Abstract
Iberian barbel (Luciobarbus bocagei Steindachner, 1864) and Iberian nase (Pseudochondrostoma polylepis Steindachner, 1864) are two Mediterranean potamodromous fish species known to perform annual upstream migrations to reach spring spawning grounds. In the Mondego River basin, at the Coimbra dam, migratory movement patterns and individual size structure were assessed through a video recording monitoring system installed on an upstream section of a vertical-slot fish pass. Visual census for these target species during two consecutive annual cycles (2013–2014) revealed alternative migratory patterns, with the first peak of upstream movements in autumn, for both barbel (October–November) and nase (November–December). Circadian movements of both species showed a diurnal preference, contrary to what is usually described for these species. Size structure analysis for individuals of both species showed significant intra-annual differences in the size of migrating fish. Boosted regression trees models applied to the 2013–2014 visual count data identified flow and temperature as the most influential environmental predictors, triggering both species’ movements in each direction in the study years. These results provide novel information on the timing of the migratory movements of these potamodromous fish, which can be used to adapt current management and conservation measures to the specificities of their migratory behaviour.
Subject terms: Animal migration, Behavioural ecology
Introduction
Many species exhibit distinct movement patterns associated with resource use, such as feeding, refuge or reproduction1–3. Although some species may restrict their activities to a well-defined area, others may exhibit longer exploratory movements in search of resources (e.g. food, spawning habitat) and incorporate these new areas into their inter-annual life cycles1. When performed exclusively in freshwater environments, such movements are referred to as potamodromous migrations1,2,4,5. Potamodromy is often associated with upstream movements in spring to reach spawning grounds, mainly by adult fish. However, this type of migration involves complex movement patterns, including upstream, downstream and lateral movements within rivers, exhibited by both adults and juveniles. These behaviors can involve entire populations or only part of them, as in the case of partial migrations [e.g.,1,4–6]. These movements can determine the success or failure of a species’ life cycle. Fish migrations are often triggered by ecological, biological, or behavioural cues1,7, closely linked to the environmental stimuli that influence them1,2. Variables such as the photoperiod, water temperature and river flow1,8,9 can trigger, suspend, or stop migratory movements due to the species-specific response to these variables. Understanding the factors that influence migratory movements is even more crucial when studying fish populations in Mediterranean rivers, which are highly torrential and characterised by warm temperate climates with dry and warm summers10), especially in the context of climate change, which may exacerbate climatic extremes.
In the context of fish migrations and global environmental change, the loss of river connectivity is a well-known reality, as most rivers are heavily impounded due to anthropogenic pressures for irrigation, hydropower generation, domestic and/or industrial water supply, navigation and flood control11. This restriction on migratory fish movements can cause the isolation of subpopulations, reduce spawning opportunities, growth and development, and interrupt key life stages1,7,12. To mitigate the negative impacts of river fragmentation on fish communities, especially on migratory fish, different types of Fish Pass Solutions (FPS) have been widely used as an essential measure to restore longitudinal river connectivity [e.g.,12–14]. Although FPS are commonly implemented, the assessment of their effectiveness and efficiency for a wide range of species, considering upstream and/or downstream migrations, has been highly questioned and often neglected, especially due to the different swimming and behavioural abilities of migratory species7,15. Even when these issues are fully addressed, most of the existing studies focus on salmonids [e.g.,16–18] and often overlook other less well-known species, such as cyprinids or leuciscids [e.g.,19,20], which are dominant in typical Mediterranean aquatic ecosystems. Implementing monitoring programmes in FPS allows the assessment of the effectiveness and efficiency of these infrastructures and can provide invaluable information on migration timing and movement patterns of target fish species.
The Iberian barbel (Luciobarbus bocagei Steindachner, 1864) and the Iberian nase (Pseudochondrostoma polylepis Steindachner, 1864) are two potamodromous species, belonging to the Cyprinidae and Leuciscidae families, respectively. The Iberian barbel occurs in all Portuguese river basins except the southernmost, Mira and Guadiana river basins, while Iberian nase occurs in all river basins between Vouga and Sado rivers21. Despite being habitat generalists, both species adopt a rheophilic profile during the spawning season, selecting habitats with higher water velocity and coarser substrate21–25. The two species are classified as of Least Concern (LC) both globally and in Portugal24,26, but the general loss of longitudinal connectivity of rivers and the inaccessibility to preferred habitats due to the presence of barriers are still major threats and have led to significant population declines8,21,26. Beside the Iberian barbel and Iberian nase, there are more species in the Iberian Peninsula belonging to the Luciobarbus and Pseudochondrostoma genus, and more potamodromous species belonging to other families, whose conservation and management may benefit from the information obtained through this study.
This study analyses a continuous visual census monitoring dataset, collected at an FPS installed at the Coimbra dam (river Mondego, Portugal). This structure was used as a monitoring tool for counting Iberian barbel and Iberian nase throughout 2013–2015 to: (1) assess the seasonal and diel activity patterns of the target species; (2) identify the migratory peak of these species; (3) characterise the size-structure of the migratory fraction of both species; and (4) identify the environmental variables that seem to trigger the upstream and downstream migrations.
Material and methods
Study area
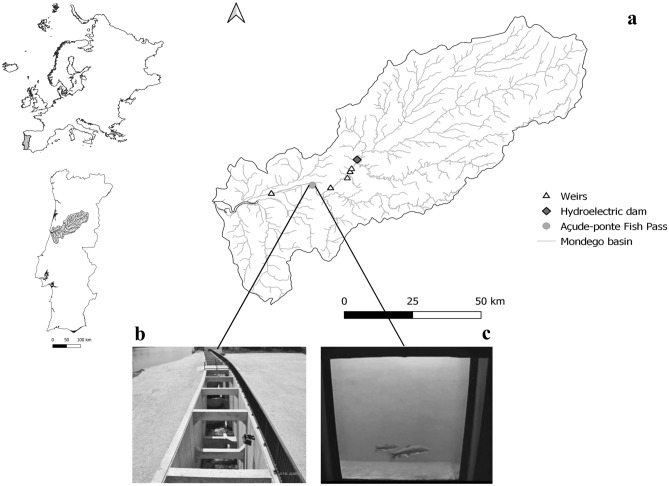
This study was carried out in the river Mondego, in central Portugal, at the FPS installed in the Coimbra dam (Fig. 1). With its source in Serra da Estrela (1 525 m of altitude), river Mondego is 258 km long and has a catchment area of approximately 6658 km227. The river basin average annual air temperature is 14.0 °C, the average annual rainfall is 1029 mm, and the average annual discharge is 2062 hm328. The Coimbra dam, part of the Hydraulic Scheme of the river Mondego, was built in 1981, mainly for flood control, water supply for agriculture, domestic and industrial purposes in the lower part of the river basin. The main stem of Mondego basin is affected by the operation of the Aguieira-Raiva-Fronhas hydroelectric systems, which were commissioned between 1981 and 1985. The downstream discharge of the Coimbra dam is mainly influenced by the flow released by these three upstream dams which, at the time this study was conducted, were limited to 6.8 m3 s−1 regarding ecological flow (4.8 m3 s−1 from the Agueira-Raiva dams plus 2.0 m3 s−1 from the Fronhas dam) and the flows released to ensure irrigation, industrial and domestic demands, that could increase up to 150 m3 s−1 during hydropeaking9,29. These flow variations are more likely to occur outside of the rainy season, whereas during flood periods, flow can reach values up to 2000 m3 s−1.
Fig. 1.
Location of the (a) study area in the river Mondego, with detail of (b) the FPS installed at the Coimbra dam and (c) the respective monitoring window used for the visual counting. The weirs showed in the study area are the ones where a nature-like fishway is installed.
Since its construction, the Coimbra dam was the first unsurmountable barrier to fish migration in the Mondego, forcing fish attempting to reach the upstream section of the river to find alternative habitats for reproduction in the 15 km of freshwater available downstream from this obstacle. In 2011, a vertical-slot fish pass was constructed at the Coimbra dam, restoring access to approximately 30 km in the river Mondego between Coimbra and the upstream Raiva dam, also providing access to two important tributaries, the rivers Ceira and Alva. This vertical-slot fish pass is a 125 m long channel with a water depth of 2 m, divided into 23 rectangular pools (4.5 × 3.0 m), connected by vertical slots (0.5 m width) with a 0.25 m head drop between the pools. At the entrance, the flow discharge is kept constant at 1.5 m3 s−1, with a velocity of about 1.5 ms−1 at the vertical slots, and the resting pools have a dissipated power of less than 150 Wm−3. Studies such as30–34 have demonstrated the effectiveness and efficiency of this FPS for a variety of migratory fish species.
Data collection
The movement and size structure of Iberian barbel and Iberian nase were assessed using the FPS monitoring system at the Coimbra dam, which is equipped with a monitoring window (125 cm × 200 cm) and a continuous video recording (Samsung SRD-470 and SCO-2080R), allowing fish to be observed, counted and identified without interfering with their natural behaviour. In 2013 and 2014, the total number of barbel and nase using the FPS, the direction of their movements (up- and downstream) and any other peculiar behaviour were analysed by trained researchers through manual visual observation of the video-recordings. The fish pass was closed for maintenance from 10 to 31 October, 18 of November and 2 of December of 2013, making total counts for this year slightly underestimated.
In 2015, a weekly subsampling was applied to measure fish and analyse size distribution of the migratory population of both target species [see32]. For measuring these fish, daily video images recorded at Coimbra dam fish pass monitoring system, were randomly sub-sampled on a weekly basis (to a total of 157 days), in which for each 1.5 h video interval, the total length of the first 20 individuals of both species was measured, considering their movement direction.
Migratory peaks and diel movement patterns
For each target species, movement direction and study year, fish counts were grouped by hour, and the bulk of migration was defined by the cumulative number of passes between the 10% and 90% percentiles, considering all movements made in 2013 and 2014. The following analyses were only performed on data within the periods identified as the bulk of migration for both species, directions, and years.
To identify migratory peaks and diel activity patterns, two matrices were compiled containing data for the bulk of the migration for each species, for each movement direction and for both study years (i.e., 2013 and 2014) combined.
Size structure
To characterise the size structure of the migratory individuals using the FPS in both directions, a total of 2181 individuals belonging to both target species were measured over 157 days in 2015, using the subsampling method previously described.
Monthly differences in the size structure (individual fish total length; TL, cm) of each species in each direction, were evaluated using a Kruskal–Wallis test (KW), followed by a post hoc Dunn’s test in case of significant differences. Only months with more than 10 measured fish, for each species and direction, were included in the analyses.
Environmental predictors
To determine which environmental variables could explain the timing of up- and downstream migration of each target species, several abiotic variables were selected to be tested as predictors, namely: Flow (discharge at Coimbra dam, in m3 s−1), Temp (water temperature, in °C), SpCond (specific conductivity of water, in µScm−1), Turb (water turbidity, in FNU), PhoPer (photoperiod, expressed in daylight hours), DayPer (phases of the day, according to twilight, i.e., NC—Night Closed; SR—Sunrise; ST—Sun Transit; SS—Sunset), Lunar Cycle (lunar phases, i.e., FM—Full Moon; TQ—Third Quarter; NM—New Moon; FQ—First Quarter) and Lunar Light Intensity (LLI, continuum variable from 0-New Moon to 1-Full Moon). Data on turbidity, salinity, specific conductivity, and temperature were obtained using a multiparameter probe (EXO2 Water Quality Probe) deployed inside the fish pass, continuously recording these physico-chemical variables every 30 min. Hourly discharge flow at the Coimbra dam was provided by the Portuguese Environmental Agency (APA), corresponding to the flow released downstream the dam and respective FPS. The photoperiod, day period and lunar cycle data for the study period were obtained from the Astronomical Observatory of Lisbon (https://oal.ul.pt/), while lunar light intensity data was obtained from Time and Date AS (https://www.timeanddate.com/). A Spearman rho coefficient was calculated to remove any redundant variables (|rho| > 0.80).
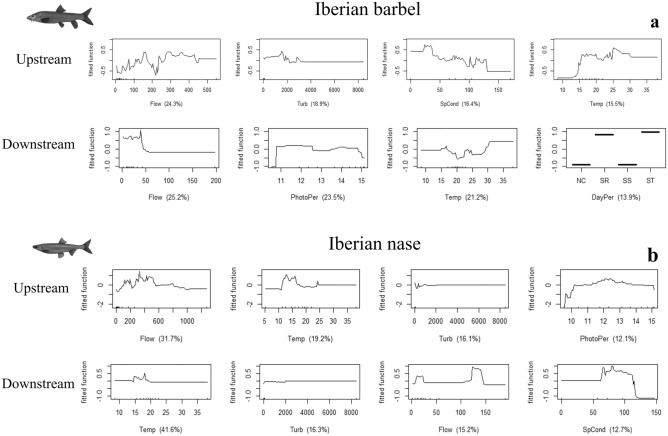
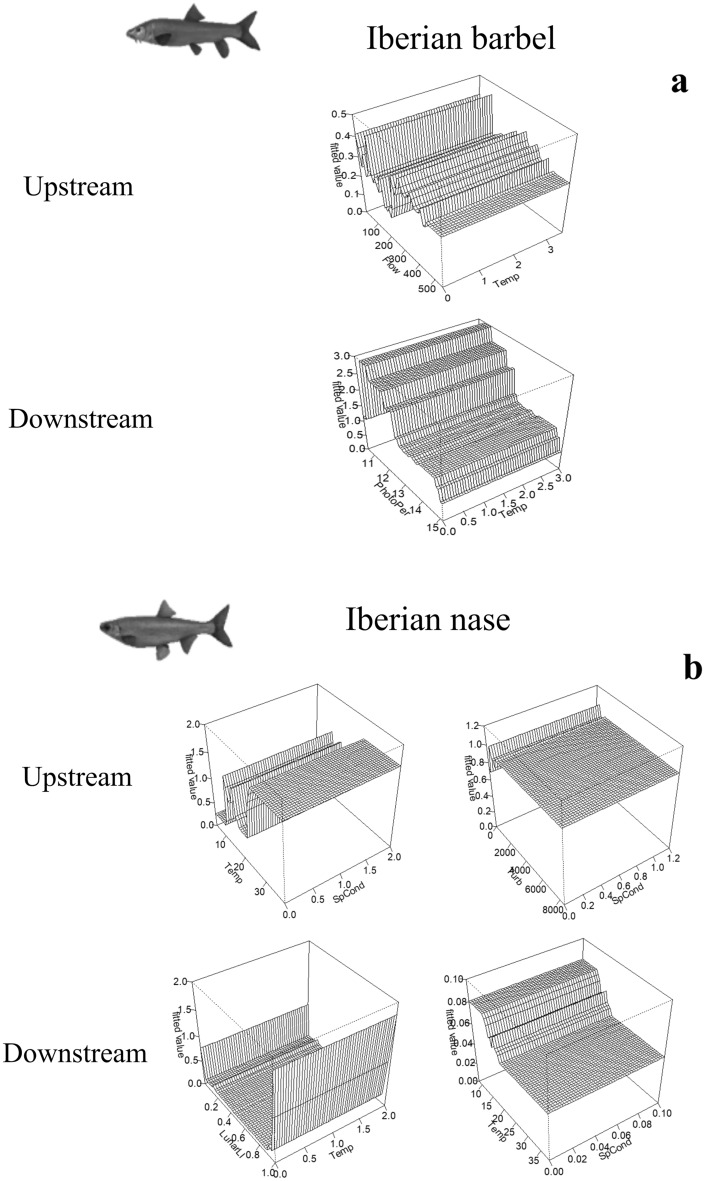
Boosted Regression Trees (BRT) analyses35,36 were conducted using the R package (R Core Team, 2020) and the gbm library37 to assess the influence of environmental variables on the upstream and downstream movements performed by each species during 2013–2014. The models were run separately for both species, considering both years together, for up- and down-stream movements during the bulk migration periods, with the hourly counts considered as the dependent variable. The best BRT models were optimised for the number of trees, tree complexity and learning rate to achieve the lowest predictive deviance. Tenfold cross-validation (cv) was performed to determine each model’s optimal number of trees. Each model was selected when the following criteria were met: (1) the smallest cv deviance (cv predictive deviance); (2) a higher percentage of explained deviance (R2), with a maximum of 1 explaining 100% of the observed variation; and (3) the highest cv correlation, a measure using Pearson’s correlation to measure the correlation between the observed data and set data35. After selecting the best model, a simplification process was applied35, which consisted of removing the non-informative predictors and re-setting the model with this new information to maximise model performance. Using the new simplified models, the interactions between predictors were analysed to identify all relationships between model predictions and all possible pairwise combinations. In addition, the relative influence (%) of each environmental predictor in the model was determined and rescaled so that the sum of all predictors in the model was 100%, with higher values indicating a greater influence and higher explained variance38 of the selected predictors on the migration patterns of the target species. Lastly, partial dependence plots were created and analysed, to visually show the effect of the response variable considering the average effects of the significant predictive variables selected by each final model, as well as for the most important interactions. The most important variables analysed in the partial dependence plots for all models were selected using a threshold cut of 75% of the total explanatory variation.
Results
Seasonal and diel movement patterns
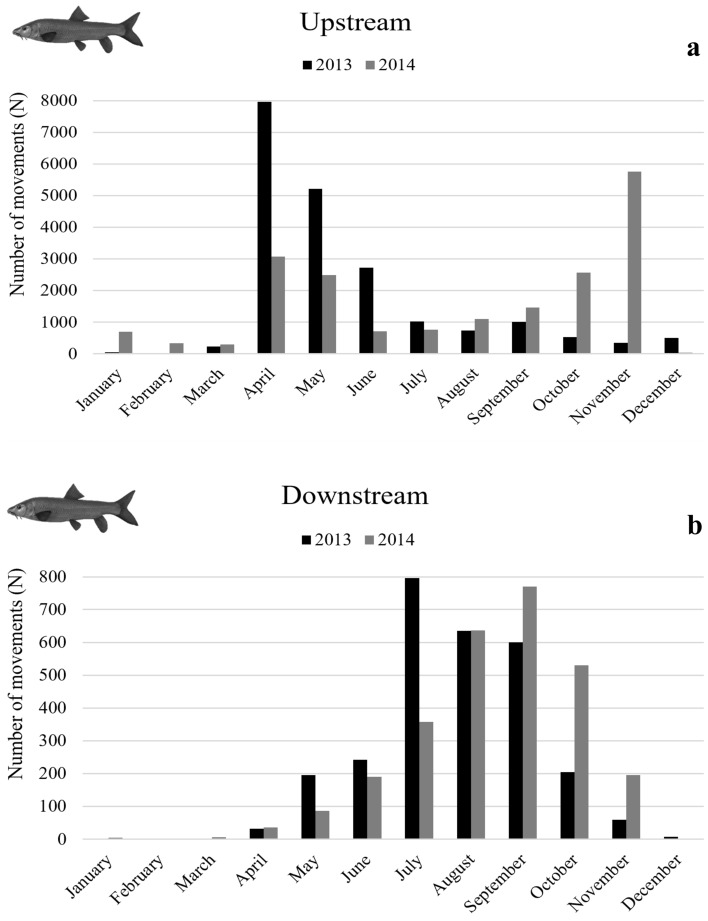
A total of 23,095 movements (20,322 upstream and 2773 downstream) of Iberian barbel were recorded in 2013 and 22,033 movements (19,223 upstream and 2810 downstream) in 2014 (Fig. 2).
Fig. 2.
Monthly counts of Iberian barbel moving through the Coimbra fish pass on the upstream (a) and downstream (b) directions during both study years (2013 and 2014).
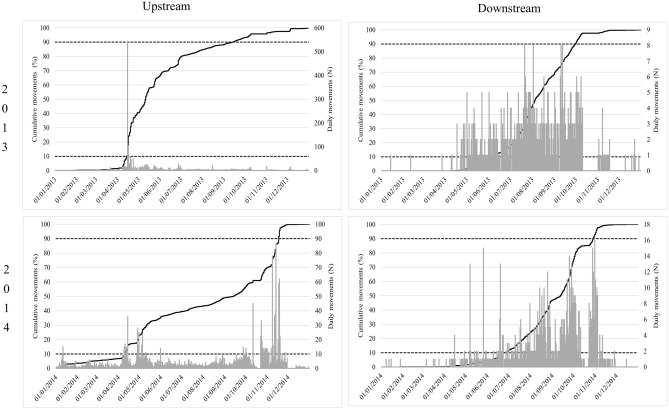
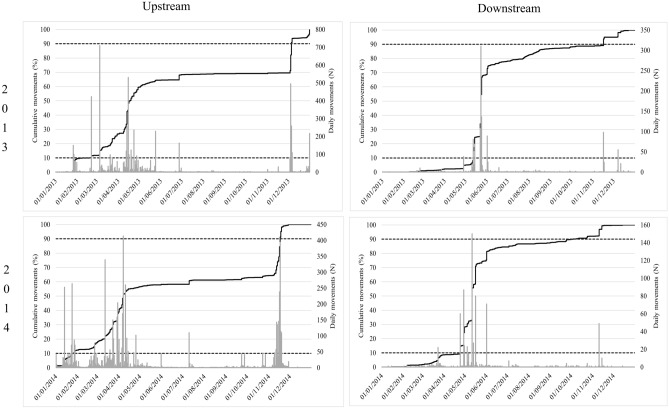
The bulk of upstream migration (10–90% of cumulative passages) for Iberian barbel was similar in both years, between April in 2013 and 2014, and September in 2013 and early November, in 2014, with the peak movements occurring between April and June. The bulk of downstream migration was similar interval in both years, between June (in 2013 and 2014) and September (in 2013) or October (in 2014), with peak movements occurring between July and October (Fig. 3).
Fig. 3.
Cumulative counts of Iberian barbel in the Coimbra dam fish pass, for each study year (2013 and 2014) and movement direction (upstream—left column; downstream—right column), with indication of what is considered the bulk of the migratory movement (10–90% of cumulative passes).
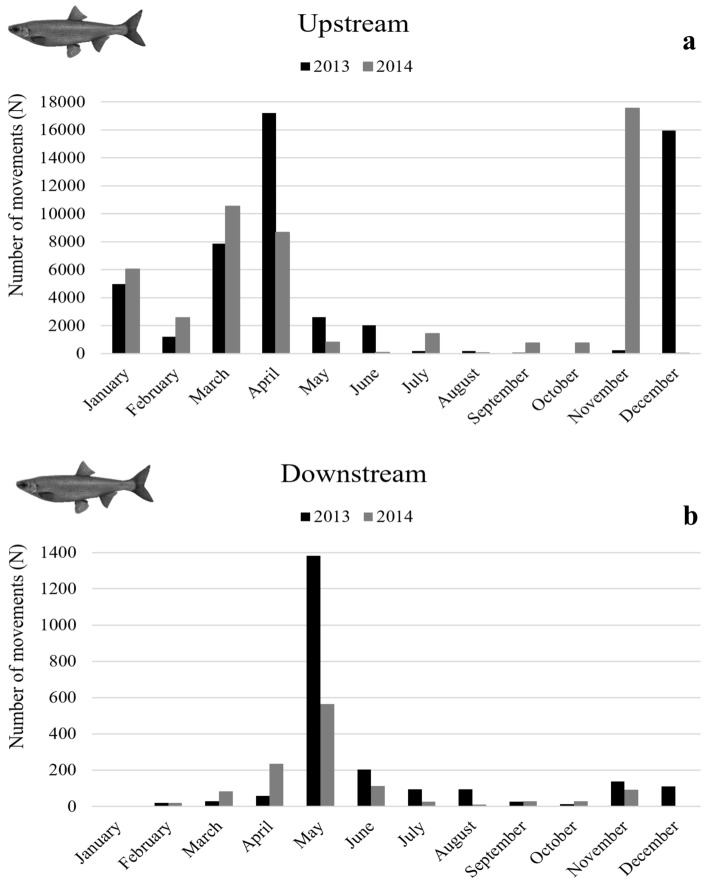
In 2013, the total number of Iberian nase movements was 54,616 (52,453 upstream and 2163 downstream), with a similar number of fish passages observed in 2014, 50,360 movements (49,162 upstream and 1198 downstream) (Fig. 4).
Fig. 4.
Monthly counts of Iberian nase moving through the Coimbra fish pass on the upstream (a) and downstream (b) direction during both the study years (2013 and 2014).
The bulk of the Iberian nase upstream migration started in January in both years, ending in December in 2013 and November in 2014. For this species, two peaks can be clearly identified in the two study years, the first occurring in November–December and the second in March–April. As far as downstream movements are concerned, it is possible to identify a strong synchrony in May in both years (Fig. 5).
Fig. 5.
Cumulative counts of Iberian nase in the Coimbra dam fish pass, for each study year (2013 and 2014) and movement direction (upstream—left column; downstream—right column), with indication of what is considered the bulk of the migratory movement (10–90% of cumulative passes).
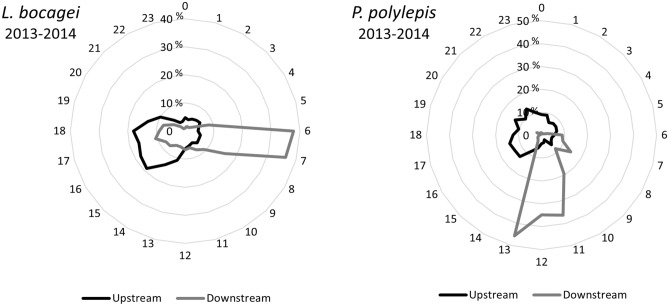
Regarding the circadian rhythm of activity, Iberian barbel upstream movements display a diurnal preference with a small peak of activity between 13 and 18 h for the aggregate of the two years, while downstream movements for this species occurred essentially between 6h00 and 7h00 (Fig. 6).
Fig. 6.
Circadian rhythm of downstream and upstream Iberian barbel (left column) and Iberian nase (right column) movements recorded for the aggregate data collected during the two-study year cycles (2013 and 2014) at the Coimbra dam FPS.
The circadian rhythm of nase upstream movements displayed a slight diurnal preference between 14 and 15 h for the two years combined. Regarding downstream movements, once again, the Iberian nase displayed a diurnal preference for these movements, with two peaks, the first and less representative between 6h00 and 8h00, and the second between 10h00 and13h00, for the two years combined (Fig. 6).
Detailed circadian rhythms for each species, movement direction, and study year are presented separately in Supplementary Material 1.
Size structure
Considering the 2015 sub-sample, a total of 857 Iberian barbel and 1377 Iberian nase were measured while moving upstream and downstream through the Coimbra dam FPS. The total length of Iberian barbel ranged from 9 to 48 cm for fish moving upstream and from 8 to 52 cm for fish moving downstream (Fig. 7). In January, no barbel moving downstream was observed. The total length of Iberian nase moving upstream ranged from 6 to 36 cm and that of those moving downstream from 6 to 31 cm (Fig. 8).
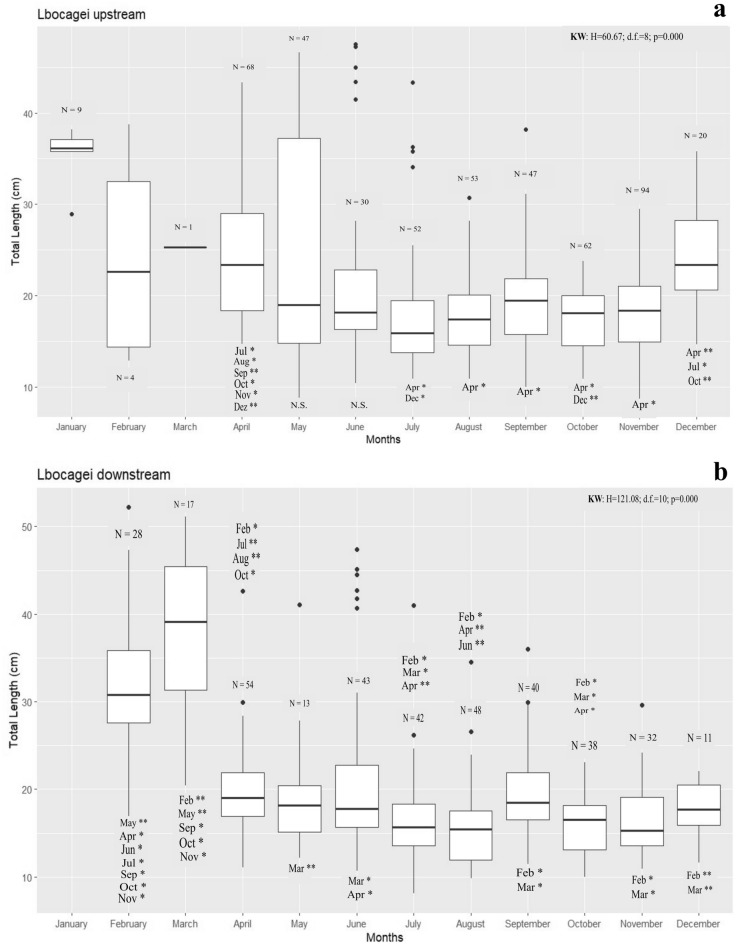
Fig. 7.
Size-range (TL, cm) of Iberian barbel moving in upstream (a) and downstream (b) directions at the Coimbra dam FPS during 2015. The number of measured individuals for each month is identified above the corresponding month (N). Dunn’s test results are identified near each month, with the identification of the level of significance: statistically highly significant, p < 0.001 (*) and statistically significant, p < 0.05 (**). In the absence of any level of statistical significance in size differences with other months, N.S. is displayed. The horizontal line represents the median value, the box the interquartile range, the whiskers 1.5 IQR, and the points are outliers.
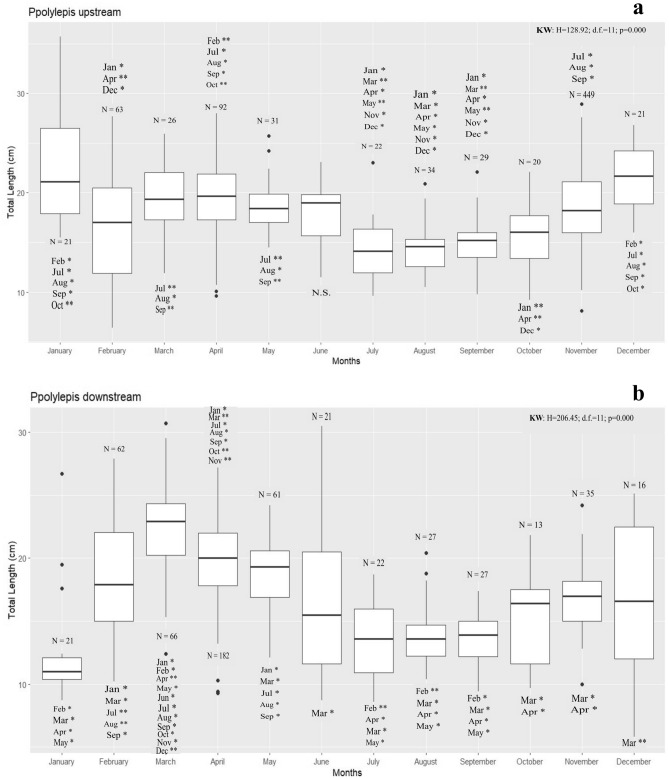
Fig. 8.
Size-range of Iberian nase moving in upstream (a) and downstream (b) directions at the Coimbra dam FPS during 2015.The number of measured individuals for each month is identified above the corresponding month (N). Dunn’s test results are identified near each month, with the identification of the level of significance: statistically highly significant, p < 0.001 (*) and statistically significant, p < 0.05 (**). In the absence of any level of statistical significance in size differences with other months, N.S. is displayed. The horizontal line represents the median value, the box the interquartile range, the whiskers 1.5 IQR, and the points are outliers.
Barbel moving upstream showed significant differences (Kruskal–Wallis test, H = 60.67; p = 0.000) in total length throughout the year, with the post-hoc Dunn’s test showing significant differences between all the months included in the analysis, except May and June, months in which barbel size classes showed no differences from the other months (Fig. 7a). The month of May presented the broadest range of barbel size classes moving upstream through the fish pass. For barbel moving downstream, the analysis showed significant differences (KW, H = 121.08; p = 0.000) in fish size between all months (Dunn’s test, Fig. 7b). In short, barbel of larger size were associated with the months of April, May and December for upstream movements, whereas when moving downstream, larger fish were mostly observed in February and March (Fig. 7).
For nase moving upstream, significant differences (KW, H = 128.92; p = 0.000) were found in size distribution between almost all months, with only June showing an absence of significant differences from the remaining months (Dunn’s test, Fig. 8a). Larger nase migrated upstream mainly in January and February, and the smaller ones in May, August, and September. For downstream movements, significant intra-annual differences were found (KW, H = 206.45; p = 0.000) in the size distribution of migrating fish, with Dunn’s test showing significant differences between all months tested (Fig. 8b). The size of the fish using the fish pass for downstream movements was highly variable throughout the year, with February, June and December being associated with the downstream movements of larger nase and January associated with the smaller individuals.
Environmental predictors of migration
Of all the predictors initially considered in the BRT analysis, no variables were excluded due to high intercorrelation (|rho| < 0.80, Spearman correlation).
The BRT models applied to barbel and nase upstream migration showed good overall performance, as evidenced by the high R2 scores of barbel (0.85) and nase (0.94) (Table 1). Overall, the BRT models show that for both Iberian barbel and Iberian nase moving upstream Flow (24.3% and 31.7%, respectively), Water Temperature (15.5% and 19.2%, respectively) and Water Turbidity (18.9% and 16.1%, respectively) are the three predictors that coincide in both models, whilst Conductivity (16.4%) and Photoperiod (12.1%) are the remaining explicative variables for Iberian barbel and Iberian nase, respectively (Fig. 9). BRT models also show important interactions between some predictors influencing these upstream movements for both species, especially for Water Temperature and Flow discharge for Iberian barbel and Conductivity with Water Temperature and Turbidity (Fig. 10). Upstream movements for both species had a high range of observed values, especially regarding Flow discharge and Temperature. Upstream movements of barbel started at a discharge of 90 m3 s−1 and stopped at 450 m3 s−1, with temperatures between 15 and 26 °C, turbidity values below 2000 FNU and conductivity below 125 µScm−1. For nase, those movements started with flow discharges of 100 m3 s−1 and stopped at 590 m3 s−1, temperatures between 12 and 25 °C, turbidity lower than 200 FNU and when the number of hours of light is 11 and 14 h.
Table 1.
Performance evaluation of BRT models for upstream and downstream movements of Iberian barbel and Iberian nase for the considered study period (2013 and 2014 combined).
| Iberian barbel | Iberian nase | |||
|---|---|---|---|---|
| Upstream | Downstream | Upstream | Downstream | |
| Number of trees | 4800 | 1200 | 5550 | 3350 |
| Tree complexity | 5 | 3 | 5 | 5 |
| Learning rate | 0.05 | 0.05 | 0.05 | 0.01 |
| Mean total deviance | 11.017 | 2.35 | 35.251 | 3.144 |
| Mean residual deviance | 1.637 | 1.195 | 2.245 | 0.484 |
| cv deviance |
3.905 SE = 0.421 |
1.630 SE = 0.047 |
11.979 SE = 1.683 |
2.260 SE = 0.596 |
| cv correlation |
0.702 SE = 0.036 |
0.435 SE = 0.024 |
0.599 SE = 0.036 |
0.337 SE = 0.089 |
| R2 | 0.85 | 0.49 | 0.94 | 0.85 |
Fig. 9.
Partial dependence plots for the most influential predictors, considering the 75% explanation threshold cut for (a) Iberian barbel and (b) Iberian nase, up- and downstream movements during the study period (2013–2014). Vertical axis observed in each plot is based on the logit scale and centred to have zero mean over the data distribution. All axes presented have different scales adapted to each case.
Fig. 10.
Main interaction between the environmental variables that influenced the most (a) Iberian barbel and (b) Iberian nase, up- and downstream movements during the study period (2013–2014).
For barbel and nase moving downstream, the BRT models showed a good performance for nase (0.85), but the lowest R2 score for barbel (0.49) (Table 1). BRT models identified for both barbel and nase that Flow (25.2% and 15.2% respectively) and Water Temperature (21.2% and 41.6%, respectively) were the two coincident variables influencing the most both species’ downstream migration, whilst Photoperiod (23.5%) and Day period (14.9%) were the remaining variables influencing this behaviour for barbel, and Water Turbidity (16.3%) and Conductivity (12.7%) for nase (Fig. 9). These downstream models also showed significant interactions between some of these predictors, with Water Temperature and Photoperiod for barbel, and Water Temperature with Lunar Light Intensity and Conductivity for nase (Fig. 10). Downstream movements occurred preferentially within more restricted ranges for all variables and both species. For barbel moving downstream, movements increased with discharge flows below 50 m3 s−1 and temperatures of 16 and 25 °C, with diurnal preferences and when the number of light hours was 11–15 h. Regarding nase moving downstream, these movements increased when the discharged flow was below 25 m3 s−1 and between 100 and 150 m3 s−1, temperatures between 15 and 20 °C, turbidity below 2000 FNU and conductivity values between 60 and 120 µScm−1.
Discussion
This study is an important addition to the understanding of seasonal migrations, diel movement patterns, and size structure of two potamodromous species widely distributed along the Iberian Peninsula rivers, the Iberian barbel and the Iberian nase. During the two complete annual cycles (2013–2014), a total of 45,128 barbels were recorded in the Coimbra dam FPS, of which 88% corresponded to upstream movements and 12% to downstream movements. For the Iberian nase, 104, 976 individuals were counted during the two-year period, of which 97% individuals were observed moving upstream and 3% moving downstream. The results for the upstream and downstream movements of both species showed a similar number of individuals counted in both 2013 and 2014. However, the differences observed in the downstream numbers in both years when compared to upstream movements are certainly underestimated due to the likely passage of individuals moving downstream through the dam sluice gates, which cannot be accounted for. Moreover, it is important to remember that downstream movements are not only made by the fish that have previously migrated upstream, but also by the juveniles that leave upstream habitats for downstream ones. Therefore, although the results obtained in this study for downstream movements of both species help provide novel insights (e.g., timing) into the ecology of these species for a typically less known life cycle phase, they should be considered with caution due to the limitations described above.
The Iberian barbel migrated upstream for a longer period than often described in most of the literature21,24,39,40 i.e., from January to June. In addition to the upstream movement peak identified in our study, which occurred most frequently in April and May, an additional migratory peak was detected in the autumn of 2014, which has also been identified in previous studies (using the genus Barbus) such as3,41,42, more precisely in September and October. Downstream movements for this species occurred during autumn and winter8,43, but started as early as April, as also described by23,33. The highest occurrence of upstream movements was more concentrated in time (April to June in 2013, and April–May and October–November, in 2014), whereas for downstream movements, they were more dispersed in the months of April and November in both years.
For the Iberian nase, our study also revealed a broader period of upstream movement than usually described in the literature. Nase were found migrating upstream throughout the year (although in smaller numbers), with two distinct and well-defined migratory peaks associated with upstream movements of this species, the first occurring in autumn (November–December), and the second occurring in spring (March–May), but only the latter was described in the literature23,24,39,40. To the best of our knowledge, this is the first time that peak winter movements have been reported for this species, which is a novel finding for nase ecology, at least in the Iberian region. Downstream movements were also observed throughout the year, but only a single migratory peak was observed in the spring, i.e., between April–June, with a residual number of passages in the remaining months. To the best of our knowledge, this behaviour has not been previously described for this species, as no other study has examined the downstream movements of Iberian nase, or other species belonging to the same genera. Other studies using this type of FPS, such as32,33 showed good results, both in terms of the number of passages and information collected, for downstream movements of the target species studied, which may indicate the suitability of this type of FPS for the downstream movements of different species. However, this can be looked upon as a complementary benefit, as designing these FPS specifically for downstream movements, may cause a decrease of efficiency for movements on the opposite direction, which is usually the main purpose of these infrastructures.
Diel patterns of Iberian barbel moving upstream were inconsistent across the sampling years. While a peak of activity of three hours in the afternoon was detected in 2013, there was no clear preferred time of the day for upstream movements in 201433,44. For the Iberian nase, no clear diel preference was found for any of the study years, as also previously observed by Mameri et al.44 with another Pseudochondrostoma species, the Northern straight-mouth nase (Pseudochondrostoma duriense, Coelho 1985) Although it is not possible to clearly identify a period of preferred passage time for Iberian barbel in 2014 and Iberian nase in both years, during this period both species displayed a preference for passages during daylight hours (55% of upstream barbel in 2014, and 55% of upstream nase movements in 2013 and 42% in 2014). This pattern is not consistent with the preference for nocturnal movements (upstream migration) usually described in the literature for the European genus of these species1,39,45,46 and may be explained by the environmental preferences of both species in the study area, characterised by warmer temperatures that occur mainly during the day and may trigger activity3,40.
The observed downstream movements showed consistent diel patterns for both species over the two study years. Barbel moving downstream clearly preferred to do so between 6h00 and 7h00 in both study years. For nase moving downstream, two peaks of activity were identified in 2013 and 2014, between 6h00–8h00 and 10h00–13h00. As downstream movements are often more challenging to study and identify, most studies disregard this aspect. Therefore, information on diel patterns of downstream movements is scarce or non-existent, making the results of our study extremely important for more complete and integrated knowledge about the movement patterns of these potamodromous species.
Our study showed evidence of size-structure seasonal segregation for upstream and downstream migrating barbel and nase. For the upstream moving barbel, larger individuals migrate in February and May, whereas for the downstream barbel, larger individuals migrate mainly in February and March. In both cases, a large variation in sizes was observed. Iberian nase of larger sizes migrated upstream in January and February, whilst the total length of the nase moving downstream was higher in February, June and December. As for barbel, a large variation in the total length of the individuals was observed, both upstream and downstream. Our upstream results are contrary to those found by47 for Barbus barbus (L.) and Chondrostoma nasus (L.), where larger barbels were associated with end of summer/early autumn migrations, whereas larger nase migrated in spring. Both studies differ in the sampling period, while47 conducted the study between August and November, also in comparison with previous spring results, and our study included two complete annual cycles, making the information more robust as it considers both inter- and intra-annual variability.
Overall, the performance of the BRT analyses carried out in this study to identify environmental variables associated with the migration of both target species provided reliable results for the upstream movements of barbel and nase, and the downstream movements of nase, despite a weaker performance (lower R2 value) when analysing the downstream movements of barbel. This weaker performance for barbel downstream movements may be because these movements are more temporally dispersed and subject to greater variation in the environmental predictors studied. These results highlight the usefulness of the BRT analysis in predicting environmental variables affecting fish movements, as shown by other authors [e.g.,31,32,48]. In all the modelling exercises carried out to assess the environmental influence on barbel and nase migration patterns, temperature and flow were the only environmental predictors that were always statistically significant, confirming the importance of these two variables as environmental triggers for Iberian barbel and Iberian nase migration1,5,23,40,41,44,49. However, other predictors also contribute to explain the observed movements. Rather, there is a direct influence of other factors such as photoperiod, water turbidity, period of the day and conductivity, which together can cause a shift in the migration speed or timing23.
The observed results showed that the preferred temperature for barbel upstream movements started at 15 °C and stopped at 26 °C, with a slightly higher temperature than that observed in previous studies with this species23,40,41. For nase upstream movements, the preferred temperature in our study started at 12 °C and had its limit at 25 °C, with these results being consistent with those of previous studies23,40,50,51. For Iberian barbel downstream movements, the preferred temperature for increasing movements started at 15 °C and stopped at temperatures of 25 °C, whereas for the Iberian nase, a clear pattern emerged with downstream movements starting at 15 °C and stopping at 20 °C.
Regarding flow, high variability was observed in both years, with values ranging from 1.91 to 1 276 m3 s−1 in 2013 and 7.85 to 1 099 m3 s−1 in 2014. For upstream moving fish, our results showed a preferential movement flow starting at 90 m3 s−1 for Iberian barbel, and for values starting at 150 m3 s−1 for the Iberian nase. Our results are in accordance with previous studies40,42,45, showing the influence of flow on the migratory patterns of barbel and nase. However, the flow values in our study, considered preferential for upstream movements, are higher than most described in the literature. For downstream movements, the preferential flow was lower than that observed for upstream movements, with Iberian barbel increasing their movements with flows below 50 m3 s−1 and Iberian nase displaying movements in two different flow intervals, 0–25 and 100–150 m3 s−1. These results are somewhat surprising, as one would expect downstream movements to coincide with high flow events1, which was not observed in our study. However, we cannot exclude the possibility that downstream movements are underestimated due to the chance of individuals passing through the dam gates during higher flow discharges and using the FPS during lower flow periods, reinforcing our previous statement that downstream results should be considered cautiously.
Conductivity, photoperiod, period of the day and turbidity were the other environmental variables found to influence and explain the movements of these species significantly. For example, photoperiod is often described as having a direct influence on the physiology and behaviour of fish by also influencing fluctuations in temperature and oxygen1, with a direct consequence on the migratory behaviour of several barbel and nase species8,40,52. Turbidity, usually associated with higher conductivity, is also a variable often associated with migratory movements, not only because an increase in turbidity is directly related to an increase in flow velocity and sediment transport, but also because migratory species may take advantage of the reduced visibility caused by increased turbidity in the water to move during diurnal periods, which usually occur at night1,52,53, a hypothesis that gains strength with the preference for movement during diurnal periods shown by the target species in this study.
The Iberian barbel that used the FPS for their migrations were between 1+ and 11+ years old29, while the Iberian nase were between 2+ and 10+54. These results show a wide range in the age of fish from these species migrating in both directions, with the surprising result of the presence of 1-year old barbel migrants, since the literature describes the reproductive migrations in barbel and nase as first occurring at the age of 2+29,55. In this study a very robust dataset was used (150,104 individuals counted, of which 45,128 were Iberian barbel and 104,976 Iberian nase), with movements in both directions (upstream and downstream) in the fish pass assessed for two years. This allowed us to identify, with a considerably high confidence statistically, the main environmental variables and, to some extent, the thresholds values that trigger the movements and migratory patterns observed.
Conclusion
This study provides novel information on the movement patterns of Iberian barbel and Iberian nase, including migratory peaks, diel activity, the size structure of the migratory fraction and the environmental variables that influence these ecological processes. The study adds important information detailing not only upstream movements, which are usually the focus of the relevant literature, but also downstream movements, a life stage process that is quite often left out of existing studies on the subject, probably because it is more difficult to study (generally smaller individuals with restrictions on tagging, more difficult to capture when moving downstream, movement often coincides with peaks in flow with difficult sampling conditions).
These results also make an important contribution to the future management of both species, by providing new information on their movement patterns, migratory periods, and related environmental triggers. In Portugal, Iberian barbel and Iberian nase are covered by both commercial and recreational fishing laws, with a closed season between 16 March and 14 June for recreational fishing and between 16 March and 30 June for commercial fishing in the study area. Both closing seasons were established to be coincident with the migratory and reproductive season of both species. Our results show a wider migratory period and, in some cases, more than one migratory peak for both species, which indicates that we are facing a misalignment between the fishing seasons currently foreseen in related legislation for the studied species, and the timing of migration and spawning, with potential implications for the survival of spawners and, consequently, the sustainability of related fishing activities. The adjustment and/or addition of new specific closed seasons, both for the traditional spring migratory peak and for the new autumn/winter migratory peak, could be measures to be implemented for maintaining or improving the Iberian barbel and Iberian nase populations. It is therefore important to deepen the existing knowledge of these migratory patterns, periods, and peaks, as well as of the migrants themselves, to better adapt and improve conservation efforts and management programmes targeting these species.
Supplementary Information
Acknowledgements
The authors would like to thank Sara Silva, Roberto Oliveira, Inês Oliveira, João Pedro Marques, Ana Filipa Silva, Joana Pereira and Rita Almeida for their assistance in visual census, fish counting and measurement. This research was financially supported by the Portuguese Environment Agency (APA) through the “Coimbra fish pass monitoring program”, the project “Habitat restoration for diadromous fish in River Mondego” (PROMAR 31-03-02-FEP-5), funded by the Ministry of Agriculture, co-funded by the European Fisheries Fund through PROMAR 2017-13 and by FEDER through the “Programa Operacional Fatores de Competitividade—COMPETE” and project “An@dromos.PT—Operational Plan for the Monitoring and Management of Anadromous Fish in Portugal” (MAR-01.03.02-FEAM-0002), funded by the Ministry of Agriculture through MAR2020 and by European Fisheries Fund. This work was also financially supported by FCT-Foundation for Science and Technology via projects UIDB/04292/2020 (https://doi.org/10.54499/UIDB/04292/2020), awarded to MARE and LA/P/0069/2020, awarded to Associated Laboratory ARNET (https://doi.org/10.54499/LA/P/0069/2020), and through the doctoral grant attributed to A. S. Rato (2021.05330.BD). Carlos M. Alexandre is supported by an open-ended public service work contract within the Institutional Call to Scientific Employment Stimulus (Institutional CEEC 2nd Edition). Catarina S. Mateus was supported by National Funds through FCT (Foundation for Science and Technology) via a Research Contract from MARE/ARNET.
Author contributions
Conceptualization: A. Rato; C.M. Alexandre; B.R. Quintella; P.R. Almeida; Methodology: C.M. Alexandre; B.R. Quintella; P.R. Almeida; Sampling: A. Rato; S. Pedro; C.S. Mateus; E. Pereira; A.F. Belo; Data analysis: A. Rato; S. Pedro; Resources: P.R. Almeida; C.M. Alexandre; B.R. Quintella; C.S. Mateus; M.F. Quadrado; A. Telhado; C. Batista; Writing-original draft preparation: A. Rato; Writing-review and editing: A. Rato; C.M. Alexandre; S. Pedro; C.S. Mateus; E. Pereira; A.F. Belo; B.R. Quintella; P.R. Almeida; Supervision: C.M. Alexandre; P.R. Almeida; Funding aquisition: C.M. Alexandre; B.R. Quintella; P.R. Almeida. All authors have read and agreed to the submitted version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74959-4.
References
- 1.Lucas, M. C. & Baras, E. Migration of Freshwater Fishes (Blackwell Science Ltd., 2001). [Google Scholar]
- 2.Tsukamoto, K., Miller, M. J., Kotake, A., Aoyama, J. & Uchida, K. The origin of fish migration: The random escape hypothesis. Am. Fish. Soc. Symp.69, 45–61 (2009). [Google Scholar]
- 3.Benitez, J.-P., Matondo, B. N., Dierckx, A. & Ovidio, M. An overview of potamodromous fish upstream movements in medium-sized rivers, by mean of fish passes monitoring. Aquat. Ecol.10.1007/s10452-015-9541-4 (2015). [Google Scholar]
- 4.Chapman, B. B. et al. Partial migration in fishes: Definitions, methodologies and taxonomic distribution. J. Fish Biol.81, 479–499. 10.1111/j.1095.8649.2021.03349.x (2012). [DOI] [PubMed] [Google Scholar]
- 5.Thurow, R. F. Life histories of potamodromous fishes. In An Introduction to Fish Migration 29–54 (Florida, 2016). [Google Scholar]
- 6.Brodersen, K. et al. Fixed and flexible: Coexistence of obligate and facultative migratory strategies in freshwater fish. PLoS ONE9(3), e90294. 10.1371/journal.pone.0090294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro-Santos, T. & Haro, A. Fish guidance and passage at barriers. Fish Locomotion.10.1201/b10190-4 (2010). [Google Scholar]
- 8.Britton, J. R. & Pegg, J. Ecology of European barbel Barbus barbus: Implications for river, fishery, and conservation management. Fish. Sci.19(4), 321–330. 10.1080/10641262.2011.599886 (2011). [Google Scholar]
- 9.Rato, A. S., Alexandre, C. M., Almeida, P. R., Costa, J. L. & Quintella, B. R. Effects of hydropeaking on the behaviour, fine-scale movements and habitat selection of an Iberian cyprinid fish. River Res. Appl.10.1002/rra.3848 (2021). [Google Scholar]
- 10.Kottek, M., Grieser, J., Beck, C., Rudolf, B. & Rubel, F. World map of the Kӧppen-Greiger climate classification updated. Meteorol. Z.15(3), 259–263. 10.1127/0941-2948/2006/0130 (2006). [Google Scholar]
- 11.ICOLD (International Commission on Large Dams), 1998. Role of Dams. www.icold-cigb.org/GB/Dams/role_of_dams.asp.
- 12.Hussain, I., Ali, A., Ahmed, I., Firdousi, N. & Bhutia, R. (2021). Prospects of fish passes in freshwater river ecology in attention to migratory fishes. Agric. Food. E-ISSN: 2581–8317.
- 13.Larinier, M. Fish passagem experience at small-scale hydro-electric power plants in France. Hydrobiologia609, 97–108. 10.1007/s10750-008-9398-9 (2008). [Google Scholar]
- 14.Katopodis, C. & Williams, J. G. The development of fish passage research in a historical context. Ecol. Eng.48, 8–18 (2012). [Google Scholar]
- 15.Ovidio, M. & Philippart, J. C. The impact of small physical obstacles on upstream movements of six species of fish. Hydrobiologia483, 55–69 (2002). [Google Scholar]
- 16.Larinier, M., Chanseau, M., Bau, F. & Croze, O. (2003). The use of radio telemetry for optimizing fish pass design. Spedicato, M.T., Lembo, G., Marmulla, G. (eds.). Aquatic telemetry: Advances and applications. in Proceeding of the Fifth Conference on Fish Telemetry held in Europe. Ustica, Italy, 9–13 June 2003. Rome, FAO/COISPA. 2005. 295p.
- 17.Izzo, L. K., Maynard, G. A. & Zydlewski, J. Upstream movements of Atlantic Salmon in the lower Penobscot River, maine following two dam removals and fish passage modifications. Mar. Coast. Fisheries8(1), 448–461. 10.1080/19425120.2016.1185063 (2016). [Google Scholar]
- 18.Keefer, M. L., Jepson, M. A., Clabough, T. S. & Caudill, C. C. Technical fishway passage structures provide high passage efficiency and effective passage for adult Pacific salmonids at eight large dams. PLoS ONE16(9), e0256805. 10.1371/journal.pone.0256805 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunt, C. M., Castro-Santos, T. & Haro, A. Performance of fish passage structures at upstream barriers to migration. River Res. Appl.10.1002/rra.1565 (2011). [Google Scholar]
- 20.Bao, J. et al. Quantitative assessment of fish passage efficiency at a vertical-slot fishway on the Daduhe River in Southwest China. J. Ecol. Eng.10.1016/j.ecoloeng.2019.105597 (2019). [Google Scholar]
- 21.Collares-Pereira, M.J., Alves, M.J., Ribeiro, F., Domingos, I., Almeida, P.R., da Costa, L., Gante, H., Filipe, A.F., Aboim, M.A., Rodrigues, P.M. & Magalhães, M.F. (2021). Guia dos peixes de água doce e migradores de Portugal Continental. Edições Afrontamento. Porto, 292 pp.
- 22.Magalhães, M. F. Feeding ecology of the Iberian cyprinid Barbus bocagei Steindachner, 1864 in a lowland river. J. Fish Biol.40, 123–133 (1992). [Google Scholar]
- 23.Rodríguez-Ruiz, A. & Granado-Lorencio, C. Spawning period and migration of three species of cyprinid in a stream with Mediterranean regimes (SW Spain). J. Fish Biol.41(4), 545–556 (1992). [Google Scholar]
- 24.Kottelat, M. & Freyhof, J. Handbook of European Freshwater Fishes (Publications Kottelat, 2007). [Google Scholar]
- 25.Santos, M. J. et al. Complex size-dependent habitat associations in potamodromous fish species. Aquat. Sci.73, 233–245. 10.1007/s00027-010-0172-5 (2011). [Google Scholar]
- 26.Magalhães, M.F., Amaral, S.D., Sousa, M., Alexandre, C.M., Almeida, P.R., Alves, M.J., Cortes, R., Farrobo, A., Filipe, A.F., Franco, A., Jesus, J., Oliveira, J.M., Pereira, J., Pires, D., Reis, M., Ribeiro, F., Robalo, J.I., Sá, F., Santos, C.S., Teixeira, A. & Domingos, I. (2023). Livro Vermelhos dos Peixes Dulciaquícolas e Diádromos de Portugal Continental. FCiências.ID & ICNF, I.P. Lisboa.
- 27.APA (Portuguese Environmental Agency) (2016). Plano de Gestão de Região Hidrográfica do Vouga, Mondego e Lis, Integrados na Região Hidrográfica 4. https://apambiente.pt/sites/default/files/_SNIAMB_Agua/DRH/PlaneamentoOrdenamento/AAE_PGRH_PGRI/2016-2021/PGRH_2_PGRI_1_RH4A_RelatorioAmbiental.pdf
- 28.APA (Portuguese Environmental Agency) (2023). Plano de Gestão de Região Hidrográfica do Vouga, Mondego e Lis. https://apambiente.pt/sites/default/files/_SNIAMB_Agua/DRH/PlaneamentoOrdenamento/PGRH/2022-2027/PTRH4A/PGRH_3_RH4A_Parte2_VolumeB.pdf.
- 29.Alexandre, C. M. et al. Effects of flow regulation on the movement patters and habitat use of a potamodromous cyprinid species. Ecohydrology9, 326–340 (2015). [Google Scholar]
- 30.Pereira, E. et al. Performance of a vertical slot fish pass for the sea lamprey Petromyzon marinus L. and habitat recolonization. River Res. Appl.33, 16–26 (2017). [Google Scholar]
- 31.Belo, A.F., Cardoso, G.R., Pereira, E., Quintella, B.R., Mateus, C.S., Alexandre, C.M., Batista, C., Telhado, A., Quadrado, M.F. & Almeida, P.R. (2021). Fish pass use by shads (Alosa alosa L. and Alosa fallax [Lacépède, 1803]): Implications for monitoring and management. Ecodydrology, e2292.
- 32.Pereira, E. et al. Temporal patterns of the catadromous thinlip grey mullet migration in freshwater (central Portugal). Ecohydrology.10.1002/eco.2345 (2021). [Google Scholar]
- 33.Sanz-Ronda, F. J., Bravo-Córdoba, F. J., Fuentes-Pérez, J. F. & Castro-Santos, T. Ascent ability of brown trout, Salmo trutta, and two Iberian cyprinids—Iberian barbel, Luciobarbus bocagei, and northern straight-mouth nase, Pseudochondrostoma duriense—In a vertical slot fishway. Knowl. Manag. Aquat. Syst.410, 10. 10.1051/kmae/2015043 (2016). [Google Scholar]
- 34.Sanz-Ronda, F. J., Fuentes-Pérez, J. F., García-Vega, A. & Bravo-Córdoba, F. J. Fishways as downstream routes in small hydropower plants: Experiences with a potamodromous Cyprinid. Water13, 1041. 10.3390/w13081041 (2021). [Google Scholar]
- 35.Elith, J., Leathwick, J. R. & Hastie, T. A working guide to boosted regression trees. J. Animal Ecol.77, 802–813 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Elith, J. & Leathwick, J.R. (2013). Boosted Regression Trees for ecological model. R documentation. https://rspatial.org/raster/sdm/9_sdm_brt.html.
- 37.Ridgeway, G. (2020). Package ‘gbm’: Generalized Boosted Regression Models. R documentation. https://cran.r-project.org/web/packages/gbm/gbm.pdf.
- 38.Froeschke, J., Stunz, G. W. & Wildhaber, M. L. Environmental influences on the occurrence of coastal sharks in estuarine waters. Mar. Ecol. Progress Series407, 279–292 (2010). [Google Scholar]
- 39.Santos, J.M., Ferreira, M.T., Godinho, F.N. & Bochechas, J. (2005). Efficacy of a nature-like bypass channel in a Portuguese lowland river. J. Appl. Ichthyol. 381–388. ISSN 0175-8659.
- 40.García-Vega, A. et al. Pre-reproductive movements of potamodromous cyprinids in the Iberian Peninsula: When environmental variability meets semipermeable barriers. Hydrobiologia.10.1007/s10750-021-04537-6 (2021). [Google Scholar]
- 41.Benitez, J.-P. & Ovidio, M. The influence of environmental factors on the upstream movements of rheophilic cyprinids according to their position in a river basin. Ecol. Freshw. Fish.10.1111/eff.12382 (2017). [Google Scholar]
- 42.Roberts, C. G., Hindes, A. M. & Britton, J. R. Factors influencing individual movements and behaviours of invasive European barbel Barbus barbus in a regulated river. Hydrobiologia830, 213–228. 10.1007/s10750-018-3864-9 (2019). [Google Scholar]
- 43.Lucas, M. C. & Batley, E. Seasonal movements and behaviour of adult barbel Barbus barbus, a riverine cyprinid fish: Implications for river management. J. Appl. Ecol.33, 1345–1358 (1996). [Google Scholar]
- 44.Mameri, D. et al. Passability of potamodromous species through a fish lift at a large hydropower plant (Touvedo, Portugal). Sustainability12, 172. 10.3390/su12010172 (2020). [Google Scholar]
- 45.Lucas, M. C. & Frear, P. A. Effects of a flow-gauging weir on the migratory behaviour of adult barbel, a riverine cyprinid. J. Fish Biol.50, 382–396 (1997). [Google Scholar]
- 46.Silva, A. T., Santos, J. M., Ferreira, M. T., Pinheiro, A. N. & Katopodis, C. Effects of water velocity and turbulence on the behaviour of Iberian barbel (Luciobarbus bocagei, Steindachner 1864) in an experimental pool-type fishway. River Res. Appl.27, 360–373. 10.1002/rra.1363 (2011). [Google Scholar]
- 47.Prchalová, M., Vetešník, L. & Slavík, O. Migrations of juvenile and subadult fish through a fishpass during late summer and fall. Folia Zool.55(2), 162–166 (2006). [Google Scholar]
- 48.Froeschke, J. T. & Froeschke, B. F. Spatio-temporal predictive model based on environmental factors for juvenile spotted seatrout in Texas estuaries using boosted regression trees. Fisheries Res.111, 131–138. 10.1016/j.fishres.2011.07.008 (2011). [Google Scholar]
- 49.Branco, P., Segurado, P., Santos, J. M., Pinheiro, P. & Ferreiras, M. T. Does longitudinal connectivity loss affect the distribution of freshwater fish?. Ecol. Eng.48, 70–78. 10.1016/j.ecoleng.2011.05.008 (2012). [Google Scholar]
- 50.Ovidio, M. & Philippart, J. C. Movement patterns and spawning activity of individual nase Chondrostoma nasus (L.) in flow-regulated and weir-fragmented rivers. J. Appl. Ichthyol.24, 256–262 (2008). [Google Scholar]
- 51.Melcher, A. H. & Schmutz, S. The importance of structural features for spawning habitat of nase Chondrostoma nasus (L.) and barbel Barbus barbus (L.) in a pre-Alpine river. River Syst. J.19(1), 33–42 (2010). [Google Scholar]
- 52.Rakowitz, G., Berger, B., Kubecka, J. & Keckeis, H. Functional role of environmental stimuli for the spawning migration in Danube nase Chondrostoma nasus (L.). Ecol. Freshw. Fish.17, 502–514. 10.1111/j.1600-0633.2008.00302.x (2008). [Google Scholar]
- 53.Fuentes-Pérez, J. F. et al. Spatial preferences of Iberian barbel in a vertical slot fishway under variable hydrodynamic scenarios. Ecol. Eng.125, 131–142. 10.1016/j.ecoleng.2018.10.014 (2018). [Google Scholar]
- 54.Lobon-Cerviá, J. (1982). Population of the Iberian nose (Chondrostoma polylepis Stein, 1865) in the Jarama River. Vie et Milieu/Life Environ. Observatoire Océanologique Laboratoire Arago. 32(3), 139–148.
- 55.Cervia, J. L. & Elvira, B. Edad, crescimiento y reproducción de la boga de rio (Chondrostoma polylepis polylepis Stein, 1865) en el embalse de pinilla (rio Lozoya). Boletín del Instituto Español de Oceanigrafia6, 199–213 (1981). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.