovirus is preferentially active in differential epithelial cells of the lungs. J Virol. 2000;74:5776–5787. doi: 10.1128/jvi.74.13.5776-5787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmarini M, Dewar P, De las Heras M, Inglis N F, Dalziel R G, Sharp J M. Epithelial tumour cells in the lungs of sheep with pulmonary adenomatosis are major sites of replication for jaagsiekte retrovirus. J Gen Virol. 1995;76:2731–2737. doi: 10.1099/0022-1317-76-11-2731. [DOI] [PubMed] [Google Scholar]
- 38.Palmarini M, Hallwirth C, York D, Murgia C, de Oliveira T, Spencer T, Fan H. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveal a different cell tropism from that of the highly related exogenous jaagsiekte sheep retrovirus. J Virol. 2000;74:8065–8076. doi: 10.1128/jvi.74.17.8065-8076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmarini M, Maeda N, Murgia C, De-Fraja C, Hofacre A, Fan H. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J Virol. 2001;75:11002–11009. doi: 10.1128/JVI.75.22.11002-11009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmarini M, Sharp J M, De las Heras M, Fan H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rai S K, Duh F M, Vigdorovich V, Danilkovitch-Miagkova A, Lerman M I, Miller A D. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulte A M, Lai S, Kurtz A, Czubayko F, Riegel A T, Wellstein A. Human trophoblast and choriocarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. Proc Natl Acad Sci USA. 1996;93:14759–14764. doi: 10.1073/pnas.93.25.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulte A M, Malerczyk C, Cabal-Manzano R, Gajarsa J J, List H J, Riegel A T, Wellstein A. Influence of the human endogenous retrovirus-like element HERV-E.PTN on the expression of growth factor pleiotrophin: a critical role of a retroviral Sp1-binding site. Oncogene. 2000;19:3988–3998. doi: 10.1038/sj.onc.1203742. [DOI] [PubMed] [Google Scholar]
- 44.Sharp J M, Herring A J. Sheep pulmonary adenomatosis: demonstration of a protein which cross-reacts with the major core proteins of Mason-Pfizer monkey virus and mouse mammary tumour virus. J Gen Virol. 1983;64:2323–2327. doi: 10.1099/0022-1317-64-10-2323. [DOI] [PubMed] [Google Scholar]
- 45.Spencer T E, Bartol F F, Bazer F W, Johnson G A, Joyce M M. Identification and characterization of glycosylation-dependent cell adhesion molecule 1-like protein expression in the ovine uterus. Biol Reprod. 1999;60:241–250. doi: 10.1095/biolreprod60.2.241. [DOI] [PubMed] [Google Scholar]
- 46.Spencer T E, Bazer F W. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–1543. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]
- 47.Spencer T E, Mirando M A, Mayes J S, Watson G H, Ott T L, Bazer F W. Effects of interferon-tau and progesterone on oestrogen-stimulated expression of receptors for oestrogen, progesterone and oxytocin in the endometrium of ovariectomized ewes. Reprod Fertil Dev. 1996;8:843–853. doi: 10.1071/rd9960843. [DOI] [PubMed] [Google Scholar]
- 48.Spencer T E, Stagg A G, Joyce M M, Jenster G, Wood C G, Bazer F W, Wiley A A, Bartol F F. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology. 1999;140:4070–4080. doi: 10.1210/endo.140.9.6981. [DOI] [PubMed] [Google Scholar]
- 49.Steele R G D, Torrie J H. Principles and procedures of statistics. New York, N.Y: McGraw-Hill; 1980. [Google Scholar]
- 50.Stewart M D, Johnson G A, Gray C A, Burghardt R C, Schuler L A, Joyce M M, Bazer F W, Spencer T E. Prolactin receptor and uterine milk protein expression in the ovine endometrium during the estrous cycle and pregnancy. Biol Reprod. 2000;62:1779–1789. doi: 10.1095/biolreprod62.6.1779. [DOI] [PubMed] [Google Scholar]
- 51.Taylor K M, Chen C, Gray C A, Bazer F W, Spencer T E. Expression of messenger ribonucleic acids for fibroblast growth factors 7 and 10, hepatocyte growth factor, and insulin-like growth factors and their receptors in the neonatal ovine uterus. Biol Reprod. 2001;64:1236–1246. doi: 10.1095/biolreprod64.4.1236. [DOI] [PubMed] [Google Scholar]
- 52.Taylor K M, Gray C A, Joyce M M, Stewart M D, Bazer F W, Spencer T E. Neonatal ovine uterine development involves alterations in expression of receptors for estrogen, progesterone, and prolactin. Biol Reprod. 2000;63:1192–1204. doi: 10.1095/biolreprod63.4.1192. [DOI] [PubMed] [Google Scholar]
- 53.Vegeto E, Allan G F, Schrader W T, Tsai M J, McDonnell D P, O'Malley B W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 54.Venables P J, Brookes S M, Griffiths D, Weiss R A, Boyd M T. Abundance of an endogenous retroviral envelope protein in placental trophoblasts suggests a biological function. Virology. 1995;211:589–592. doi: 10.1006/viro.1995.1442. [DOI] [PubMed] [Google Scholar]
- 55.Villarreal L P. On viruses, sex, and motherhood. J Virol. 1997;71:859–865. doi: 10.1128/jvi.71.2.859-865.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wooding F B. The role of the binucleate cell in ruminant placental structure. J Reprod Fertil Suppl. 1982;31:31–39. [PubMed] [Google Scholar]
- 57.York D F, Vigne R, Verwoerd D W, Querat G. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J Virol. 1992;66:4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]