Abstract
In this triple-blind, randomized clinical trial, 60 menopausal women between the ages of 45 and 65 were randomized to receive 78% dark chocolate (12 g/day) or milk chocolate (12 g/day) for eight weeks. The primary outcome was depression scores. Secondary outcomes included sleep quality and anthropometric indices. ANCOVA with baseline adjustment showed that the mean depression score after the intervention in the group receiving dark chocolate was significantly reduced compared to the milk chocolate group (mean difference: -2.3; 95% confidence interval: -3.9 to -0.8; p = 0.003; Cohen’s d = -0.54). However, no statistically significant difference in the overall sleep quality score and its subdomains was observed between the two groups after the intervention (p > 0.05). Furthermore, after the intervention, there was no statistically significant difference between the two groups in terms of anthropometric indices, including weight (p = 0.075), BMI (p = 0.137), waist circumference (p = 0.463), and hip circumference (p = 0.114). The study suggests that consuming 78% dark chocolate for eight weeks may contribute to improvements in depression scores, but it does not appear to improve sleep quality or anthropometric indices.
Trial registration: IRCT20220926056046N1; December 2022.
Keywords: Chocolate, Polyphenols, Menopause, Depression, Randomized Controlled Trial
Subject terms: Psychology, Health care, Medical research
Introduction
Menopause is a unique experience in a woman’s life1. According to the definition of the World Health Organization, natural menopause refers to the cessation of menstruation due to ovarian follicular function decline without the presence of other physiological or pathological factors2. Natural menopause age is influenced by factors such as demographic factors (such as education level and employment status), menstruation and reproduction history (such as parity and use of contraceptive pills), familial and genetic factors, and lifestyle factors (such as smoking, weight, physical activity, and nutrition)3. In a meta-analysis conducted on 36 studies from various countries, the average age of menopause was around 49 years4, and in Iran, it has been reported as 49.2 years5.
Menopause causes a reduction in estrogen levels that affects both the body and the brain6. Approximately 74 to 80% of women have menopausal symptoms7. The main symptoms are vasomotor symptoms, including hot flashes and night sweats8. Menopause is associated with problems such as poor sleep quality9 and an increase in body fat percentage10. Additionally, menopausal women are more likely to have negative mood symptoms such as anxiety and depression11.
The perimenopause describes the phase of transition from regular menstruation to its cessation, characterized by significant hormonal changes12. A systematic review and meta-analysis conducted on 55 studies reported the global prevalence of depression to be approximately 34% in perimenopausal women and around 35% in postmenopausal women13. Many common symptoms experienced during the menopause transition, such as low energy, sleep disturbances, and sexual problems overlap with the presentation of depression during this stage, complicating diagnosis14. Depression is also a prevalent psychological issue in postmenopausal women15, with approximately 60% reported in Iran16. In addition to hormonal changes, vasomotor symptoms, previous history of depression, high body mass index (BMI), and sleep problems are considered risk factors for menopausal depression17. Depressed women had lower levels of estradiol hormone, an estrogen precursor, which causes more severe menopausal symptoms18. Depression decreases quality of life and work productivity while increasing health and medical care expenditure19.
Multiple pharmacological and non-pharmacological treatments are used for treating depression in menopausal women20. Hormone therapy is recognized as an effective method for managing menopausal symptoms in women under 60 years old with less than 10 years since menopause onset, but it is not recommended as a treatment for depression in postmenopausal women21. Antidepressant medications are used as an effective method for treating depression, although they come with side effects such as gastrointestinal issues, sudden heat stroke, swelling, and dry mouth22. Menopausal women have a strong tendency towards using natural or safer approaches for the treatment or relief of menopausal symptoms23.
Polyphenols are micronutrients that can improve depression24. Cocoa is one of the food items rich in polyphenols. Among different types of chocolates, dark chocolate is rich in cocoa and has minimal additives like sugar, thus maximizing the benefits of cocoa25. Furthermore, dark chocolate has the highest antioxidant properties compared to other types of chocolates, such as milk and white chocolate26.
Oxidative stress is one of the consequences of decreased estrogen during menopause27, which may be associated with depression in menopausal women28. In conditions of oxidative stress and lack of efficient antioxidant response, excessive production of reactive oxygen species can precipitate depression by causing processes such as inflammation, neurodegeneration and death of neurons in the brain29. Polyphenols with antioxidant, anti-inflammatory activity, and an impact on gut microbiota can contribute to depression treatment30. A clinical trial on women aged 40 to 60 has shown that consuming cocoa-containing beverages for eight weeks could improve mood31. Additionally, clinical trial results indicate the positive effects of cocoa products in reducing the severity of depression among trainee nurses32 and cancer patients33.
The sleep quality of menopausal women is influenced by factors such as hormonal changes like estrogen, melatonin, and cortisol levels, vasomotor symptoms like hot flashes and night sweats, and mood disorders like depression and anxiety34. Results from a cross-sectional study on 3,600 American adults have shown that higher levels of oxidative stress are associated with lower sleep quality, and an antioxidant-rich diet improves sleep quality35. The effect of cocoa on sleep quality may be through its content of tryptophan and flavonoids. Tryptophan may enhance the quality of sleep by aiding in the secretion of melatonin36. Additionally, flavonoids increase nitric oxide production in vascular walls and improve endothelial function, increasing blood flow to the brain37.
Low estrogen levels lead to changes in lipid metabolism and, consequently, a rise in fat accumulation in menopausal women38. In a cross-sectional study, abdominal fat in postmenopausal women was 49% higher than in premenopausal women39. Polyphenols’ anti-obesity mechanism includes reducing lipogenesis, increasing lipolysis, inhibiting the differentiation and growth of fat cells, reducing inflammatory responses, and suppressing oxidative stress40. Additionally, chocolate consumption leads to decreased appetite and energy intake41.
The population of menopausal women is expected to reach 1.2 billion by 2030 as life expectancy increases42. Furthermore, given the significant individual and societal consequences of depression18,19 and the high prevalence of depression among Iranian menopausal women16, the current study aimed to determine the effect of 78% dark chocolate on depression scores (primary outcome), sleep quality, and anthropometric indices such as weight, BMI, waist circumference, and hip circumference (secondary outcomes) among Iranian menopausal women.
Materials and methods
Study design
In this parallel, randomized, triple-blind clinical trial, the participants, data collector, and data analyst were unaware of the allocation sequence. The study was conducted from December 2022 to November 2023 at healthcare centers in Tabriz, Iran.
Inclusion and exclusion criteria
The study’s inclusion criteria were natural menopause (defined as absence of menstruation for at least 12 months), an age range of 45 to 65, mild depression (score between 14 and 19), or moderate depression (score between 20 and 28) based on the Beck Depression Inventory (BDI) and confirmed by a clinical psychologist, literacy in reading and writing or accompanied by a literate family member, and willingness to consume chocolate.
The exclusion criteria included cardiovascular diseases, diabetes mellitus, hypertension, dyslipidemia, liver diseases, kidney diseases, fibrocystic breast disease, neurological or neuropsychological disorders, depression under treatment, severe depression according to BDI (score above 28), positive response to the suicidal ideation question in BDI, current hormone therapy, chronic daily consumption of coffee or cocoa products, habitual use of herbal mood-enhancing drugs, and intolerance/allergic reaction to cocoa.
Sample size calculation
Using the G*Power 3.1.9.7 software (www.gpower.hhu.de), the sample size was calculated based on the sample size formula considering α = 0.05 and β = 0.9 for comparing the means in two groups. The mean (standard deviation) of depression obtained from the Hospital Anxiety and Depression Scale in the study by Lua and Wong32 was reported as 5.4 (2.4) for the intervention group and 7.3 (1.7) for the control group. Each group’s initial participant count of 27 was raised to 30 after accounting for a 10% dropout rate.
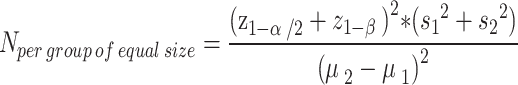 |
Sampling
The list of women aged 45 to 65 was obtained by the researcher (first author) from health centers in Tabriz, Iran. It was screened for inclusion and exclusion criteria (through checking electronic medical records and telephone interviews). During telephone calls, women were informed that the purpose of the study is to investigate the effects of cocoa-containing chocolate on depression scores, sleep quality, and anthropometric indices such as weight, BMI, waist circumference, and hip circumference in menopausal women. Additionally, they were informed that each participant would be randomly placed in either the milk chocolate or dark chocolate group. Women were invited to attend the health center on a specified day if they were eligible and willing to participate. During the face-to-face visit, the BDI was completed through interviews. Additionally, the presence and severity of depression were confirmed by a clinical psychology specialist (fifth author) through a clinical interview. The psychologist’s diagnosis was based on the final conclusion drawn from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for depression and the BDI. To ensure that illiterate participants were fully informed about the contents of the written informed consent, they were asked to attend the health center with a literate family member. The study did not include individuals with systolic blood pressure equal to or greater than 140 mmHg or diastolic blood pressure equal to or greater than 90 mmHg.
Randomization
To minimize bias, the allocation sequence was conducted by an individual not involved in the study’s implementation stages using a random number list. Subsequently, participants were assigned to intervention or control groups using random block sizes of six (block randomization method) and a 1:1 allocation ratio. Dark and milk chocolates were of identical shape (square) and size and packaged in aluminum foils. For the purpose of allocation concealment, the chocolate packages were put in sealed opaque packets sequentially numbered. The packets were numbered from 1 to 60 based on the predetermined random number sequence. The first participant received packet number one, and the second received packet number two, which continued until the sixtieth participant. Due to the differences in color and taste between dark and milk chocolates, all outcomes were assessed by a trained individual not involved in the study stages. This ensured the blinding of participants, the researcher involved in participant selection, and the outcome assessor to the randomization status and treatment assignment.
Intervention
In the present study, 78% dark chocolate was utilized for the intervention group, while milk chocolate was used for the control group. According to previous studies, dark chocolate containing over 40% cocoa possesses polyphenol-related benefits, with higher cocoa percentages leading to greater effectiveness43. However, due to the excessive bitterness associated with chocolates with higher cocoa percentages, 78% dark chocolate comprising was chosen for the intervention group. The contents of dark and milk chocolates were evaluated by a nutritional consultant (fourth author) of the study, and the chocolates were sourced from a chocolate factory located in Tabriz, Iran. The nutritional contents of dark and milk chocolates per 6 g were as follows: energy: 32 kilocalories, carbohydrates: 3.2 g, fat: 2.03 g, salt: 0.025 g, and trans-fatty acids: 0 g. The cocoa content in milk chocolates was less than 2%.
The intervention group consumed 12 g of 78% dark chocolate daily at a specified time for eight weeks, while those in the control group consumed 12 g of milk chocolate daily for the same duration. To ensure correct chocolate consumption, participants were initially given chocolates for four weeks and asked to complete a chocolate consumption checklist. After obtaining the participants’ checklist at the conclusion of the fourth week of the intervention and confirming appropriate consumption, chocolates were supplied to them to be consumed over the next four weeks. The researcher made weekly reminder phone calls to participants to enhance compliance with chocolate consumption. At the beginning of the study, participants were instructed to contact the researcher in case of any adverse events. Additionally, participants were questioned about side effects at the end of the fourth and eighth weeks after intervention. The intervention was discontinued in case of severe or intolerable side effects, and the participant was referred to a physician.
Data collection tools
Socio-demographic and obstetrics characteristics questionnaire
The researcher-designed questionnaire included questions regarding age, menopausal age, marital status, employment status, monthly income adequacy for living expenses, educational level, spouse’s educational level, housing status, gravida, parity, number of living children, history of abortion, and history of infertility, which were completed through face-to-face interviews with the participants.
Beck Depression Inventory-second edition (BDI-II)
BDI consists of 21 items, each with four options. Responses to the questions are scored from 0 to 3, and the total score ranges from 0 to 63. The severity of depression is estimated based on the total score, with higher scores indicating more severe depression. Scores from 0 to 13 indicate no or minimal depression, 14 to 19 indicate mild depression, 20 to 28 indicate moderate depression, and 29 to 63 indicate severe depression44. The psychometric properties of the Persian version of the questionnaire have been investigated and confirmed, with a Cronbach’s alpha coefficient of 0.9245. The questionnaire was completed twice, once at the beginning of the study and again at the end of the eighth week after the intervention, using a structured interview method. Individuals who had a score ranging from 14 to 28 on the questionnaire and were diagnosed with mild to moderate depression by a clinical psychologist were initially enrolled in the study. At the end of the trial, the clinical psychologist (fifth author), who was blinded to the questionnaire-based depression results, assessed the severity of depression through clinical interviews with participants, categorizing it as mild, moderate, or severe. Then, the correlation coefficient was determined between the BDI results after the intervention and the psychologist’s assessment.
Pittsburgh Sleep Quality Index (PSQI)
PSQI comprises 18 questions and seven components, including subjective sleep quality, sleep latency, sleep duration, sleep efficacy, sleep disturbances, use of sleep medication, and daytime dysfunction. Each question is scored from 0 to 3, and the total score ranges from 0 to 21. Higher scores indicate poorer sleep quality, and obtaining a score greater than 5 means low sleep quality46. The psychometric properties of the Persian version of this questionnaire have been examined and confirmed, with a Cronbach’s alpha coefficient of 0.5547. PSQI was completed twice, once at the beginning of the study and again at the end of the eighth week after the intervention, using structured interviews.
International Physical Activity Questionnaire (IPAQ)
IPAQ is designed for individuals aged 18 to 65 and assesses the duration and intensity of physical activity performed by the individual in the past seven days48. According to the IPAQ guidelines, the frequency and duration of physical activities (vigorous, moderate, walking, and sitting) were recorded in minutes per week for each individual. Then, the total score of MET-minutes/week (metabolic equivalence test) was calculated by summing up the scores obtained from each of the moderate, vigorous, and walking activities. The validity and reliability of the Persian version of the questionnaire have been confirmed, with a Cronbach’s alpha of 0.7049. The short form of IPAQ was completed twice, once at the beginning and once at the end of the intervention, using structured interviews.
Food frequency questionnaire
This questionnaire assessed participants for energy and dietary intakes. At the beginning of the study, participants were instructed not to change their usual diet and physical activity throughout the study period. Participants were also asked to report all food items that were consumed except water during the day (two regular days and one weekend day), once at the beginning of the study and again at the end of the study. Energy intake in kilocalories and macronutrients (protein, carbs, and fat) in grams were calculated using the Kelpiesoft Food File 1.0.5 software (www.kelpiesoft.com). The average of three days’ data was considered as participants’ actual intakes50.
Anthropometric indices checklist
In this study, weight, height, BMI, waist circumference, and hip circumference were measured once at the beginning and again at the end of the intervention. Weight measurement was conducted with minimal clothing and without shoes. A digital scale with a precision of 0.1 kg was used for this purpose. Participants’ height was measured in a standing position, without shoes and hats, using a wall-mounted standard meter with a precision of 0.1 centimeters. Waist circumference was measured at the narrowest part of the waist, and hip circumference was measured at the widest part of the hips. Both measurements were taken using a flexible, non-stretchable tape measure. Participants stood with their feet close together during the measurements, and no pressure was applied to the body.
Confounding variables
During the study design phase, we determined that if there were statistically significant differences between the two study groups in terms of socio-demographic and obstetrics characteristics, baseline anthropometric indices, dietary data including energy, protein, carbohydrates, and fats (saturated fats, monounsaturated fatty acids, and polyunsaturated fatty acids), and physical activity level, would be considered as confounding variables affecting the results of anthropometric indices after the intervention.
Ethical considerations
All stages of this study followed the guidelines and regulations outlined in the Declaration of Helsinki. Before the intervention, the researcher explained the objectives, methods, and potential side effects in detail to the participants. All participants signed an informed consent form before participating in the study. The protocol of this study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.807) and registered at the Iranian Registry of Clinical Trials (www.IRCT.IR) with registration number IRCT20220926056046N1; 12/10/2022.
Statistical analysis
Data was analyzed using SPSS-26 software (SPSS Inc., Chicago, IL, USA). The normal distribution of each variable was assessed using the Kolmogorov–Smirnov test. Descriptive statistics were used to report the data. The independent t-test, Fisher’s exact test, chi-square for trend, and Chi-square were used to compare socio-demographic and obstetric characteristics between the two groups. The independent t-test was used to compare the mean scores of depression and anthropometric indices between the two groups before the intervention. After the intervention, an ANCOVA test with baseline adjustment was employed to compare the mean scores of depression and anthropometric indices. Before and after the intervention, the mean total sleep quality scores were compared using the independent t-test and the Mann-Whitney U test, respectively. Chi-square for trend and independent t-test were used to compare physical activity levels and dietary intake before and after the intervention. The effect size was calculated using Cohen’s d index, which is determined by the formula: the difference in means between intervention and control groups divided by the pooled standard deviation. According to the guidelines for interpreting effect size, a value of 0.2 is considered small, 0.5 medium, and 0.8 or greater large. Intention-to-treat analysis was performed. P-values less than 0.05 were considered statistically significant.
Results
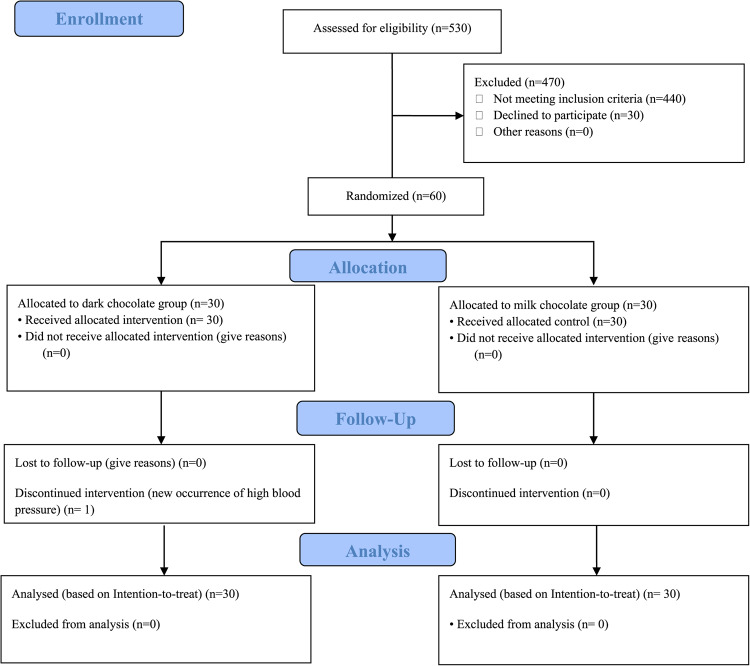
Out of 530 menopausal women aged 45 to 65 who were initially assessed, 440 individuals were excluded due to ineligibility, and 30 individuals declined to participate in the study. Ultimately, 60 menopausal women were enrolled in the study. One participant in the dark chocolate group discontinued the intervention in the second week due to newly developed hypertension. Consequently, 60 participants were included in the analysis (Fig. 1).
Fig. 1.
CONSORT study flow diagram. 30 out of 60 participants were allocated to the dark chocolate group and 30 participants were allocated to the milk chocolate group. One participant in the dark chocolate group discontinued the intervention, but analysis was done on 60 participants based on the intention to treat (ITT) principals.
Participant characteristics
The mean (standard deviation) age of participants in the dark chocolate and milk chocolate groups was 53.6 (3.9) and 53.9 (4.0) years, respectively. The mean (standard deviation) age at menopause at the time of study entry in the dark chocolate and milk chocolate groups was 49.8 (2.8) and 50.1 (2.3) years, respectively. The two groups had no statistically significant difference in socio-demographic and obstetric characteristics (Table 1).
Table 1.
Sociodemographic and obstetrics characteristics in study groups.
| Variable | Dark chocolate (n = 30) | Milk chocolate (n = 30) | P-value |
|---|---|---|---|
| Age (year) | 53.6 ± 3.9 | 53.9 ± 4.0 | 0.820a |
| Menopause age (year) | 49.8 ± 2.8 | 50.1 ± 2.3 | 0.725a |
| Marital status | 1.000b | ||
| Married | 27.0 (90.0) | 28.0 (93.3) | |
| Single | 3.0 (10.0) | 2.0 (6.7) | |
| Occupation | 0.506b | ||
| Housewife | 23.0 (76.7) | 26.0 (86.7) | |
| Employed | 7.0 (23.3) | 4.0 (13.3) | |
| Monthly income adequacy | 0.699c | ||
| Adequate | 8.0 (26.7) | 11.0 (36.7) | |
| Relatively adequate | 18.0 (60.0) | 14.0 (46.7) | |
| Inadequate | 4.0 (13.3) | 5.0 (16.7) | |
| Education | 0.782c | ||
| Illiteracy | 4.0 (13.3) | 3.0 (10.0) | |
| Primary school | 7.0 (23.3) | 8.0 (26.7) | |
| Secondary school | 2.0 (6.7) | 2.0 (6.7) | |
| High school | 2.0 (6.7) | 1/0 (3.3) | |
| Diploma | 8.0 (26.7) | 7.0 (23.3) | |
| University education | 7.0 (23.3) | 9.0 (30.0) | |
| Spouse’s Education | 0.741c | ||
| Illiteracy | 0.0 (0.0) | 1.0 (3.6) | |
| Primary school | 6.0 (21.4) | 6.0 (21.4) | |
| Secondary school | 2.0 (7.1) | 1.0 (3.6) | |
| High school | 4.0 (14.3) | 2.0 (7.1) | |
| Diploma | 8.0 (28.6) | 6.0 (21.4) | |
| University education | 8.0 (28.6) | 12.0 (42.9) | |
| Housing | 1.000b | ||
| Personal | 29.0 (96.7) | 28.0 (93.3) | |
| Rental/ Living with relatives | 1.0 (3.3) | 2.0 (6.7) | |
| Gravida | 0.657d | ||
| 2 or less | 9.0 (30.0) | 6.0 (20.0) | |
| 3–4 | 12.0 (40.0) | 13.0 (43.3) | |
| 5 or more | 9.0 (30.0) | 11.0 (36.7) | |
| Parity | 0.774b | ||
| 2 or less | 12.0 (30.0) | 9.0 (40.0) | |
| 3–4 | 14.0 (46.7) | 17.0 (56.7) | |
| 5 or more | 4.0 (13.3) | 4.0 (13.3) | |
| Living children | 1.000b | ||
| 2 or less | 12.0 (40.0) | 12.0 (40.0) | |
| 3–4 | 15.0 (50.0) | 14.0 (46.7) | |
| 5 or more | 3.0 (10.0) | 4.0 (13.3) | |
| Abortion history | 0.606d | ||
| Yes | 14.0 (46.7) | 16.0 (53.3) | |
| No | 16.0 (53.3) | 14.0 (46.7) | |
| Infertility history | 1.000b | ||
| Yes | 1.0 (3.3) | 1.0 (3.3) | |
| No | 29.0 (96.7) | 29.0 (96.7) |
Data were presented as mean ± standard deviation or number (percent), a Independent ttest, bFisher’s exact test, cChi-square for trend test, dChi-square test.
Outcome variables
The mean (standard deviation) depression score before the intervention in the dark chocolate and milk chocolate groups was 18.3 (3.9) and 18.8 (4.4), respectively. According to the independent t-test, there was no statistically significant difference in depression scores before the intervention between the two study groups (p = 0.683). The mean (standard deviation) depression score after the intervention in the dark chocolate and milk chocolate groups was 15.3 (4.8) and 18.1 (5.5), respectively. ANCOVA test with baseline adjustment showed that the mean depression score after the intervention in the dark chocolate group was significantly reduced compared to the milk chocolate group (mean difference: -2.3; 95% confidence interval: -3.9 to -0.8; p = 0.003). The effect size, calculated as Cohen’s d, was − 0.54, indicating a moderate effect size (Table 2).
Table 2.
Comparison of depression between study groups.
| Variable | Dark chocolate (n = 30) | Milk chocolate (n = 30) | Intergroup difference | p-value | ES |
|---|---|---|---|---|---|
| Baseline | 18.3 ± 3.9 | 18.8 ± 4.9 | 0.5 (-1.8 to 2.7) | 0.683a | - |
| After 8 weeks | 15.3 ± 4.8 | 18.1 ± 5.5 | -2.3 (-3.9 to -0.8) | 0.003b | -0.54 |
Data were presented as mean ± standard deviation or mean difference (95% confidence interval), a Independent t test, b ANCOVA adjusted for baseline scores, ES: effect size (Cohen’s d).
Furthermore, using the Spearman correlation test, a substantial and statistically significant direct correlation coefficient was observed between the severity of depression after the intervention, as assessed by the BDI, and clinical diagnosis by the psychologist (r = 0.916; p < 0.001).
Based on the intergroup analysis, after eight weeks of intervention, there was no statistically significant difference between the two groups in terms of overall sleep quality score (p = 0.546) (Cohen’s d = -0.07) and its subdomains, including subjective sleep quality (p = 0.780) (Cohen’s d = -0.09), sleep latency (p = 0.145) (Cohen’s d = -0.19), sleep duration (p = 0.260) (Cohen’s d = 0.35), sleep efficacy (p = 0.715) (Cohen’s d = 0.00), sleep disturbances (p = 0.684) (Cohen’s d = 0.17), use of sleep medications (p = 0.655) (Cohen’s d = -0.18), and daytime dysfunction (p = 0.134) (Cohen’s d = -0.35) (Table 3).
Table 3.
Comparison of sleep quality and its subscales between study groups.
| Variable (Score range) | Dark chocolate (n = 30) | Milk chocolate (n = 30) | P-value | ES | ||
|---|---|---|---|---|---|---|
| Global score (0–21) | ||||||
| Baseline | 8.4 ± 4.2 | 8.0 (4.8 to 11.0) | 8.4 ± 4.2 | 8.0 (5.0 to 12.0) | 0.988a | - |
| After 8 weeks | 7.3 ± 4.2 | 5.5 (4.8 to 10.0) | 7.6 ± 4.1 | 6.5 (5.0 to 10.3) | 0.546b | -0.07 |
| Subjective sleep quality (0–3) | ||||||
| Baseline | 1.3 ± 1.1 | 1.0 (0.8 to 2.0) | 1.2 ± 1.0 | 1.0 (0.0 to 2.0) | 0.815 b | - |
| After 8 weeks | 1.1 ± 1.1 | 1.0 (0.0 to 2.0) | 1.2 ± 1.1 | 1.0 (0.0 to 2.0) | 0.780 b | -0.09 |
| Sleep latency (0–3) | ||||||
| Baseline | 1.2 ± 0.8 | 1.0 (0.8 to 2.0) | 1.3 ± 1.0 | 1.0 (0.8 to 2.0) | 0.596 b | - |
| After 8 weeks | 1.1 ± 1.1 | 1.0 (0.0 to 1.3) | 1.3 ± 1.0 | 1.0 (0.8 to 2.0) | 0.145 b | -0.19 |
| Sleep duration (0–3) | ||||||
| Baseline | 1.8 ± 0.8 | 2.0 (1.0 to 2.0) | 1.7 ± 1.0 | 2.0 (1.0 to 2.3) | 0.863 b | - |
| After 8 weeks | 1.6 ± 0.9 | 2.0 (1.0 to 2.0) | 1.3 ± 0.8 | 1.5 (1.0 to 2.0) | 0.260 b | 0.35 |
| Sleep efficacy (0–3) | ||||||
| Baseline | 0.8 ± 1.2 | 0.0 (0.0 to 2.0) | 0.7 ± 1.1 | 0.0 (0.0 to 1.3) | 0.850 b | - |
| After 8 weeks | 0.6 ± 0.9 | 0.0 (0.0 to 1.0) | 0.6 ± 1.1 | 0.0 (0.0 to 1.0) | 0.715 b | 0.00 |
| Sleep disturbances (0–3) | ||||||
| Baseline | 1.6 ± 0.6 | 2.0 (1.0 to 2.0) | 1.5 ± 0.6 | 1.5 (1.0 to 2.0) | 0.720 b | - |
| After 8 weeks | 1.4 ± 0.6 | 1.0 (1.0 to 2.0) | 1.3 ± 0.6 | 1.0 (1.0 to 2.0) | 0.684 b | 0.17 |
| Use of sleep medication (0–3) | ||||||
| Baseline | 0.2 ± 0.8 | 0.0 (0.0 to 0.0) | 0.2 ± 0.7 | 0.0 (0.0 to 0.0) | 0.688 b | - |
| After 8 weeks | 0.1 ± 0.6 | 0.0 (0.0 to 0.0) | 0.2 ± 0.5 | 0.0 (0.0 to 0.0) | 0.655 b | -0.18 |
| Daytime dysfunction (0–3) | ||||||
| Baseline | 1.6 ± 0.8 | 2.0 (1.0 to 2.0) | 1.7 ± 0.9 | 2.0 (1.0 to 2.3) | 0.614 b | - |
| After 8 weeks | 1.4 ± 0.8 | 1.0 (1.0 to 2.0) | 1.7 ± 0.9 | 2.0 (1.0 to 2.0) | 0.134 b | -0.35 |
Data were presented as mean ± standard deviation or median (percentile 25 to percentile 75), a Independent t-test, b Mann–Whitney U test, ES: effect size (Cohen’s d).
There was no statistically significant difference between the two groups in terms of physical activity levels before the intervention (p = 0.063) and after the intervention (p = 0.250) (Table 4). Additionally, there was no statistically significant difference in energy and dietary intakes before and after the intervention (p > 0.05) (Table 5).
Table 4.
Comparison of physical activity level between study groups.
| Variable | Dark chocolate (n = 30) | Milk chocolate (n = 30) | p-value a |
|---|---|---|---|
| Baseline | 0.063 | ||
| Low | 15.0 (50.0) | 20.0 (66.7) | |
| Moderate | 11.0 (36.7) | 10.0 (33.3) | |
| High | 4.0 (13.3) | 0.0 (0.0) | |
| After 8 weeks | 0.250 | ||
| Low | 17.0 (56.7) | 20.0 (66.7) | |
| Moderate | 11.0 (36.7) | 10.0 (33.3) | |
| High | 2.0 (6.7) | 0.0 (0.0) |
Data were presented as number (percent), a Chi-square for trend test.
Table 5.
Comparison of dietary intake between study groups.
| Variable | Dark chocolate (n = 30) | Milk chocolate (n = 30) | p-value a |
|---|---|---|---|
| Baseline | |||
| Energy (kcal/day) | 2254.4 ± 759.7 | 2176.7 ± 815.8 | 0.704 |
| Protein (g/day) | 61.6 ± 22.7 | 68.7 ± 33.0 | 0.333 |
| Carbohydrate (g/day) | 317.3 ± 132.6 | 285.7 ± 127.9 | 0.352 |
| Fat (g/day) | 86.1 ± 31.1 | 88.0 ± 43.4 | 0.844 |
| After 8 weeks | |||
| Energy (kcal/day) | 2028.7 ± 688.9 | 2158.0 ± 784.9 | 0.500 |
| Protein (g/day) | 57.0 ± 23.3 | 61.0 ± 25.2 | 0.525 |
| Carbohydrate (g/day) | 284.9 ± 127.1 | 281.1 ± 129.3 | 0.911 |
| Fat (g/day) | 89.2 ± 33.8 | 87.3 ± 32.5 | 0.825 |
Data were presented as mean ± standard deviation, a Independent t- test.
There was no statistically significant difference in anthropometric indices before the intervention between the two groups (p > 0.05). Furthermore, there was no statistically significant difference between the two groups in terms of weight (p = 0.075) (Cohen’s d = 0.30), BMI (p = 0.137) (Cohen’s d = 0.20), waist circumference (p = 0.463) (Cohen’s d = 0.26), and hip circumference (p = 0.114) (Cohen’s d = 0.32) after the intervention (Table 6).
Table 6.
Comparison of the anthropometric indicators between study groups.
| Variable | Dark chocolate (n = 30) | Milk chocolate (n = 30) | Intergroup difference | p-value | ES |
|---|---|---|---|---|---|
| Body weight (kg) | |||||
| Baseline | 77.19 ± 12.35 | 73.21 ± 11.95 | -3.98 (-10.26 to 2.30) | 0.210a | - |
| After 8 weeks | 77.03 ± 12.27 | 73.34 ± 12.05 | -0.29 (-0.61 to 0.03) | 0.075b | 0.30 |
| BMI (kg/m 2 ) | |||||
| Baseline | 30.98 ± 4.71 | 29.87 ± 5.17 | -1.10 (-3.66 to 1.45) | 0.391a | - |
| After 8 weeks | 30.92 ± 4.67 | 29.92 ± 5.22 | -0.10 (-0.23 to 0.03) | 0.137b | 0.20 |
| Waist circumference (cm) | |||||
| Baseline | 98.93 ± 12.84 | 94.73 ± 11.28 | -4.20 (-10.45 to 2.05) | 0.184a | - |
| After 8 weeks | 97.90 ± 12.07 | 94.77 ± 11.97 | -0.80 (-2.96 to 1.37) | 0.463b | 0.26 |
| Hip circumference (cm) | |||||
| Baseline | 116.90 ± 12.14 | 111.17 ± 11.81 | -5.73 (-11.92 to 0.46) | 0.069 a | - |
| After 8 weeks | 115.47 ± 10.95 | 111.87 ± 11.50 | -1.51 (-3.39 to 0.38) | 0.114b | 0.32 |
Data were presented as mean ± standard deviation or mean difference (95% confidence interval), BMI: body mass index, a Independent ttest, b ANCOVA adjusted for baseline scores, ES: effect size (Cohen’s d).
Regarding side effects, one participant in the milk chocolate group reported experiencing a mild skin rash in the eighth week of the intervention.
Discussion
The current study demonstrated that daily consumption of 10 g of 78% dark chocolate for eight weeks improved depression among menopausal women. Based on our knowledge, this is the first study in Iran to investigate the effects of dark chocolate on depression in menopausal women. The findings of this study may contribute to improving the mental health of this group of people.
The results of our study are consistent with studies that have examined the effects of cocoa-containing products on depression or mood. In a cross-sectional study by Smith et al.51 involving 13,626 adult Americans, individuals who consumed dark chocolate were less likely to exhibit clinically relevant depressive symptoms. In a systematic review and meta-analysis by Gabbiadini et al.52, nine clinical interventions were examined, with five interventions involving single-dose cocoa consumption, two involving 3-day consumption, and two involving consumption for more than one week. According to the results, cocoa-rich products had a significant short-term effect on depressive symptoms. In a systematic review by Scholey and Owen53, 5 out of 8 studies concluded that chocolate or its constituents positively affect mood. In a clinical trial conducted by Natsume et al.31, consuming a beverage containing cacao flavanols for eight weeks improved negative mood indicators (such as depression, fatigue, and irritability) and overall mood disturbance scores in middle-aged women. Trials conducted by Lua and Wong on trainee nurses32 and cancer patients33 demonstrated that consuming 50 g of dark chocolate for three days could reduce depression and anxiety.
One of the potential ways dark chocolate may affect menopausal women’s depression is through its polyphenols. Dietary polyphenols act as signaling molecules, increasing nitric oxide bioactivity and creating antioxidant and anti-inflammatory properties54. Tryptophan is another amino acid found in cocoa that functions as a precursor to the serotonin hormone and may enhance mood55. Another possible mechanism could be influencing gut microbiota and the gut-brain axis. The gut influences brain function through mechanisms such as aiding in producing brain-affecting substances and neural signals56. In menopausal women, due to decreased estrogen hormone, there is a reduction in gut microbiota diversity57, which is associated with an increased risk of psychological disorders such as depression and anxiety58. A clinical trial reported that consuming 30 g per day of 85% dark chocolate concurrently improved mood and increased gut microbial diversity, leading to the conclusion that dark chocolate has prebiotic effects on the gut and may alleviate negative emotional states through the gut-brain axis59.
According to the results of this study, daily consumption of 78% dark chocolate for eight weeks failed to improve sleep quality in menopausal women. These findings contradict those of a clinical trial conducted by Hernandez-Gonzalez et al.41, which demonstrated that daily consumption of 100 g of milk chocolate for two weeks in the evening may regulate the timing of sleep episodes in postmenopausal women. Additionally, the results of the current study are in contrast to a trial by Okauchi et al.60, which showed that a diet containing 2% cocoa improved sleep in mice induced with chronic sleep disorder for 30 days. Furthermore, Espitia-Bautista et al.61 reported that consuming chocolate in the morning may prevent sleep disturbances resulting from night shift work and jet lag. The discrepancy in the results of our study and the mentioned studies may be due to differences in the study population, the percentage of cocoa consumed by participants, variations in measurement tools, and the examination of different aspects of sleep. Additionally, some studies have suggested that consumption of caffeine-containing chocolate may even decrease sleep quality62,63. However, according to the results of this study, no adverse changes in sleep quality were observed in participants in any of the study groups.
This study showed that daily consumption of 10 g of 78% dark chocolate, compared to milk chocolate, did not have a beneficial effect on weight, BMI, waist circumference, and hip circumference in menopausal women. These findings are consistent with a systematic review conducted by Kord-Varkaneh et al.64, which meta-analyzed 35 clinical trials and determined that cocoa supplements did not induce changes in weight, BMI, and waist circumference. In a clinical trial conducted by Garcia-Yu et al.65, daily consumption of 10 g of 99% dark chocolate for six months reduced fat mass and percentage of fat in the body, arms, and legs of menopausal women. Still, it did not induce changes in weight and BMI. However, the results of our study are contradictory to the findings of a randomized clinical trial by Hernandez-Gonzalez et al.41 on 19 postmenopausal women, which showed that daily consumption of 100 g of milk chocolate in the morning for two weeks led to a decrease in energy intake by 300 kilocalories per day, as well as a reduction of fasting blood sugar, waist circumference, and an increase in fat oxidation. Differences in study results may be due to variations in the amount of cocoa used, duration of intervention, type of anthropometric measurement index used, and even the timing of cocoa consumption. A subgroup analysis by Kord-Varkaneh et al. reported weight and BMI reduction in studies with cocoa supplement consumption with intervention durations of 4–8 weeks and over 30 g per day64. Additionally, in the trial by Hernandez‐Gonzalez et al., chocolate consumption in the morning led to a more significant decrease in waist circumference41.
One of the strengths of this study is its adherence to all principles of clinical trials, including random allocation, allocation concealment, and blinding of participants, investigators, and outcome assessors. Another strength is that the two types of chocolate used in this study had the same energy content and were produced from single-origin cocoa beans. Using the same cocoa type in both chocolates allows attributing differences in the results of the two groups to the benefits of cocoa itself rather than differences in cocoa type, as variations in cocoa type, geographical region, and preparation method can lead to differences in chocolate compositions66. Additionally, the depression outcome was assessed not only through the BDI but also once by a clinical psychologist, and the correlation coefficient determined can be considered a strength of the study. Measuring inflammatory markers in the blood and changes in gut microbiota and determining their correlation with BDI results could have been interesting but not feasible. Another limitation of this study is the lack of control over the amount of polyphenols individuals consumed from their daily diet during the study, although randomization (likely creating similar conditions in both groups) and considering daily consumption of chocolate and coffee as one of the exclusion criteria may have mitigated this limitation. At the end of the study, participants were not asked to guess their assigned group, as this awareness could influence their self-reporting of depression. Furthermore, since this study was conducted on postmenopausal women aged 45 to 65 years with relatively few chronic medical issues, the results cannot be generalized to all menopausal women.
Conclusion
The results obtained from this study showed that daily consumption of 10 g of 78% dark chocolate has beneficial effects on mild and moderate depression scores in menopausal women. These findings can be helpful in nutritional education related to lifestyle modification for menopausal women, as chocolate consumption is an easy and enjoyable complementary method. More research is required to identify the precise ingredient in dark chocolate and the mechanism by which it helps menopausal women with depression. It will also be essential to find the optimal amount and duration of the intake of dark chocolate to reap the benefits for this population.
Acknowledgements
We are grateful to all participants for their cooperation, Tabriz University of Medical Sciences, Tabriz, Iran (Code: 70312) for financial support, and the Clinical Research Development Unit of Taleghani Hospital, Tabriz University of Medical Sciences, Tabriz, Iran.
Abbreviations
- BMI
Body mass index
- BDI
Beck Depression Inventory
- PSQI
Pittsburgh Sleep Quality Index
- IPAQ
International Physical Activity Questionnaire
- ANCOVA
Analysis of covariance
Authors’ contributions
Study design: SGH, MM, ER, LP. Data gathering: EA and EN. Statistical analysis: MM. Drafting the manuscript: EA and SGH. Critically revised the manuscript: SGH, MM, ER, LP. All authors have read and approved the final manuscript before submission.
Funding
This study has been approved and funded by Tabriz University of Medical Sciences, Tabriz, Iran (Code: 70312).
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Declarations
Consent for publication
All authors approved the final version of the manuscript and agreed to publish all aspects of the work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Talaulikar, V. Menopause transition: physiology and symptoms. Best Pract. Res. Clin. Obstet. Gynaecol.81, 3–7 (2022). [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Research on the menopause in the 1990s: report of a WHO scientific group (1996). [PubMed] [Google Scholar]
- 3.Gold, E. B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. North. Am.38, 425–440 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenaker, D. A., Jackson, C. A., Rowlands, J. V. & Mishra, G. D. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int. J. Epidemiol.43, 1542–1562 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamaniyan, M. et al. Age of natural menopause and related factors among the tabari cohort. J. Menopausal Med.26, 18–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro, N., Roeca, C., Peters, B. A. & Neal-Perry, G. The menopause transition: signs, symptoms, and Management options. J. Clin. Endocrinol. Metab.106, 1–15 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Palacios, S., Henderson, V., Siseles, N., Tan, D. & Villaseca, P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 13, 419–428 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Nappi, R. E. et al. Global cross-sectional survey of women with vasomotor symptoms associated with menopause: prevalence and quality of life burden. Menopause. 28, 875–882 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zolfaghari, S. et al. Effects of menopause on sleep quality and sleep disorders: Canadian longitudinal study on aging. Menopause. 27, 295–304 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Fenton, A. Weight, shape, and body composition changes at menopause. J. Midlife Health. 12, 187–192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gava, G. et al. Cognition, mood and sleep in menopausal transition: the role of menopause hormone therapy. Medicina. 55, 668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaspers, L. et al. Health in middle-aged and elderly women: a conceptual framework for healthy menopause. Maturitas. 81, 93–98 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Jia, Y., Zhou, Z., Xiang, F., Hu, W. & Cao, X. Global prevalence of depression in menopausal women: a systematic review and meta-analysis. J. Affect. Disord. 358, 474–482 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Willi, J. & Ehlert, U. Symptoms assessed in studies on perimenopausal depression: a narrative review. Sex. Reprod. Healthc.26, 100559 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Li, J. et al. Prevalence and associated factors of depression in postmenopausal women: a systematic review and meta-analysis. BMC Psychiatry. 24, 431 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshari, P., Manochehri, S., Tadayon, M., Kianfar, M. & Haghighizade, M. Prevalence of depression in postmenopausal women. Jundishapur J. Chronic Dis. Care. 4, 27521 (2015). [Google Scholar]
- 17.Maki, P. M. et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J. Womens Health. 28, 117–134 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Cekmez, Y., Torun, F., Göçmen, A. & Şanlıkan, F. A hidden reason for menopausal symptoms in premenopausal aged women: depression. Int. J. Clin. Exp. Med.8, 6277–6281 (2015). [PMC free article] [PubMed] [Google Scholar]
- 19.Jain, S., Gupta, S., Li, V. W., Suthoff, E. & Arnaud, A. Humanistic and economic burden associated with depression in the United States: a cross-sectional survey analysis. BMC Psychiatry. 22, 542 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares, C. N. Depression and menopause: an update on current knowledge and clinical management for this critical window. Med. Clin. North. Am.103, 651–667 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Pinkerton, J. V. Hormone therapy for postmenopausal women. N Engl. J. Med.382, 446–455 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Ramic, E., Prasko, S., Gavran, L. & Spahic, E. Assessment of the antidepressant side effects occurrence in patients treated in primary care. Mater. Sociomed. 32, 131–134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posadzki, P. et al. Prevalence of complementary and alternative medicine (CAM) use by menopausal women: a systematic review of surveys. Maturitas. 75, 34–43 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Lin, K., Li, Y., Toit, E. D., Wendt, L. & Sun, J. Effects of polyphenol supplementations on improving depression, anxiety, and quality of life in patients with depression. Front. Psychiatry. 12, 765485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glicerina, V., Balestra, F., Dalla Rosa, M. & Romani, S. Microstructural and rheological characteristics of dark, milk and white chocolate: a comparative study. J. Food Eng.169, 165–171 (2016). [Google Scholar]
- 26.Zujko, M. E. & Witkowska, A. M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. Int. J. Food Prop.17, 86–92 (2014). [Google Scholar]
- 27.Moreau, K. L., Hildreth, K. L., Klawitter, J., Blatchford, P. & Kohrt, W. M. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience. 42, 1699–1714 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandankhede, M., Gupta, M. & Pakhmode, S. Assessment of psychological status and oxidative stress in postmenopausal women: a cross-sectional study. J. Menopausal Med.27, 155–161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt, S., Nagappa, A. N. & Patil, C. R. Role of oxidative stress in depression. Drug Discov Today. 25, 1270–1276 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Winiarska-Mieczan, A. et al. Anti-inflammatory, antioxidant, and neuroprotective effects of polyphenols—polyphenols as an element of diet therapy in depressive disorders. Int. J. Mol. Sci.24, 2258 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami, R., Natsume, M., Ito, K., Ebihara, S. & Terauchi, M. Effect of flavanol-rich cacao extract on the profile of mood state in healthy middle-aged Japanese women: a randomized, double-blind, placebo-controlled pilot study. Nutrients. 15, 3843 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lua, P. L. & Wong, S. Y. Can dark chocolate alleviate anxiety, depressive and stress symptoms among trainee nurses? A parallel, open-label study. Asian J. Psychiatr. 4, 1–13 (2011).23050907 [Google Scholar]
- 33.Lua, P. & Wong, S. Dark chocolate consumption on anxiety, depression and health-related quality of life of patients with cancer: a randomised clinical investigation. Asian J. Psychiatr. 21, 10–24 (2012). [Google Scholar]
- 34.Ameratunga, D., Goldin, J. & Hickey, M. Sleep disturbance in menopause. Intern. Med. J.42, 742–747 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Lei, X., Xu, Z. & Chen, W. Association of oxidative balance score with sleep quality: NHANES 2007–2014. J. Affect. Disord. 339, 435–342 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Sutanto, C. N., Loh, W. W. & Kim, J. E. The impact of tryptophan supplementation on sleep quality: a systematic review, meta-analysis, and meta-regression. Nutr. Rev.80, 306–316 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Rendeiro, C., Rhodes, J. S. & Spencer, J. P. The mechanisms of action of flavonoids in the brain: direct versus indirect effects. Neurochem Int.89, 126–139 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Seong-Hee, K. & Hyun-Sook, K. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. 12, 202 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth, M. J., Tchernof, A., Sites, C. K. & Poehlman, E. T. Effect of menopausal status on body composition and abdominal fat distribution. Int. J. Obes. Relat. Metab. Disord. 24, 226–231 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-Pérez, C., Segura-Carretero, A. & del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: a review. Crit. Rev. Food Sci. Nutr.59, 1212–1229 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Hernández-González, T. et al. Timing of chocolate intake affects hunger, substrate oxidation, and microbiota: a randomized controlled trial. FASEB J.35, 21649 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Hill, K. The demography of menopause. Maturitas. 23, 113–127 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Jaćimović, S. et al. Antioxidant activity and multi-elemental analysis of dark chocolate. Foods. 11, 1445 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubley, A. M. Beck depression inventory in Encyclopedia of Quality of Life and Well-Being Research (ed Maggino, F.) 1–11 (Springer, (2021). [Google Scholar]
- 45.Hamidi, R. et al. Validity and reliability Beck Depression Inventory-II among the Iranian elderly population. J. Sabzevar Univ. Med. Sci.22, 189–198 (2015). [Google Scholar]
- 46.Smyth, C. The Pittsburgh Sleep Quality Index (PSQI). J. Gerontol. Nurs.25, 10–11 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Nazifi, M., Mokarami, H., Akbaritabar, A., Kalte, H. O. & Rahi, A. Psychometric properties of the Persian translation of Pittsburgh Sleep Quality Index. Health Scope. 3, 15547 (2014). [Google Scholar]
- 48.Craig, C. et al. International physical activity questionnaire-short form. J. Am. Coll. Health. 65, 492–501 (2017).28641040 [Google Scholar]
- 49.Moghaddam, M. B. et al. The Iranian version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl. Sci. J.18, 1073–1080 (2012). [Google Scholar]
- 50.Venter, C. et al. Reliability and validity of a maternal food frequency questionnaire designed to estimate consumption of common food allergens. J. Hum. Nutr. Diet.19, 129–138 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Jackson, S. E. et al. Is there a relationship between chocolate consumption and symptoms of depression? A cross-sectional survey of 13,626 US adults. Depress. Anxiety. 36, 987–995 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Fusar-Poli, L. et al. The effect of cocoa-rich products on depression, anxiety, and mood: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr.62, 7905–7916 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Scholey, A. & Owen, L. Effects of chocolate on cognitive function and mood: a systematic review. Nutr. Rev.71, 665–681 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Pechanova, O., Dayar, E. & Cebova, M. Therapeutic potential of polyphenols-loaded polymeric nanoparticles in cardiovascular system. Molecules. 25, 3322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liaqat, H., Parveen, A. & Kim, S. Y. Neuroprotective natural products’ regulatory effects on depression via gut–brain axis targeting tryptophan. Nutrients. 14, 3270 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osadchiy, V., Martin, C. R. & Mayer, E. A. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin. Gastroenterol. Hepatol.17, 322–332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters, B. A., Santoro, N., Kaplan, R. C. & Qi, Q. Spotlight on the gut microbiome in menopause: current insights. Int. J. Womens Health. 14, 1059–1072 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong, M. L. et al. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Mol. Psychiatry. 21, 797–805 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin, J. H. et al. Consumption of 85% cocoa dark chocolate improves mood in association with gut microbial changes in healthy adults: a randomized controlled trial. J. Nutr. Biochem.99, 108854 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Oishi, K., Okauchi, H., Yamamoto, S. & Higo-Yamamoto, S. Dietary natural cocoa ameliorates disrupted circadian rhythms in locomotor activity and sleep-wake cycles in mice with chronic sleep disorders caused by psychophysiological stress. Nutrition. 75, 110751 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Escobar, C. et al. Chocolate for breakfast prevents circadian desynchrony in experimental models of jet-lag and shift-work. Sci. Rep.10, 6243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watson, E. J., Coates, A. M., Kohler, M. & Banks, S. Caffeine consumption and sleep quality in Australian adults. Nutrients. 8, 479 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lodato, F. et al. Caffeine intake reduces sleep duration in adolescents. Nutr. Res.33, 726–732 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Kord-Varkaneh, H., Ghaedi, E., Nazary-Vanani, A., Mohammadi, H. & Shab-Bidar, S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr.59, 2349–2362 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Yu, I. A. et al. Cocoa-rich chocolate and body composition in postmenopausal women: a randomised clinical trial. Br. J. Nutr.125, 548–556 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Kongor, J. E. et al. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int.82, 44–52 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.