Abstract
Cognitive impairment is frequently seen in patients with type 2 diabetes (T2DM), ranging from mild impairment to dementia. However, our knowledge of the specific profiles and risk factors for these different levels of impairment is limited. In this study involving 152 patients with T2DM, cognitive function was assessed using the Montreal Cognitive Assessment test. The Fuzzy C-means clustering algorithm was utilized to group individuals with similar cognitive characteristics. The study evaluated how well clinical parameters could classify characteristics of clusters using the Classification and Regression Trees algorithm. ROC analysis was then used to assess the classification success. Three distinct cognitive clusters were identified. Cluster 1 had the poorest cognitive performance and was characterized by more women, lower education levels, and lower levels of iron, hemoglobin, and creatine. Cluster 3, the amnestic cluster, was distinguished by low TSH levels. The decision tree model highlighted several parameters, including education level, hemoglobin, duration of diabetes mellitus (DM), iron, TSH, gender, family history of diabetes, and microalbumin/creatinine ratio, as significantly affecting the distinction of cognitive clusters. Diabetes-associated cognitive impairment stems from multifaceted pathophysiological mechanisms influenced by complex risk factors, resulting in diverse types of cognitive deficits.
Keywords: Diabetes mellitus, Cognitive dysfunction, Cluster analysis, Decision trees
Subject terms: Cognitive neuroscience, Diabetes
Introduction
The complications of diabetes are traditionally categorized into macrovascular such as cardiovascular and cerebrovascular diseases, and microvascular problems including nephropathy, retinopathy, and neuropathy. There is a growing recognition of cognitive impairment, spanning from mild cognitive impairment to dementia, as a potential complication of diabetes. The rising prevalence of diabetes, coupled with population aging, may lead to a significant upsurge in cognitive impairment among individuals with diabetes1,2.
Clinical and epidemiological investigations indicate connections between type 2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD), as well as vascular dementias in general. A meta-analysis of 30 studies reported an increased risk of cognitive impairment, not just dementia, in people with T2DM3. However, the precise causal relationship between diabetes and specific cerebral alterations, as well as the underlying mechanisms, remain largely unknown4. For instance, while there is significant epidemiological evidence linking diabetes to a heightened risk of dementia, including AD, there doesn’t seem to be an increased occurrence of AD neuropathology in individuals with diabetes5,6. Moreover, although diabetes is correlated with cerebrovascular disease and an elevated risk of stroke7, this association doesn’t seem to be the primary explanation for the heightened risk of dementia. Diabetes-related mechanisms impact both neurodegenerative and vascular processes, thereby influencing both Alzheimer’s-like cognitive impairment, characterized by memory deficits, and cerebrovascular-type cognitive impairment, which involves executive cognitive function impairment2. Hence, it is crucial to identify the different types of impairments linked to diabetes and to elucidate their underlying causes.
In the literature, there is considerable emphasis on T2DM elevating the risk of mild cognitive impairment (MCI) and dementia, with particular attention to its association with Alzheimer’s disease (AD) and vascular dementias. Nevertheless, there remains a dearth of studies investigating the cognitive profiles of patients with T2DM and identifying the risk factors for deterioration within these profiles. In this study, we aimed to group individuals with similar cognitive profiles using fuzzy clustering analysis and to identify the clinical parameters important for predicting these cognitive groups through decision tree analysis. Our hypothesis is that cognitive impairment, as a complication of diabetes, may manifest in distinct patterns, reflecting the heterogeneous clinic profiles of these patients. The advantage of the fuzzy clustering algorithm is to provide consistent results when clusters are not distinctly separated or when there are uncertainties in memberships. It is applicable for both qualitative and quantitative variables. On the other hand, the decision tree method was applied to determine the clinical parameters that could be effective for the clusters obtained through clustering. By using the decision tree algorithm, which is a classification algorithm, the probabilities of belonging to Montreal Cognitive Assessment (MoCA) score clusters were evaluated based on clinical parameters (inputs).
Materials and methods
Participants
The sample comprised 152 patients with T2DM, of whom 54.6 (n = 83) were male, with a mean age of 51.95 years (SD = 8.53). The study retrospectively included individuals who applied to Bezmialem Vakif University Faculty of Medicine, Department of Internal Medicine outpatient clinic between January 2022 and December 2023 and were diagnosed with T2DM according to ADA (American Diabetes Association) criteria. T2DM patients aged between 30 and 65 years with at least primary school education were included in our study. The exclusion criteria were as follows: (1) insulin use (Insulin therapy was taken as an exclusion criterion to avoid the effect of hypoglycemia on cognition), (2) severe psychiatric or neurologic disorder (e.g., major depressive disorder, neurdogenerative diseases, stroke or brain tumor), (3) vision and hearing problem, (4) B12 and folic acid deficiency, (5) Alcohol, substance addiction. Clinical data and laboratory results of the patients were accessed through the hospital system. All participants signed written informed consent and approval was obtained from Bezmialem Vakif University Non-interventional Research Ethics Committee (2023/343 − 06.12.2023). Informed consent was obtained from all participants and all methods were carried out in accordance with the Declaration of Helsinki.
Cognitive assessment
The Montreal Cognitive Assessment (MoCA) is a widely used cognitive screening tool designed to assess various cognitive domains, including visuospatial-executive functions, naming, memory, attention, language, abstraction, and orientation. It was developed by Dr. Nasreddine and has become a popular instrument for detecting MCI and early signs of dementia, such as Alzheimer’s disease8. Scores on the MoCA can range from 0 to 30, with higher scores indicating better cognitive function. A score of 26 or above is generally considered normal, while scores below 26 may suggest cognitive impairment. However, the interpretation of scores may vary based on factors such as age, education, and cultural background. Based on a validation study conducted with the Turkish population9,10, a score of 21 or higher was considered to indicate normal cognition, while a score below 21 was classified as impaired cognition.
Statistical analysis
Descriptive statistics for qualitative variables in the study were presented as counts and percentages, while descriptive statistics for quantitative variables were provided as mean, standard deviation, median, minimum, and maximum. The assumption of normal distribution was evaluated using the Kolmogorov-Smirnov test. Levene’s test was used to assess the homogeneity of variance. One-way analysis of variance (ANOVA) was employed for comparing means among more than two groups. Multiple comparisons were evaluated using the Tukey test. The Kruskal-Wallis test was utilized for comparing medians among more than two groups. Pairwise comparisons were conducted using the Dunn test as a post-hoc test. Pearson’s chi-square analysis was employed for comparing the occurrence rates of relevant qualitative variables among groups, with Bonferroni correction applied for multiple comparisons.
To evaluate the missing data in the dataset, a missing data analysis was conducted. The randomness of the missing data was tested using Little’s MCAR (Missing Completely at Random) test. It was determined that the structure of the dataset with missing data was MCAR. The Expectation Maximization (EM) method was employed for imputing missing values. In the EM method, the E-step estimates the missing data conditionally based on the available parameters. These estimates are then replaced with the missing data. The M-step selects the values with the highest likelihood estimation that are appropriate for the missing data. Finally, after convergence is achieved, an EM variance-covariance matrix is formed, and the imputed dataset is saved.
In the second phase of the study, clustering analysis was conducted using the obtained scores from the MoCA assessments to cluster individuals with similar characteristics based on their scores. The Fuzzy C-means (FCM) clustering algorithm was employed for clustering analysis.
In the FCM method, the belonging of observations to the cluster is determined by fuzzy logic instead of classical logic. According to classical logic, observations either belong to a cluster or they do not. However, according to fuzzy logic, the membership degree of observations is calculated separately for each cluster. For whichever cluster the observation has the highest membership, it is assigned to that cluster. The general use of the method is with the Fanny algorithm developed by Kaufman and Rousseuw11. Here, memberships are non-negative (Eq. 2) and the sum of the membership degrees of the observations to the clusters is 1 (Eq. 3). Accordingly, the objective function and constraints of the algorithm are given as follows (Eq. 1).
| 1 |
| 2 |
| 3 |
It is desired that the objective Eq. 1 is minimum. For this purpose, the objective function is minimized with an iterative algorithm and as a result, the coefficients matrix is obtained. Thus, the probabilities of each observation belonging to clusters are calculated. After this stage, there is an important problem encountered, which is to decide the number of clusters. In order to overcome this problem, the Elbow method and various cluster validity indices have been proposed in the literature. The Elbow method is defined as a graph formed by plotting the percentage of variance explained by clusters against the number of relevant clusters12. Silhouette statistics can also be used to determine how reliable or valid the clustering is. In this technique, the distances within and between clusters of observations are compared. In order for the cluster structure to be considered appropriate, the Silhouette statistics value is expected to be 0.50.
After applying FCM to the data, it was determined that the optimal number of clusters as k = 3 according to the Elbow method. The within-cluster sum of squares shows the highest numerical decrease between k = 2 and k = 3. It can be seen that for cluster numbers after k = 3, the within-cluster sum of squares tend to change slowly. As a result of FCM analysis, the Silhouette statistics is also obtained as 0.504835 for k = 3. Moreover, the observations are distributed as 39 in the 1st cluster, 59 in the 2nd cluster and 54 in the 3rd cluster.
In the third phase of the study, the classification success of clinical parameters in terms of the characteristics of the generated clusters was evaluated using the classification and regression trees (CRT) algorithm. CRT is a non-parametric statistical method proposed by Breiman et al.13, suitable for both categorical and numerical variables. The shape of the tree created depends on the algorithm used in the decision tree, leading to different classification results with different tree structures. The method employs a tree diagram, starting from the root node and progressing downwards through sequential nodes until reaching a leaf. The root node is the main node, encompassing all observations related to the relevant independent variable, while intermediate nodes are decision nodes and are recursively split based on the test result of the relevant variable. Terminal nodes represent leaf nodes. Branching criteria were based on entropy criteria14.
In the final stage, to evaluate the classification success, ROC (Receiver Operating Characteristic) analysis was conducted to assess the performance of the predicted probability values obtained from the decision tree in distinguishing clusters. For classification performance evaluation, the area under the ROC curve (AUC) was calculated. An AUC value greater than 0.70 was determined as an indicator of success. Confidence intervals (CI) for the AUC values, with both lower and upper bounds at 95%, were provided.
Cluster analysis and decision tree were applied to data. A significance level of 0.05 was chosen for statistical analysis. SPSS (version 28.0, Armonk, NY IBM Corp.) software package and R program (version 4.2.3) were used for calculations.The packages required to perform this analysis in the R program are: library(cluster), library(fclust), library(factoextra), library(ppclust), library(haven).
Results
Descriptives for the sample
The majority of our patient cohort exhibited lower levels of education, with 48% (n = 73) having completed primary school and 16.4% (n = 25) having completed secondary school. Moreover, a predominant proportion of patients (72.4%, n = 110) reported no family history of diabetes, and the average duration of diabetes was 6.29 years (SD = 6.82). Additional concurrent conditions included hypertension in 46.1% (n = 70), hyperlipidemia in 51.3% (n = 78), and thyroid disorders in 11.8% (n = 18). The mean MoCA score of our patients was 22.07 (SD = 4.171). This indicates that 67.7% (n = 103) of the patients with T2DM in our study demonstrated normal cognitive status, while 32.3% (n = 49) exhibited impaired cognitive function.
Fuzzy clustering procedure
During clustering analysis, our initial aim was to group individuals with similar characteristics based on MoCA scores, placing those with similar sub-dimension scores in the same clusters and those with different characteristics into separate clusters according to their MoCA scores. The goal was to group individuals into similar clusters based solely on MoCA sub-dimension scores.
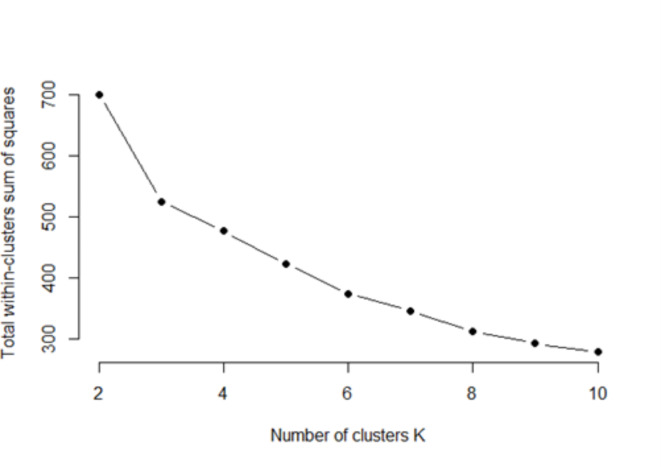
In the study, Elbow methos used for detect optimum cluster size. In the Elbow method, it is necessary to observe how the percentage of explained variance changes with different numbers of clusters to determine the appropriate number of clusters. As shown in the Fig. 1, the total within-cluster sum of squares tends to gradually change after k = 3. Therefore, according to the Elbow method, k = 3 is a good candidate for the optimal number of clusters. Here, since the total within-cluster sum of squares decreases from 700.172 to 525.011 when the number of clusters is 3, this breaking point provides the most optimal number of clusters .
Fig. 1.
Elbow method results.
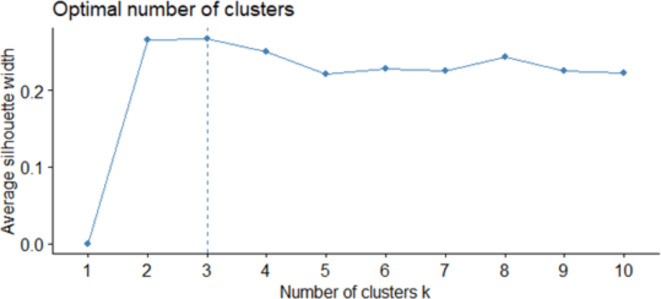
Additionally, Silhouette statistics are provided for all clusters. We have provided Silhouette statistics for a wider range of cluster numbers. The point with the highest Silhouette statistic value indicates the optimal number of clusters. The results for Silhouette statistic value was 0.4824 for k = 2; 0.5048 for k = 3; 0.4146 for k = 4; 0.4157 for k = 5; 0.4493 for k = 6; 0.4571 for k = 7; 0.4605 for k = 8; 0.4567 for k = 9; 0.4577 for k = 10. (Fig. 2). Based on both methods, the conclusion is that the optimal number of clusters is three.
Fig. 2.
Silhouett statistics of results.
The optimal solution automatically selected by the system was the three-cluster grouping, which achieved the highest measure of cohesion/separation (Silhouette = 0.50). The Silhouette index was in the fair range, with evidence of an adequate cluster structure in this subsample. To decide on the appropriate number of clusters, it is necessary to observe how the percentage of explained variance changes with different numbers of clusters. This method is called the elbow method. According to the resulting event structure, after k = 3, the total within-cluster sum of squares shows a slow change trend. Therefore, k = 3 is a good candidate for the optimal number of clusters according to the elbow method.
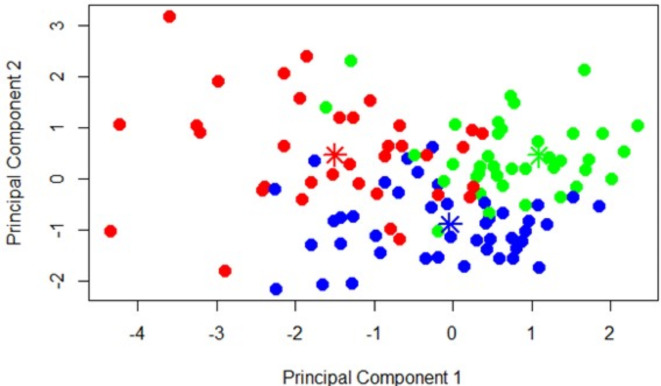
Cluster 1 (identified as “severe non-amnestic”) included n = 39 patients (25.7%), Cluster 2 (identified as “mild non-amnestic”) included n = 59 (38.8%), and Cluster 3 (denominated as “amnestic” included n = 54 (35.5%). Figure 3 was obtained to visualize the separation of three clusters in a two-dimensional space. In the coloring, observations with the same color belong to the same cluster, while observations colored differently belong to different clusters.
Fig. 3.
Fuzzy c-means clustering result.
Comparison between groups
Table 1 presents a comparison of sociodemographic and clinical characteristics among the groups. There was no significant age difference between the clusters (p > 0.05). Cluster 1, predominantly comprising women, exhibits the lowest level of education and the highest prevalence of hypertension. In contrast, Cluster 2, characterized by the highest patient count, is associated with a shorter duration of T2DM.
Table 1.
Comparison of sociodemographic and clinical variables of fuzzy clusters.
| 1 | 2 | 2 | p | |
|---|---|---|---|---|
| n = 39 | n = 59 | n = 54 | ||
| Age | 53.59 ± 6.73 | 50.37 ± 7.993 | 52.50 ± 10 | 0.159 |
| Gender | < 0.001 | |||
| Female | 31 (79.5)a | 22 (37.3)b | 16 (29.6)b | |
| Male | 8 (20.5)a | 37 (62.7)b | 38 (70.4)b | |
| Education Level | < 0.001 | |||
| Primary | 32 (81)a | 22 (37.9)b | 19 (35.2)b | |
| Secondary | 6 (15.4) | 7 (12.1) | 12 (22.2) | |
| High School | 1 (2.6)a | 18 (31)b | 17 (31.5)b | |
| University | 0a | 11 (19)b | 6 (11.1)a, b | |
| T2DM duration (years) | 6.50 (0-7.49) | 2 (0–21) | 5 (0–30) | 0.016 * |
| Family History of T2DM | 34 (87.2)a | 36 (61)b | 40 (74.1) | 0.017 |
| Disease | ||||
| Hypertension | 29(74.3)a | 21 (35.6)b | 20 (37)b | < 0.001 |
| Thyroid Disorders | 9 (23)a | 6 (10.1)a, b | 3 (5.5)b | 0.028 |
| Hyperlipidemia | 19(48.7) | 31 (52.5) | 28 (51.8) | 0.936 |
Normally distributed continuous variables were presented as mean ± SD, non-normally distributed continuous variables were presented as median (min-max), categorical variables were presented as counts (percentages). For categorical variables, the presence of the same letter in the upper index signifies a lack of significant difference between the categories, whereas a distinct letter denotes a statistically significant difference among the categories. T2DM: Type 2 diabetes mellitus.
*post hoc 1vs2 p = 0.01, 2vs3 p = 0.023.
Table 2 illustrates the comparison of laboratory results among the clusters. Notably, Cluster 1 is distinguished by low levels of iron, hemoglobin, and creatine, while Cluster 3 is characterized by reduced thyroid-stimulating hormone (TSH) levels.
Table 2.
Comparison of demographic and clinical data between fuzzy clusters.
| 1 | 2 | 3 | p | Post hoc | |||
|---|---|---|---|---|---|---|---|
| n = 39 | n = 59 | n = 54 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||
| Fasting Glicose(mg/dL) | 137.99 (94–286) | 141 (87–344) | 137.5 (72–381) | 0.843 | |||
| HbA1c (%) | 7.10 (5.79–10.46) | 7.23 (5.26–13.20) | 7.27 (5.21–11.38) | 0.592 | |||
| Iron (µg/dL) | 83.90 (28–114) | 94 (23–160) | 96.19 (20–208) | 0.006 | 0.014 | 0.002 | |
| ALT (U/L) | 21.00 (11–94) | 28.46 (10–74) | 25 (12–92) | 0.273 | |||
| LDL (mg/dL) | 124 (63-216.7) | 120 (55,9-192) | 152.5 (55.7-389.6) | 0.456 | |||
| TSH (mIU/L) | 2.21(0.64–47.03) | 1.97 (0.52–5.15) | 1.56 (0.52–10.72) | 0.023 | 0.008 | 0.054 | |
| Triglycerides (mg/dL) | 153 (76–455) | 139 (55–722) | 152 (54–591) | 0.396 | |||
| B12 (ng/L) | 355.76 (135–580) | 318 (183–1440) | 361.52 (174–899) | 0.805 | |||
| Folic acide (µg/L) | 7.82 (3.9–14.1) | 8.21 (2.5–17.3) | 7.79 (3.4–11.8) | 0.599 | |||
| 25(OH)D (µg/L) | 19.00 ± 7.95 | 21.31 ± 8.42 | 21.20 ± 10.54 | 0.412 | |||
| Ca (mg/dL) | 9.63 ± 0.38 | 9.636 ± 0.32 | 9.56 ± 0.25 | 0.384 | |||
| Na (mmol/L) | 139 (132–144) | 139 (137–143) | 139.15 (134–143) | 0.635 | |||
| Mg (mg/dL) | 1.84 ± 0.15 | 1.90 ± 0.12 | 1.91 ± 0.17 | 0.058 | |||
| Urea (mg/dL) | 29.99 ± 5.44 | 31.66 ± 6.42 | 32.66 ± 7.10 | 0.245 | |||
| eGFR(mL/min/1.73m2) | 93 (52–112) | 93 (65–112) | 96 (52–118) | 0.856 | |||
| Creatinine (mg/dL) | 0.77 (0.58–1.29) | 0.97 (0.62–1.22) | 0.85 (0.55–1.39) | 0.002 | 0.001 | 0.001 | |
| Neutrophil (10*3/ µL) | 4.28 (2.59-8) | 4.34 (1.89–8.68) | 4.05 (2.23–7.84) | 0.849 | |||
| Lymphocyte (10*3/ µL) | 2.65 (0.81–4.11) | 2.63 (1.05–5.01) | 2.57 (1.56–6.34) | 0.706 | |||
| Hemoglobin (g/dL) | 13.46 ± 1,06 | 14.68 ± 1.57 | 14.65 ± 1.56 | < 0.001 | < 0.001 | < 0.001 | |
| Platelet (10*3/µL) | 277 (166–368) | 257.59 (146–540) | 251.50 (155–391) | 0.076 | |||
| Microalbumin (mg/L) | 20 (3-1312.7) | 20 (1.4–870) | 20 (3-482) | 0.381 | |||
| Microalbumin /Creatine | 17.30(2.45–638.3) | 16.47 (1.4–765) | 45.21 (3.90-361.80) | 0.172 | |||
Normally distributed continuous variables were presented as mean ± SD, non-normally distributed continuous variables were. presented as median (min-max), Categorical variables were presented as counts (percentages). HbA1c: The hemoglobin A1c; ALT: Alanine aminotransferase; LDL: Low-density lipoprotein; TSH: Thyroid stimulating hormone; 25(OH)D: 25-hydroxycholecalciferol; Ca: Calcium; Na: Sodium; Mg: Magnesium; eGFR: estimated glomerular filtration rate.
Table 3 presents a comparison of MoCA total and sub-scores among the clusters. Significant differences were observed in the MoCA total scores, with cluster 1 displaying the most substantial decline in cognitive function. The orientation domain did not exhibit discriminatory power among the clusters. Cluster 3 outperformed Cluster 1 in total and all other subdomains; however, its memory score was significantly lower than that of Cluster 1.Cluster 3 was characterized as an ‘amnestic’ cognitive impairment profile due to its markedly poorer performance in the memory domain, despite achieving more moderate scores in other domains. Given that both Cluster 1 and 2 demonstrated better memory scores, and Cluster 1 displayed poorer performance than Cluster 2 across all cognitive domains, Cluster 1 was categorized as severe non-amnestic, while Cluster 2 was identified as moderate non-amnestic.
Table 3.
Comparison of cognitive scores of fuzzy clusters.
| Cognitive Scores | 1 | 2 | 3 | p | Post hoc | ||
|---|---|---|---|---|---|---|---|
| n = 39 | n = 59 | n = 54 | 1–2 | 1–3 | 2–3 | ||
| MoCA total |
18.08 ± 3.45 (11–24) |
25.44 ± 2.76 (19–30) |
21.26 ± 2.74 (16–26) |
< 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Visuospatial/Executive |
2.87 ± 1.15 3 (0–5) |
3.83 ± 1.177 4 (1–5) |
3.41 ± 0.981 3 (1–5) |
< 0.001 | 0.001 | 0.028 | 0.035 |
| Naming |
2.03 ± 0.58 2 (1–3) |
2.75 ± 0.477 3 (1–3) |
2.61 ± 0.492 3 (2–3) |
< 0.001 | 0.001 | 0.001 | |
| Attention |
2.79 ± 1.12 3 (0–4) |
5.47 ± 0.704 6 (4–6) |
5.26 ± 0.782 5 (3–6) |
< 0.001 | 0.000 | 0.001 | |
| Language |
1.05 ± 1.02 1 (0–3) |
1.93 ± 0.980 2 (0–3) |
1.48 ± 1.023 1 (0–3) |
< 0.001 | 0.001 | 0.058 | 0.023 |
| Abstraction |
1.05 ± 0.72 1 (0–2) |
1.73 ± 0.485 2 (0–2) |
1.35 ± 0.731 1 (0–2) |
< 0.001 | 0.001 | 0.035 | 0.005 |
| Memory |
2.69 ± 1.23 3 (0–5) |
3.81 ± 0.776 4 (3–5) |
1.30 ± 0.861 1 (0–3) |
< 0.001 | 0.001 | < 0.001 | 0.000 |
| Orientation |
5.74 ± 0.59 6 (4–6) |
5.88 ± 0.326 6 (5–6) |
5.83 ± 0.376 6 (5–6) |
0.602 | |||
Scores are given as mean ± SD median (min-max). MoCA: The Montreal Cognitive Assessment.
Decision tree model
The decision tree analysis was used for comparing the clusters with clinical parameters. Decision trees were derived from the significant clinical parameters identified. Therefore, the clustering results obtained from MoCA scores were considered, and the effects of clinical parameters were evaluated using the classification method of decision trees. In conclusion, decision tree results can provide an initial assessment of clinical parameters for predicting patients’ cognitive groups (diagnosis). Future studies could enhance these predictive methods to offer predictions about patients’ cognitive progression processes based on clinical data.
All clinical parameters were subjected to comparison among the three clusters identified through cluster analysis. Only those with p-values exceeding 0.25 were incorporated into the decision tree model. Consequently, the parameters integrated into the model encompassed family history of diabetes, hypertension (HT), iron, magnesium (Mg), thyroid-stimulating hormone (TSH), creatinine, hemoglobin, age, duration of diabetes (DM) in years, thyroid disorders, urea, platelet-lymphocyte ratio (PLR), microalbumin/creatinine, gender, and education level. The decision trees yielded a total of 11 nodes, with six being terminal nodes. The resultant decision tree model is visually depicted in Fig. 4. Notably, education level, hemoglobin, duration of DM, iron, TSH, gender, family history of diabetes, and microalbumin/creatinine ratio were identified as parameters exerting over 50% influence within the decision tree model, respectively. Table 4 illustrates the predictive accuracy of the decision tree model for fuzzy clusters. As depicted, the decision tree algorithm achieves a prediction accuracy of 63.2% for all model and 89.7% for fuzzy cluster 1.
Fig. 4.

Desicion tree of clusters of cognitive profile in diabetes.
Table 4.
Correct percent classification of the fuzzy clusters.
| Observed | Predicted | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Percent Correct | |
| 1 | 35 | 1 | 3 | 89.7% |
| 2 | 10 | 23 | 26 | 39.0% |
| 3 | 6 | 10 | 38 | 70.4% |
| Overall Percentage | 33.6% | 22.4% | 44.1% | 63.2% |
As a result of the decision trees, predicted probability values were obtained for each individual. Afterwards, ROC analysis was performed to evaluate the success of predicted probability values in distinguishing cluster clusters. In ROC analysis, predicted probability values were taken as the test variable and the presence (presence/absence) of cluster 1, 2 and 3 were taken as the state variable and ROC curves were drawn. ROC analyses of the clusters are presented in Fig. 5. The ROC analysis showed that the predicted probability value of cluster 1 obtained as a result of decision tree showed a very high discrimination success for cluster 1. The area under the curve AUC value was 91% (95% CI 0.858–0.960, p < 0.001). Area under the curve AUC values for clusters 2 and 3 were 74% (95% CI 0.655–0.817, p < 0.001) and 75% (95% CI 0.673–0.832, p < 0.001), respectively.
Fig. 5.
ROC curve.
Discussion
This study aims to elucidate the cognitive profiles of patients with T2DM based on MoCA subscores. Utilizing the results of fuzzy clustering analysis, three distinct clusters were identified. All cognitive subdomains, except orientation, exhibited statistically significant discriminatory power among these groups. Cluster 1 exhibited the poorest overall cognitive performance and characterized by a higher proportion of women, lower education levels, and lower levels of iron, hemoglobin, and creatine. Cluster 3, identified as the amnestic cluster, is characterized by more prominent memory impairment and was distinguished by low TSH levels. In the decision tree model, education level, hemoglobin, duration of DM, iron, TSH, gender, family history of diabetes, and microalbumin/creatinine ratio were identified as parameters significantly affecting the distinction of cognitive clusters.
A meta-analysis conducted in patients with T2DM revealed small to moderate performance decrements in every cognitive domain examined, including motor function, executive function, processing speed, verbal memory, visual memory, and attention, when compared to without diabetes controls15. Moreover, the results of a 6-year longitudinal study, which included detailed cognitive screening, indicated that individuals with diabetes performed poorly on an executive function test at baseline. Furthermore, language (phonemic verbal fluency) and executive function showed sharper declines over time in individuals with diabetes compared to those without diabetes. Overall, individuals with diabetes consistently performed worse than those without diabetes16. There is considerable evidence in the literature showing cognitive impairment in diabetes by comparing T2DM patients with healthy subjects. However, findings from various studies regarding the link between T2DM and cognitive subtypes have been inconsistent. Some studies suggest a higher degree of memory impairment17, while others describe a cognitive profile marked by deficits in executive attention and other functions, without amnestic changes18. However, studies investigating the phenotypes of cognitive impairment in patients with T2DM and the underlying clinical correlates are limited. In this study, we focused on this gap and defined 3 different types of cognitive clusters in diabetes. MCI and other cognitive processes, as well as normal aging, are dynamic process with biological heterogeneity19,20. Longitudinal follow-up of patients with MCI has shown heterogeneous cognitive trajectories in terms of cognitive trajectories and progression to AD, ranging from mild changes to aggressive declines, as well as no change in cognitive function21,22. It is important to acknowledge the heterogeneity of the clusters identified in this study’s cross-sectional design, which may vary across different trajectories. Future longitudinal studies tracking cognitive decline in patients with T2DM could make significant contributions to the literature by shedding light on the diverse and complex nature of cognitive changes, revealing different cognitive trajectories over time.
The findings regarding the relationship between T2DM and subtypes of MCI and dementia are not entirely congruent across studies. Moreover, beyond the established association between diabetes and non-amnestic disorders in the literature23, it has been suggested that this relationship may manifest differently across genders and cognitive domains17. In men, diabetes was linked to a heightened risk of amnestic mild cognitive impairment (aMCI) and multi-domain aMCI, with a twofold increase in the risk of multi-domain non-amnestic MCI. Conversely, in women, diabetes exhibited a robust association with single-domain naMCI17. Our findings indicate the presence of distinct profiles of cognitive impairment in individuals with diabetes, with various demographic and clinical factors posing risks for these different types. We observed that the majority of individuals in the amnestic cluster were male, while the majority of individuals in the non-amnestic cluster were female, which supports the findings of Roberts et al. It is hypothesized that T2DM may elevate the risk of amnestic cognitive impairment by inciting pathology linked to AD, as well as non-amnestic MCI through vascular pathology mechanisms2. The association of vascular risk factors such as hypertension with cluster 1-non amnestic impairment-supports the hypothesis that vascular mechanisms underlie the cognitive impairment profile seen in this cluster.
Our findings uncovered a noteworthy association between thyroid function and cognitive performance in individuals with T2DM, with TSH levels notably lower in the cluster exhibiting an amnestic-type impairment. Thyroid dysregulation is significant in metabolic syndromes and neurological disorders due to the involvement of thyroid hormones in various critical processes including regulating cell death responses, modulating neuronal function, neurotransmitter production, glucose metabolism, and lipid metabolism24. While studies have reported associations between thyroid function and MCI as well as AD, the literature concerning the role of thyroid function in cognitive impairment related to diabetes remains limited. Analyzing data from the Human Connectome Project, Santhanam revealed a potential link between elevated serum TSH levels and higher fluid intelligence scores among young women25. Furuto-Kato et al. also reported in a Japanese population that thyroid function was higher (lower TSH and higher FT4) in individuals without cognitive impairment, with the exception of men in their 90s26. However, conflicting results have been reported in studies examining hypothyroidism and its impact on cognitive function27. Patients with hypothyroidism showed altered hippocampal activation, leading to working memory loss, and cerebral blood flow impairment28. Parsaik et al. found no association between clinical or subclinical hypothyroidism and MCI in a population-based cohort of elderly individuals29. Pasqualetti et al. reported that a relationship between hypothyroidism and cognitive impairment only in individuals < 75 years old with elevated TSH concentrations30. Rieben et al. also reported that subclinical hyperthyroidism may be associated with an increased risk of dementia, while subclinical hypothyroidism is not31. Moon et al. showed that a low serum TSH level in the reference range was independently associated with risk of cognitive impairment32. Moreover, consistent with our results, existing literature suggests that the association between thyroid function and cognition may be domain-specific, focusing on areas such as working memory, executive function, or verbal memory33–35. The association between thyroid hormone deficiency and hippocampal changes, such as decreased cell number in the dentate gyrus and abnormal neuronal migration and maturation, leading to memory impairments, has also been documented36. Additionally, Accorroni proposed that reduced levels of thyroid hormone in the central nervous system may elevate the expression of amyloid precursor protein, subsequently resulting in increased levels of Aβ peptide and β-amyloid, potentially predisposing individuals to AD37. Yu et al. showed a negative correlation between MoCA score and TSH in their study in T2DM patients, but subtypes of cognitive impairment were not analyzed38. In support of this, our study found that cluster 1, which exhibited the poorest cognitive status, had a higher mean TSH value. Our study corroborates these findings, indicating that low TSH levels are linked to an amnestic-type cognitive impairment profile among patients with T2DM.
Cross-sectional studies in the literature report that higher A1C levels are associated with lower cognitive function in individuals with diabetes39. The systematic review conducted by Geijselaers et al. observed that there was a negative association between high HbA1c concentration and glucose variability with cognitive function. However, they found that the strength of this association was weak, explaining less than 10% of the cognitive variance40. However, what is noteworthy about our findings is that despite the absence of differences in fasting glucose and HbA1c levels, patients exhibited distinct profiles of deterioration. The development of cognitive impairment in individuals with diabetes is influenced by a variety of interconnected vascular, metabolic and psychosocial factors and may affect cognitive abilities through various pathophysiological pathways41. According to our results, Cluster 1, which performed the non-amnestic severe cognitive impairment, encompassed predominantly female patients, lower education levels, an extended history of diabetes, and a high prevalence of hypertension. The literature has previously highlighted the association of these factors with cognitive impairment41–44. Anemia may be linked to cognitive decline, as low hemoglobin levels can lead to inadequate cerebral oxygenation and reduced aerobic capacity45,46. In support of the literature, Cluster-1 with the most impaired cognitive performance had the lowest hemoglobin levels. Our findings highlight that diabetes is associated with various types of cognitive impairment, each linked to distinct pathophysiological mechanisms related to these factors. In diabetes-related cognitive impairment, where numerous factors interact in a complex manner, our analysis using the decision tree method revealed that variables such as education level, hemoglobin, duration of DM, iron, TSH, gender, family history of diabetes and microalbumin/creatinine ratio were highly discriminating factors in identifying distinct clusters. According to the ROC analysis, the decision tree model was found to be 90% successful especially in predicting cluster 1.
While our study leverages robust statistical analysis methods, its generalizability is limited by the relatively small sample size. The restriction of our patient cohort to individuals aged 30–65 means that the number of patients with dementia in our study is lower than what might be observed in the broader T2DM population. However, expanding the study to include patients over 65, in this retrospectively designed investigation, would introduce a heterogeneous mix of patients, including those with different types of dementia and those whose hyperglycemia may be related to dementia care issues.
In summary, cognitive impairment in diabetes is believed to stem from a multifaceted pathophysiological mechanism influenced by various risk factors. This condition, increasingly recognized as a significant complication of diabetes, manifests in different types of cognitive impairments. Future studies that focus on the distinct types of cognitive impairment in diabetes, as identified by clustering analysis, could provide valuable insights into the underlying pathophysiology. By studying these subtypes rather than heterogeneous populations, researchers may uncover more targeted treatment approaches and strategies for earlier diagnosis. This approach has the potential to improve outcomes for patients with T2DM and cognitive impairment.
Acknowledgements
The authors would like to express their sincere gratitude to Bezmialem Vakıf University Hospital for their invaluable support and contribution in sharing data for this research.
Author contributions
BSŞ: conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, Final approval of the version to be publishedOP: statistical analysis and interpretation of data, Final approval of the version to be publishedDE: acquisition of dataSG: statistical analysisAS: conception and design, acquisition of data, Final approval of the version to be published.
Funding
None.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koekkoek, P. S., Kappelle, L. J., van den Berg, E., Rutten, G. E. & Biessels, G. J. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 14 (3), 329–340. 10.1016/s1474-4422(14)70249-2 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Luchsinger, J. A., Cabral, R., Eimicke, J. P., Manly, J. J. & Teresi, J. Glycemia, diabetes status, and cognition in hispanic adults aged 55–64 years. Psychosom. Med. 77 (6), 653–663. 10.1097/psy.0000000000000208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, X., Jiang, X., Han, S., Liu, Q. & Zhou, J. Type 2 diabetes Mellitus is Associated with the risk of cognitive impairment: a Meta-analysis. J. Mol. Neuroscience: MN 68(2), 251–260. 10.1007/s12031-019-01290-3 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Biessels, G. J. & Whitmer, R. A. Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia 63(1), 3–9. 10.1007/s00125-019-04977-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abner, E. L. et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s Dementia: J. Alzheimer’s Assoc. 12(8), 882–889. 10.1016/j.jalz.2015.12.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dos Santos Matioli, M. N. P. et al. Diabetes is not Associated with Alzheimer’s Disease Neuropathology. J. Alzheimer’s Dis: JAD 60(3), 1035–1043. 10.3233/jad-170179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luitse, M. J., Biessels, G. J., Rutten, G. E. & Kappelle, L. J. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 11(3), 261–271. 10.1016/s1474-4422(12)70005-4 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x (2005). [DOI] [PubMed] [Google Scholar]
- 9.Selekler, K., Cangöz, B. A., & Sait, U. L. Power of discrimination of Montreal cognitive assessment (MoCA) scale in Turkish patients with mild cognitive impairment and Alzheimer’s disease. Turk. J. Geriatr. 13(3) (2010).
- 10.Ozdilek, B. & Kenangil, G. Validation of the Turkish version of the Montreal Cognitive Assessment Scale (MoCA-TR) in patients with Parkinson’s disease. Clin. Neuropsychol. 28 (2), 333–343. 10.1080/13854046.2014.881554 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Kaufman, L. & Rousseeuw, P. Finding groups in data: An Introduction To Cluster Analysis. (1990).
- 12.Bholowalia, P. & Kumar, A. J. I. EBK-Means: A Clustering Technique based on Elbow Method and K-Means in WSN. J. C. A 105, 17–24 (2014). [Google Scholar]
- 13.Breiman, L., Friedman, J. H., Olshen, R. A. & Stone, C. J. J. B. Classification and Regression Trees. 40, 874 (1984).
- 14.Akman, M. Veri madenciliğine Genel bakış ve Random Forests yönteminin Incelenmesi: sağlık alanında bir Uygulama (Ankara University, 2010).
- 15.Palta, P., Schneider, A. L., Biessels, G. J., Touradji, P. & Hill-Briggs, F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. 20(3), 278–291. 10.1017/s1355617713001483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palta, P. et al. Diabetes and cognitive decline in older adults: the Ginkgo evaluation of memory study. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 12(1), 123–130. 10.1093/gerona/glx076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, R. O. et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimer’s Dementia: J. Alzheimer’s Assoc. 10(1), 18–26. 10.1016/j.jalz.2013.01.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung, S. E., Fischer, A. L. & Dixon, R. A. Exploring effects of type 2 diabetes on cognitive functioning in older adults. Neuropsychology 23(1), 1–9. 10.1037/a0013849 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nettiksimmons, J., DeCarli, C., Landau, S. & Beckett, L. Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimer’s Dementia: J. Alzheimer’s Assoc. 10(5), 511-521e1. 10.1016/j.jalz.2013.09.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu, Z., Phyo, A. Z. Z., Al-Harbi, T., Woods, R. L. & Ryan, J. Distinct cognitive trajectories in Late Life and Associated predictors and outcomes: a systematic review. J. Alzheimer’s Dis. Rep. 24(1), 459–478. 10.3233/adr-200232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, X., Ye, T., Zhou, W. & Zhang, J. Uncovering heterogeneous cognitive trajectories in mild cognitive impairment: a data-driven approach. Alzheimer’s Res. Ther. 20(1), 57. 10.1186/s13195-023-01205-w (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang, Q. et al. Signatures of neuropsychological test results in the Long Life Family Study: a cluster analysis. J. Alzheimer’s Dis: JAD 93(4), 1457–1469. 10.3233/jad-221025 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenza, S. et al. Mild cognitive impairment subtypes and type 2 diabetes in Elderly subjects. J. Clin. Med. 30 (7). 10.3390/jcm9072055 (2020). [DOI] [PMC free article] [PubMed]
- 24.Kim, H. K. & Song, J. Hypothyroidism and diabetes-related dementia: focused on neuronal dysfunction, insulin resistance, and Dyslipidemia. Int. J. Mol. Sci. 10 (6). 10.3390/ijms23062982 (2022). [DOI] [PMC free article] [PubMed]
- 25.Santhanam, P., Nath, T., Lindquist, M. A. & Cooper, D. S. Relationship between TSH levels and cognition in the Young Adult: an analysis of the human Connectome Project Data. J. Clin. Endocrinol. Metab. 16(7), 1897–1905. 10.1210/clinem/dgac189 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Furuto-Kato, S. et al. Relationship between the thyroid function and cognitive impairment in the Elderly in Japan. . Intern. Med. (Tokyo, Japan) 15(20), 3029–3036. 10.2169/internalmedicine.9034-21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie, M. & Yeap, B. B. Thyroid hormone: influences on mood and cognition in adults. Maturitas 81(2), 266–275. 10.1016/j.maturitas.2015.03.016 (2015). [DOI] [PubMed] [Google Scholar]
- 28.He, X.-S. et al. Functional magnetic resource imaging assessment of altered brain function in hypothyroidism during working memory processing. Eur. J. Endocrinol. 164(6), 951–959. 10.1530/EJE-11-0046 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Parsaik, A. K. et al. Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA Neurol. 71(2), 201–207. 10.1001/jamaneurol.2013.5402 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasqualetti, G., Pagano, G., Rengo, G., Ferrara, N. & Monzani, F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J. Clin. Endocrinol. Metabol. 100(11), 4240–4248. 10.1210/jc.2015-2046 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Rieben, C. et al. Subclinical thyroid dysfunction and the risk of Cognitive decline: a Meta-analysis of prospective cohort studies. J. Clin. Endocrinol. Metabol. 101(12), 4945–4954. 10.1210/jc.2016-2129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon, J. H. et al. Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean longitudinal study on Health and Aging (KLoSHA). J. Clin. Endocrinol. Metabol. 99(2), 424–432. 10.1210/jc.2013-3385 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Wekking, E. M. et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur. J. Endocrinol. 153(6), 747–753. 10.1530/eje.1.02025 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Schraml, F. V., Goslar, P. W., Baxter, L. & Beason-Held, L. L. Thyroid stimulating hormone and cognition during severe, transient hypothyroidism. Neuroendocrinol. Lett. 32(3), 279–285 (2011). [PMC free article] [PubMed] [Google Scholar]
- 35.Szlejf, C. et al. Thyrotropin level and cognitive performance: baseline results from the ELSA-Brasil Study. Psychoneuroendocrinology 87, 152–158. 10.1016/j.psyneuen.2017.10.017 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Bavarsad, K., Hosseini, M., Hadjzadeh, M. A. & Sahebkar, A. The effects of thyroid hormones on memory impairment and Alzheimer’s disease. J. Cell. Physiol. 234(9), 14633–14640. 10.1002/jcp.28198 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Accorroni, A. et al. Thyroid hormone levels in the cerebrospinal fluid correlate with disease severity in euthyroid patients with Alzheimer’s disease. Endocrine 55(3), 981–984. 10.1007/s12020-016-0897-6 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Yu, Z. W. et al. Impaired sensitivity to thyroid hormones is Associated with mild cognitive impairment in Euthyroid patients with type 2 diabetes. Clin. Interv. Aging. 18, 1263–1274. 10.2147/cia.S413584 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cukierman-Yaffe, T. et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32(2), 221–226. 10.2337/dc08-1153 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geijselaers, S. L. C., Sep, S. J. S., Stehouwer, C. D. A. & Biessels, G. J. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 3(1), 75–89. 10.1016/s2213-8587(14)70148-2 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Feinkohl, I., Price, J. F., Strachan, M. W. & Frier, B. M. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res. Ther. 7 (1), 46. 10.1186/s13195-015-0130-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, R.-H. et al. Risk factors of mild cognitive impairment in middle aged patients with type 2 diabetes: A cross–section study. Ann. d’Endocrinol. 73(3), 208–212. 10.1016/j.ando.2012.04.009 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Bruce, D. G. et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia 51(2), 241–248. 10.1007/s00125-007-0894-7 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Kloppenborg, R. P., van den Berg, E., Kappelle, L. J. & Biessels, G. J. Diabetes and other vascular risk factors for dementia: Which factor matters most? A systematic review. Eur. J. Pharmacol. 585(1), 97–108. 10.1016/j.ejphar.2008.02.049 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Qin, T. et al. Association between anemia and cognitive decline among Chinese middle-aged and elderly: evidence from the China health and retirement longitudinal study. BMC Geriatr. 19, 305. 10.1186/s12877-019-1308-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottesman, R. F. et al. Patterns of regional cerebral blood flow associated with low hemoglobin in the baltimore longitudinal study of aging. J. Gerontol. Ser A 67(9), 963–969. 10.1093/gerona/gls121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.