Abstract
Patients with chronic kidney disease (CKD) are at a high risk of cardiovascular (CV) complications. In these patients, sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce CV events. Mineralocorticoid receptor antagonists (MRAs) exert similar benefits in diabetic CKD, though their effects in non-diabetic CKD remain unclear. This study aimed to evaluated whether the combination of Dapagliflozin (DAPA) and Eplerenone (EPLE) would have positive effects on cardiorenal functions in a non-diabetic CKD model. CKD was induced in rats via 5/6 nephrectomy, followed by treatment with DAPA (5 mg/kg/day PO), EPLE (100 mg/kg/day PO) or the combination for 3 months following CKD induction. Cardiorenal functions were assessed after the treatment period. All treated groups showed reduced kidney fibrosis though plasma creatinine and urea levels remained unchanged. Compared to untreated CKD, EPLE or DAPA/EPLE reduced left ventricle (LV) end-diastolic pressure and LV end-diastolic pressure volume relationship, whereas DAPA alone did not achieve significant reductions. Compared to untreated CKD, EPLE and DAPA/EPLE improved cardiac perfusion but DAPA alone did not. Cardiac fibrosis in CKD was blunted by either DAPA or EPLE alone, with the combination showing an additive effect. In conclusion, co-treatment with DAPA and EPLE enhances diastolic function, cardiac perfusion and reduces myocardial fibrosis in non-diabetic CKD rats.
Keywords: Cardiorenal, Chronic kidney disease, Mineralocorticoid receptor antagonist, Sodium-glucose cotransporter-2 inhibitor, 5/6 nephrectomy
Subject terms: Circulation, Kidney, Cardiology, Nephrology
Introduction
Cardiovascular (CV) and chronic kidney disease (CKD) are both on the rise and pose significant public health challenge. These two pathologies evolve slowly, with a progressive decline in cardiac and renal function over time and are closely interrelated, with the deterioration of one function leading to deterioration of the other. Both CV and CKD share similar risk factors, including fluid overload, diabetes and atherosclerosis. Moreover, they mutually exacerbate one another: heart failure can cause renal dysfunction through reduced renal perfusion, while kidney failure can contribute to heart failure through the accumulation of uremic toxins and increased afterload1–3. Notably, cardiovascular events account for 50% of deaths in patients with CKD4, underscoring the urgent need for treatments that can slow the progression of CKD and prevent its cardiovascular complications.
Hypertension and diabetes are the leading causes of CKD. A significant advancement in treatment emerged with the introduction of sodium-glucose cotransporter-2 inhibitors (SGLT2i), which were originally designed as hypoglycemic agents. These drugs have been shown to improve cardiovascular and renal outcomes when combined with standard care using renin-angiotensin-system inhibitors (RASi), in both diabetic and non-diabetic patients, as demonstrated in clinical trials like EMPEROR, DAPA-HF, and DAPA-CKD. SGLT2is, such as empagliflozin (EMPA), canagliflozin, and dapagliflozin (DAPA), have been shown to reduce hospitalizations for cardiovascular events5–7. DAPA and other SGLT2is have been recently approved in the United States and Europe for the treatment of chronic kidney disease (CKD) for patients with and without type 2 diabetes8.
Another therapeutic approach involves the use of mineralocorticoid receptor (MR) antagonists, as activation of the MR is involved in inflammation and fibrosis, accelerating the progression of CKD9,10. Clinical trials, such as FIGARO-DKD and FIDELIO-CKD, have recently demonstrated that the non-steroidal MR antagonist (MRA) finerenone can slow kidney failure progression and reduce the incidence of cardiovascular events in diabetic patients compared to placebo11,12. However, the use of MRAs in CKD patients has been limited due to the increased risk of hyperkalemia. Interestingly, finerenone a new MRA showed a low risk of hyperkalemia in CKD patients with diabetes in these trials12,13. The potential benefits of combining an SGLT2i with an MRA remain unknown. However, the ROTATE-3 crossover trial suggested that short-term co-treatment with an SGLT2i (DAPA) and an MRA (eplerenone; EPLE) could reduce albuminuria more effectively than either treatment alone, while also lowering the risk of MRA-induced hyperkalemia14. These findings suggest a potential synergistic effect of the co-treatments with both SGLT2i and MRA.
This study aimed to compare the effects of SGLT2i and MRA both individually or in combination, on cardiorenal function in a rat model of non-diabetic CKD.
Materials and methods
The data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Experimental design
Experiments were approved by the Darwin ethics committee of Sorbonne University (#22206–2019110515362968 v4), conducted according to the INSERM animal care and are in compliance with the DIRECTIVE 2010/63/EU of the European Parliament. Animals were housed in a climate-controlled facility with a 12-h light/12-h dark cycle with free access to water and food.
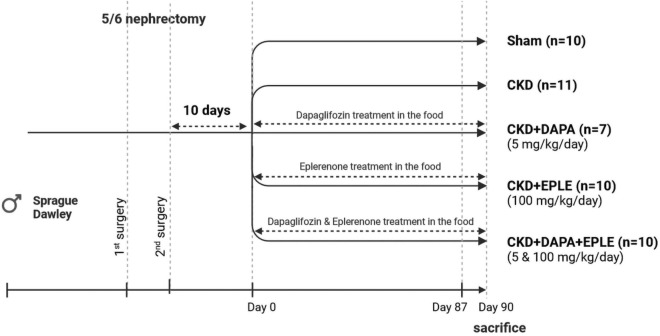
CKD was induced by subtotal nephrectomy in 10-week-old Sprague Dawley rats purchased from Charles River. The procedure consisted of 5/6 subtotal nephrectomy by removing 2/3 of the right kidney during a first surgery followed 10 days later by the removal of the left kidney in a second surgery. For the Sham group, sham surgery was carried out on anesthetized rats in which the left and right kidneys were exposed for the same time as that of the rats with subtotal nephrectomy. Ten days after the second surgery plasmatic creatinine and urea were measured to assess renal dysfunction. Rats where then randomized into four groups with homogeneous renal dysfunction (as assessed by creatinine levels) before starting treatments. The CKD groups were as follows: untreated, treated with DAPA (5 mg/kg/day), EPLE (100 mg/kg/day), or both DAPA and EPLE (5 and 100 mg/kg/day, respectively). The treatments were mixed in the food of the animals and given for three months starting 10 days after the last surgery (Fig. 1).
Fig. 1.
Schematic presentation of experimental design.
Echocardiography
Echocardiography was performed one week before the end of the three-month treatment period. Rats were anesthetized with isoflurane (ISO-VET®, 2.5% for induction, 1.5% for maintenance) and the echocardiography performed using a Vivid 7 ultrasound echograph equipped with an M12L probe, as described previously15. Briefly, a two-dimensional short-axis view of the LV was obtained at the level of the papillary muscles to record M-mode tracings. LV diameters were measured by the American Society of Echocardiology method as previously described16. In addition, LV outflow velocity was measured by pulsed-wave Doppler and cardiac output, calculated as CO = aortic VTI (velocity time interval) × [π × (LV outflow diameter/2)2] × heart rate. Echocardiography was done in blinded manner and the same day for all animals.
Left ventricular hemodynamics and blood pressure
Left ventricular hemodynamics were assessed 3 months and 10 days after the last surgery before euthanasia using LV pressure volume curves, as previously described15. Animals were anesthetized with methohexital (40 mg/kg) and a conductance-micromanometer catheter (model SPR-819, Millar Instruments) connected to a pressure-conductance unit and advanced in the retrograde direction via the carotid artery into the LV. Systolic and diastolic blood pressure were measured in the carotid before advancing the catheter in the LV. LV pressure–volume loops were recorded at baseline and during loading by gently occluding the abdominal aorta with a cotton swab. Data were stored and analyzed using Millar conductance data acquisition/analysis software. The following parameters were measured/calculated from the pressure–volume curves: LV end-systolic and end-diastolic pressures (LVESP, LVEDP), LV end-systolic pressure–volume relationship and end-diastolic pressure–volume relationship (LVESPVR, LVEDPVR), and the LV relaxation constant Tau.
Myocardial perfusion
The last week before the end of the protocol myocardial perfusion was measured in methohexital (40 mg/kg) anesthetized animals using a Bruker Biospec 4.7 Tesla MRI. T1 sequence acquisition was done without contrast agent, as previously described17,18.
Histology
The heart and kidneys were harvested at the end of the study. Sirius red staining was used to determine interstitial fibrosis, as previously described19. Renal and LV fibrosis was calculated as the percentage of the area containing collagen to the total area of the image of the staining. Masson’s trichrome staining of paraffin-embedded kidney sections was used for the analysis of glomerular and tubular injuries. Ten images per sections were obtained under a microscope (Zeiss) at 20 × magnification. (ZEN 3.1—Zeiss software). A minimum of 20 glomeruli (range: 20–30) in each specimen glomerular and tubular injury was graded from 0 to 4 related to morphological alteration observed in the structure, as previously described20. All analyses were blinded.
Biochemical studies
Twenty-four-hour urine collection was performed for all studied groups 10 weeks after the last surgery using metabolic cages. Plasma urea and creatinine levels, as well as urinary creatinine levels, were determined using an automatic analyzer (Konelab 20i; Thermo Fisher Scientific, Vernon Hills, IL, United States). Albuminuria has been measured using an Albumin ELISA Kit at the end of the protocol (Crystal Chem, Elk Grove Village, IL, United States #80630).
Statistical analysis
The results are presented as the mean values ± SEM. All analyses were performed using GraphPad Prism 8.0.2 (GraphPad Software, La Jolla, CA). (1) To determine the alteration in the CKD rats a two-tailed unpaired Student’s t-test was done (Sham vs CKD). (2) Then, the effects of treatments with DAPA and EPLE alone or in combination compared to CKD were analyzed by two-tailed unpaired Student’s t-test (CKD vs CKD + DAPA, CKD vs CKD + EPLE and CKV vs CKD + DAPA + EPLE). (3) Lastly to determine a differential effect between treatments, all treated CKD groups were compared by one way ANOVA. The normality and homogeneity of variances were verified by Shapiro–Wilk and Bartlett tests. If distribution was not normal, analyses were done using nonparametric Kruskal–Wallis test instead of ANOVA or Mann Whitney test instead of Student’s t-test. Results were considered significant for p < 0.05.
Results
Kidney function parameters
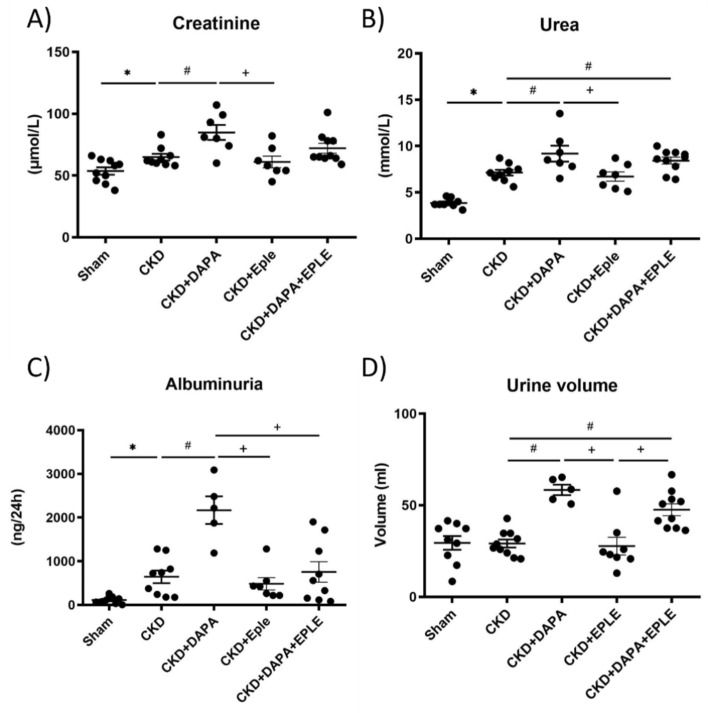
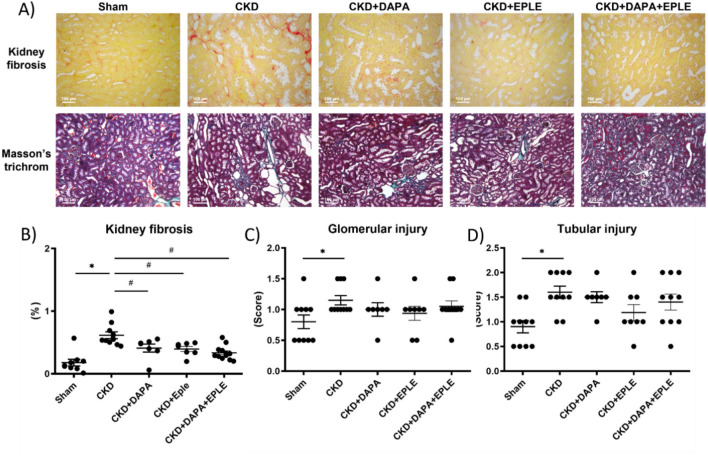
Three months after the last surgery, CKD rats exhibited significantly higher plasma creatinine and urea levels compared to Sham animals (Fig. 2A–B). Among the CKD rats, those treated with DAPA alone showed higher plasma creatinine levels than untreated CKD rats, while treatment with EPLE alone did not result in elevated creatinine. (Fig. 2A–B). Additionally, 24-h albuminuria was elevated in the CKD group relative to Sham (Fig. 2C). Of note, 24 h-albuminuria and 24-h urinary volume were elevated in CKD rats treated with DAPA but not with EPLE. DAPA-EPLE combination increased urine volume without increasing creatinuria and albuminuria (Fig. 2C–D). Similar results were observed for Urinary Albumin Creatinine Ratio (UACR) when albuminuria was normalized to urinary creatinine, with an increase of UACR in CKD versus Sham rats and a further increase in the DAPA group but not in EPLE alone or in the DAPA-EPLE combination (supplementary Fig. S1). Kidney fibrosis was more pronounced in CKD rats than in Sham rats (Fig. 3A). Treatment with DAPA, EPLE, or their combination significantly reduced kidney fibrosis compared to untreated-CKD rats, though no significant differences were noted between treated groups. Renal glomerular and tubular structures were impaired in CKD rats, with no substantial improvement observed following treatment with DAPA, EPLE or their combination (Fig. 3A,C–D).
Fig. 2.
Kidney function parameters. Plasma Creatinine (A) and Urea (B) levels. 24 h Albuminuria (C) and Urine volume (D). Unpaired Student’s t-test or one way ANOVA was used for statistical analysis, mean ± SEM, n = 5–10. * p < 0.05 Sham vs. CKD; # p < 0.05 CKD vs. DAPA/EPLE; + p < 0.05 DAPA + EPLE vs. DAPA/EPLE or DAPA vs. EPLE.
Fig. 3.
Combination of DAPA-EPLE treatment improved kidney fibrosis but not kidney structure. Representative pictures (20 × magnification) of rat kidney sections stained with Sirius red (upper A panel) and Masson’s trichrome (lower A panel). Quantification of Kidney fibrosis (B), Glomerular injury (C) and Tubular injury (D). Unpaired Student’s t-test or one way ANOVA was used for statistical analysis, mean ± SEM, n = 7–10. * p < 0.05 Sham vs. CKD; # p < 0.001 CKD vs. EPLE.
Cardiac function parameters
Three months after CKD induction, we assessed various cardiac function parameters. There were no significant differences between Sham and CKD rats in left ventricular weight, systolic and diastolic blood pressure, fractional shortening, cardiac output, stroke volume, left ventricular end-diastolic diameter (LVEDD), or left ventricular end-systolic diameter (LVESD) (Table 1). Fractional shortening was increased only in the EPLE-treated group, with no significant changes were observed in the DAPA or DAPA + EPLE groups. Other echocardiographic parameters remained unaffected by any treatment (Table 1).
Table 1.
Cardiovascular parameters.
| Group | Sham | CKD | CKD + DAPA | CKD + EPLE | CKD + DAPA + EPLE |
|---|---|---|---|---|---|
| LVEDD (mm) | 8.9 ± 1.2 | 8.7 ± 0.3 | 8.1 ± 0.2 | 8.8 ± 0.4 | 8.1 ± 0.2 |
| LVESD (mm) | 4.7 ± 0.2 | 4.5 ± 0.1 | 4.3 ± 0.2 | 4.1 ± 0.3 | 4.3 ± 0.2 |
| Fractional shortening (%) | 47.7 ± 1.4 | 47.7 ± 1.3 | 47.8 ± 2.2 | 54.1 ± 1.7# | 47.3 ± 2.3 |
| Stroke volume (ml) | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.35 ± 0.02 | 0.34 ± 0.02 | 0.35 ± 0.01 |
| Cardiac output (ml/min) | 126 ± 4 | 128 ± 5 | 126 ± 8 | 122 ± 6 | 125 ± 5 |
| Systolic blood pressure (mmHg) | 148.6 ± 4.4 | 154.5 ± 5.3 | 141.5 ± 7.1 | 141.0 ± 6.3 | 134.2 ± 3.7 |
| Diastolic blood pressure (mmHg) | 116.3 ± 4.0 | 115.3 ± 4.7 | 108.7 ± 6.1 | 109.2 ± 4.9 | 101.7 ± 4.0 |
| LV weight (g) | 1.07 ± 0.03 | 1.13 ± 0.06 | 1.10 ± 0.10 | 1.05 ± 0.04 | 1.03 ± 0.03 |
Left ventricular (LV) end diastolic diameter (LVEDD), LV end systolic diameter (LVESD), LV fractional shortening, Stroke volume and cardiac output were assessed after 90 days of treatment. Systolic blood pressure, diastolic blood pressure and LV weight. One way ANOVA was used for statistical analysis, mean ± SEM, n = 9–10. #p < 0.05 vs. CKD.
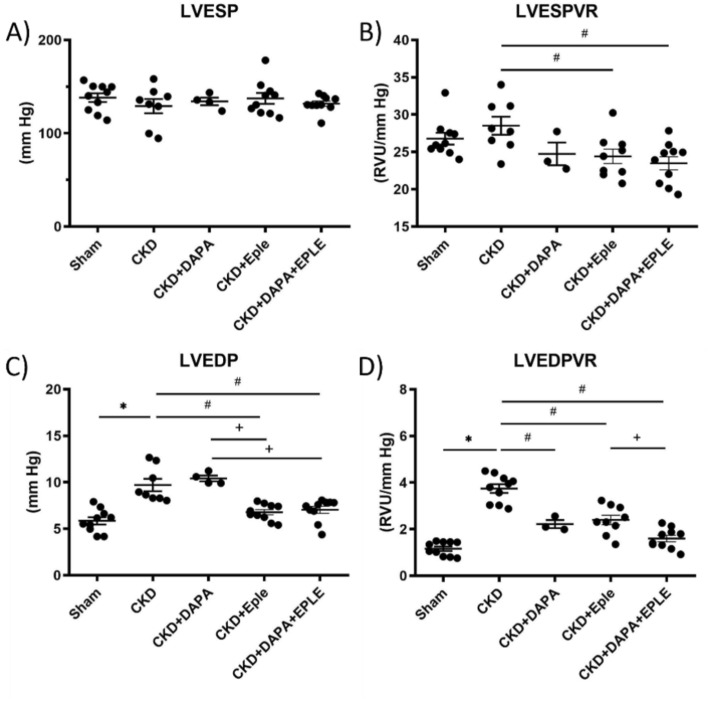
Hemodynamic studies showed that LV end systolic pressure (LVESP) is not modified by CKD or by, DAPA, EPLE, or their combination (Fig. 4A). However, the left ventricular end-systolic pressure–volume relationship (LVESPVR) was significantly lower in the CKD groups treated with EPLE or the DAPA-EPLE combination compared to the untreated CKD group, while DAPA alone had no significant effect (Fig. 4B). Diastolic hemodynamic parameters were modified in the CKD rats, with a higher LV end diastolic pressure (LVEDP) and LV end diastolic pressure–volume relationship (LVEDPVR) which are indices of filling pressure and LV compliance, respectively compared to Sham rats (Fig. 4C, D). DAPA treatment did not modify the increased LVEDP but mitigated the elevated LVEDPVR compared to the untreated CKD group. In contrast, EPLE treatment significantly improved both LVEDP and LVEDPVR (Fig. 4C, D) compared to the untreated CKD group. Treatment with the DAPA-EPLE combination improved LVEDP and LVEDPVR when compared to either treatment alone. Therefore, treatment with the DAPA-EPLE combination was more effective at improving cardiac filling pressure or cardiac compliance compared to CKD rats treated by DAPA or EPLE alone (Fig. 4C–D).
Fig. 4.
Hemodynamics parameters in CKD rats were improved by DAPA-EPLE combination. Left ventricular (LV) end systolic pressure (A), LV end systolic pressure volume relationship (B), LV end diastolic pressure (C). LV end diastolic pressure volume relationship (D). Unpaired Student’s t-test or one way ANOVA was used for statistical analysis, mean ± SEM, n = 3–10. * p < 0.05 Sham vs. CKD; # p < 0.05 CKD vs. DAPA/EPLE; + p < 0.05 DAPA + EPLE vs. DAPA/EPLE or DAPA vs. EPLE.
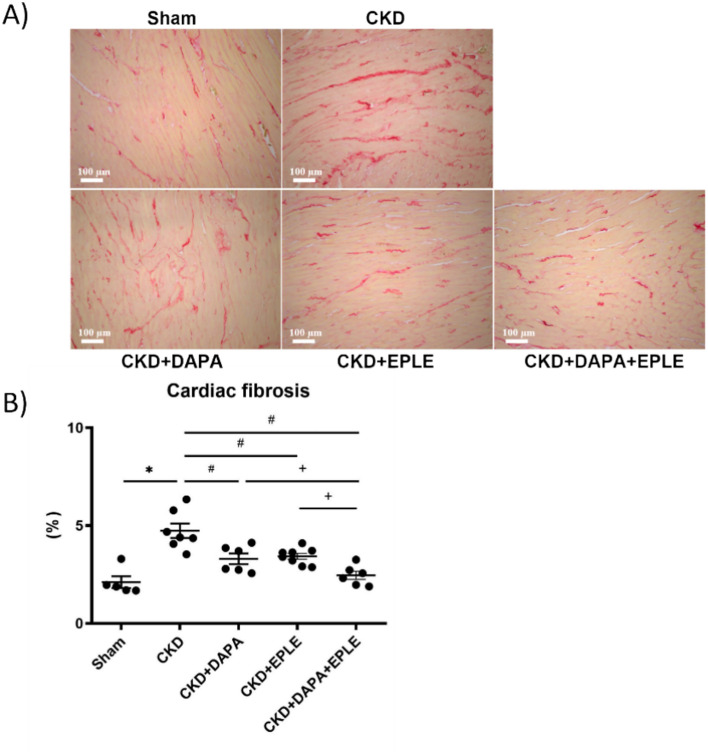
Interstitial cardiac fibrosis
Interstitial cardiac fibrosis, revealed by Sirius red staining, was higher in CKD rats compared to Sham rats (Fig. 5A–B). Treatment of CKD rats with either DAPA or EPLE alone effectively reduced the extent of cardiac fibrosis compared to the untreated CKD rats, while the combination therapy provided additional benefits beyond those achieved by each treatment alone (Fig. 5A–B).
Fig. 5.
Cardiac fibrosis is blunted by DAPA-EPLE combination. Representative pictures (20 × magnification) of rat cardiac sections stained with Sirius red (A). Quantification of cardiac fibrosis (B). Unpaired Student’s t-test or one way ANOVA was used for statistical analysis, mean ± SEM, n = 5–10 *: * p < 0.05 Sham vs. CKD; # p < 0.05 CKD vs. DAPA/EPLE; + p < 0.05 DAPA + EPLE vs. DAPA/EPLE.
Cardiac perfusion
Cardiac perfusion, which plays a crucial role in diastolic function and cardiovascular health, was not significantly modified in the CKD rats relative to Sham (Fig. 6). DAPA alone did not affect it, whereas EPLE alone or in combination with DAPA enhanced perfusion relative to untreated CKD rats. However, the combination therapy did not offer any additional benefits over EPLE alone (Fig. 6).
Fig. 6.
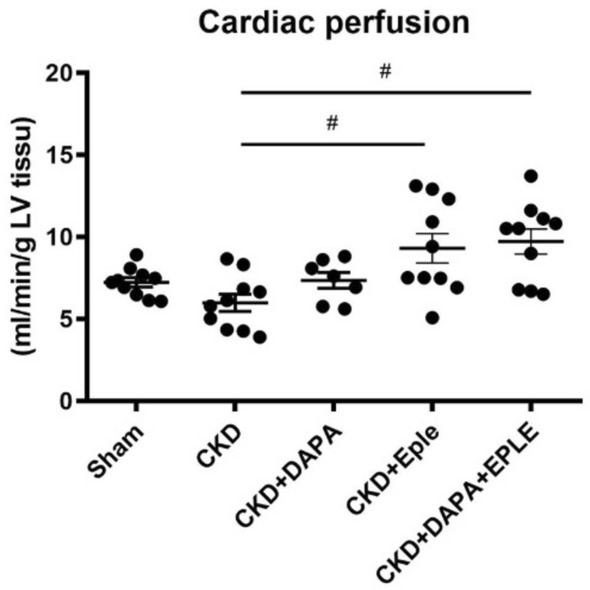
DAPA-EPLE combination improved LV perfusion in the CKD rat model. LV perfusion. Unpaired Student’s t-test or one way ANOVA was used for statistical analysis, mean ± SEM, n = 7–10 *: # p < 0.05 CKD vs. EPLE.
Discussion
Our study demonstrates that the combination of DAPA and EPLE limits the development of diastolic dysfunction and cardiac fibrosis while improving cardiac perfusion in a non-diabetic CKD rat model.
Although this combination therapy reduced renal fibrosis, it did not significantly alter the overall deterioration of gross renal structure. DAPA alone paradoxically increased 24-h albuminuria and UACR in this model, likely due to a combination of increased urine volume and the relatively short duration of treatment, which was also ineffective in preventing renal injury. Similar results have been observed with EMPA, another SGLT2i, in several rat CKD models21 and with DAPA in hypertensive Dahl salt-sensitive rats on a high-salt diet22.
The DAPA-CKD trial reported a reduced relative risk of adverse cardiovascular and kidney outcomes in a mixed population of diabetic and non-diabetic CKD patients treated with DAPA23. The FIDELIO-DKD and FIGARO-DKD trials using finerenone, a non-steroidal MRA, showed similar benefits for patients with diabetic kidney disease12,13. Given that approximately 50% of CKD patients are non-diabetic24,25, it is crucial to assess the potential benefits of both drugs, alone or in combination, for treating non-diabetic renal failure. Indeed, analysis of the results of the DAPA-CKD trial showed that the benefit of DAPA is independent of diabetes, as non-diabetic CKD patients also benefited from SGLT2 inhibition23. The FINEARTS-HF trial recently showed that, in patients with heart failure and mildly reduced (HFmrEF) or preserved ejection fraction (HFpEF), finerenone resulted in a significantly lower rate of a composite of total worsening heart failure events and death from CV causes than placebo26.
A key unresolved question is whether a combination therapy that associates an MRA and SGLT2i would be beneficial over monotherapy treatment for non-diabetic CKD patients.
This study was specifically designed to address this question using a non-diabetic rat CKD model, avoiding any confounding anti-diabetic effects of SGLT2i. Diastolic dysfunction, commonly reported in the rodent 5/6 nephrectomy model, was evident in our CKD rats, with unaltered systolic function, preserved ejection fraction, altered diastolic hemodynamics and increased cardiac fibrosis27–31. These features are typical cardiac parameters of HFpEF which is commonly observed in CKD patients32. The DAPA-EPLE combination significantly improved these diastolic parameters compared to the untreated CKD group. Both DAPA and EPLE significantly reduced cardiac fibrosis compared to the untreated CKD group, with the combination providing an even greater effect.
It has been reported that EMPA, a SGLT2i, also reduced cardiac fibrosis in two different preclinical studies in hypertensive rats33,34. The MRAs spironolactone and finerenone both improved diastolic function and reduced cardiac fibrosis in diabetic rats20,35, similar findings have been observed with finerenone in non-diabetic CKD mice36.
Coronary microvascular dysfunction is a proposed mechanism underlying HFpEF37,38. The PROMIS-HFpEF trial found decreased coronary reserve in 75% of patients, correlating strongly with elevated albumin/creatinine ratios and highlighting the role of coronary dysfunction in HFpEF development in CKD39. In our study, while DAPA alone did not modify cardiac perfusion, EPLE alone or combined with DAPA improved myocardial perfusion compared to the untreated CKD group. Cardiac perfusion predominantly occurs during diastole and depends in part on the difference in left ventricle diastolic pressure (LVEDP) from the aortic diastolic pressure40 as well as coronary vascular dilator tonus, i.e. vascular NO bio-availability. This emphasizes that the lower LVEDP pressure in EPLE or combination treated rats could improve cardiac perfusion. Moreover, MRA finerenone has been reported to improve endothelial function in the Munich Wistar Frömter rat, a CKD model, via a reduction in oxidative stress41 and spironolactone improves endothelial function by reducing oxidative stress via inhibition of the AGE/RAGE pathway in a 5/6 nephrectomy rat CKD model similar to that used in the present study42. Furthermore, we recently shown an improved cardiac NO production for rats treated with finerenone43. This supports a role of reduced oxidative stress and improved endothelial function among the benefits of MRA that could contribute to the increased coronary perfusion observed in the present study. SGLT2i also improved endothelial dysfunction partly due to recovery of impaired NO bioavailability44. Taken together the combination therapy may be beneficial on coronary microvascular dysfunction associated to CKD and improving HFpEF.
Emerging evidence suggests that combining an MRA with an SGLT2i may benefit renal outcomes in diabetic CKD patients14. A meta-analysis reported a reduction in adverse cardiovascular outcomes for diabetic patients treated with both an MRA and SGLT2i41. Additionally, recent studies have shown improved survival rate and decreased creatinine levels, proteinuria, and blood pressure in hypertensive mice treated with the combination, whereas a low dose of EMPA or finerenone alone had an intermediate or no impact45. The triple RAS/SGLT2/MR blockade (using ramipril, empagliflozin, finerenone, respectively) improved survival and renal outcomes in a mouse model of Alport syndrome with an additive effect of the MRA finerenone on interstitial fibrosis over other treatment46.
The use of a SGLT2i may also mitigate the hyperkalemic effect of MR antagonism for patients with reduced renal function, suggesting that the combination of MRA and SGLT2i will be of interest. In fact, the ROTATE 3 crossover trial reported less hyperkalemia among patients treated with both DAPA and EPLE than those treated only with EPLE14. The ongoing CONFIDENCE (COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with CKD and type 2 diabetes using an UACR Endpoint study) trial will assess this hypothesis in T2D patients with CKD by combining EMPA and finerenone47 and will study their impact on the UACR and potassium level after six months of treatment with the drugs alone or in combination. However, the long-term effects of combining SGLT2i and MRA on renal and cardiovascular outcomes still need further investigation.
Variabilities between individual drugs from each class (SGLT2i or MRA) may influence clinical outcomes. The pharmacological selectivity of SGLT2i to SGLT2 over SGLT1 is variable. A recent metanalysis showed no statistical differences between SGLT2i with high or low selectivity on cardiorenal outcomes48 while SGLT2i with lower selectivity have a more pronounced benefit to reduce fatal and nonfatal strokes in high-risk type 2 diabetic patients48. Steroidal (spironolactone, eplerenone) and non-steroidal MRAs (finerenone, esaxerenone for example) differ in their selectivity for steroidal receptors leading to lower risk of sexual side effects of the non-steroidal MRAs. Moreover, non-steroidal MRAs may have less hyperkalemic risk than steroidal MRAs49.
A limitation of our study is that analysis of fractional shortening and ejection fraction is not the best surrogate markers to assess cardiac contractility. Another limitation is the use of high doses of DAPA and EPLE. In preclinical studies, it is difficult to assess several doses, even more in variable combination. Of note in the Confidence trial47, interventions will be once-daily finerenone 10 mg or 20 mg (target dose) plus empagliflozin 10 mg, or empagliflozin 10 mg alone, or finerenone 10 mg or 20 mg (target dose) alone, doses that are similar, but not lower, to the Empa-kidney trial50 or the Fidelio-DKD trial12. In the current study we used DAPA an EPLE doses that have been previously used and showed to be efficient in rodent models. These high doses may have made it more challenging to observe a distinct additive effect from the combination treatment.
The present study shows that the DAPA-EPLE combination in a non-diabetic CKD rat model is effective to limit the development of diastolic dysfunction and fibrosis, and to improve cardiac perfusion. Further clinical research is necessary to determine the relevance of these findings in non-diabetic patients with renal dysfunction.
Supplementary Information
Acknowledgements
We thank the team of the CEF of the Cordeliers Research Center for their support with the animal care.
Abbreviations
- CV
Cardiovascular
- DAPA
Dapagliflozin
- EMPA
Empagliflozin
- EPLE
Eplerenone
- HFpEF
Heart failure with preserved ejection fraction
- HFmrEF
Heart failure with mid-range ejection fraction
- LV
Left ventricle
- LVEDP
LV end diastolic pressure
- LVEDPVR
LV end diastolic pressure volume relationship
- MRA
Mineralocorticoid receptor antagonist
- MRI
Magnetic resonance imaging
- NO
Nitric oxide
- SGLT2i
Sodium-glucose cotransporter-2 inhibitor
Author contributions
F.J. and P.M. provided the concept and design of research M.S., M.D., Y.S., I.L.P., R.P.R., L.N., P.M., and F.J., performed experiments and prepared figures. M.S., Y.S., I.L.P., N.A.L., P.M., and F.J., analyzed data and interpreted results of experiments. M.S. and F.J. drafted the manuscript. M.S., M.D., Y.S., I.L.P., R.P.R., L.N., N.A.L., P.M., and F.J. approved the final version of the manuscript.
Funding
This work was funded by grants from the Institut National de la Santé et de la Recherche Médicale, Fight-HF Avenir investment program (ANR-15-RHUS-0004), ANR NGAL-HT (ANR-19-CE14-0013) and IRP INSERM Miravac-CKD grant.
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: P. Mulder and F. Jaisser.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74934-z.
References
- 1.Ronco, C., Cicoira, M. & McCullough, P. A. Cardiorenal syndrome type 1: Pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J. Am. Coll. Cardiol.60, 1031–1042 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Tumlin, J. A. et al. Cardiorenal syndrome type 4: Insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol.182, 158–173 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Metra, M., Cotter, G., Gheorghiade, M., Dei Cas, L. & Voors, A. A. The role of the kidney in heart failure. Eur. Heart J.33, 2135–2142 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Heywood, J. T. et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J. Card. Fail.13, 422–430 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Packer, M. et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med.383, 1413–1424 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Spertus, J. A. et al. The SGLT2 inhibitor canagliflozin in heart failure: The CHIEF-HF remote, patient-centered randomized trial. Nat. Med.28, 809–813 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med.381, 1995–2008 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Wheeler, D. C. et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: Baseline characteristics. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc.35, 1700–1711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantong, B., Kratschmar, D. V., Nashev, L. G., Balazs, Z. & Odermatt, A. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells. J. Neuroinflammation9, 260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel, V., Joharapurkar, A. & Jain, M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev. Res.82, 341–363 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Ruilope, L. M. et al. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am. J. Nephrol.50, 345–356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med.383, 2219–2229 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Pitt, B. et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med.385, 2252–2263 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Provenzano, M. et al. Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: A randomized crossover clinical trial. J. Am. Soc. Nephrol. JASN33, 1569–1580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang, Y. et al. Improvement of left ventricular diastolic function induced by β-blockade: A comparison between nebivolol and metoprolol. J. Mol. Cell. Cardiol.51, 168–176 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Sahn, D. J., DeMaria, A., Kisslo, J. & Weyman, A. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation58, 1072–1083 (1978). [DOI] [PubMed] [Google Scholar]
- 17.Waller, C. et al. Myocardial perfusion imaging using a non-contrast agent MR imaging technique. Int. J. Cardiovasc. Imaging17, 123–132 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Nahrendorf, M. et al. In vivo assessment of cardiac remodeling after myocardial infarction in rats by cine-magnetic resonance imaging. J. Cardiovasc. Magn. Reson.2, 171–180 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Henri, O. et al. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation133, 1484–1497 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Lima-Posada, I. et al. Benefits of the non-steroidal mineralocorticoid receptor antagonist finerenone in metabolic syndrome-related heart failure with preserved ejection fraction. Int. J. Mol. Sci.24, 2536 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hojná, S. et al. Empagliflozin is not renoprotective in non-diabetic rat models of chronic kidney disease. Biomedicines10, 2509 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kravtsova, O. et al. SGLT2 inhibition effect on salt-induced hypertension, RAAS, and Na+ transport in Dahl SS rats. Am. J. Physiol. Renal Physiol.322, F692–F707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler, D. C. et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol.9, 22–31 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Bonner, R., Albajrami, O., Hudspeth, J. & Upadhyay, A. Diabetic kidney disease. Prim. Care47, 645–659 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Lin, Y.-C., Chang, Y.-H., Yang, S.-Y., Wu, K.-D. & Chu, T.-S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. Taiwan Yi ZhiBold">117, 662–675 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Solomon, S. D. et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med.10.1056/NEJMoa2407107 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Hamzaoui, M. et al. 5/6 nephrectomy induces different renal, cardiac and vascular consequences in 129/Sv and C57BL/6JRj mice. Sci. Rep.10, 1524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams, V. et al. Targeting MuRF1 by small molecules in a HFpEF rat model improves myocardial diastolic function and skeletal muscle contractility. J. Cachexia Sarcopenia Muscle13, 1565–1581 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winzer, E. B. et al. empagliflozin preserves skeletal muscle function in a HFpEF rat model. Int. J. Mol. Sci.23, 10989 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang, D. et al. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp. Biol. Med. Maywood NJ246, 2511–2521 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sárközy, M. et al. Chronic kidney disease induces left ventricular overexpression of the pro-hypertrophic microRNA-212. Sci. Rep.9, 1302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mark, P. B. et al. Left ventricular dysfunction with preserved ejection fraction: The most common left ventricular disorder in chronic kidney disease patients. Clin. Kidney J.15, 2186–2199 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruckert, C. et al. Empagliflozin prevents angiotensin II-induced hypertension related micro and macrovascular endothelial cell activation and diastolic dysfunction in rats despite persistent hypertension: Role of endothelial SGLT1 and 2. Vascul. Pharmacol.146, 107095 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Lee, H.-C. et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc. Diabetol.18, 45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachaux, M. et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes. Metab.20, 2399–2407 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Bonnard, B. et al. Mineralocorticoid receptor antagonism improves diastolic dysfunction in chronic kidney disease in mice. J. Mol. Cell. Cardiol.121, 124–133 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Paulus, W. J. & Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol.62, 263–271 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Nelson, M. D., Wei, J. & Bairey Merz, C. N. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: The chicken or the egg? Eur. Heart J.39, 850–852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah, S. J. et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J.39, 3439–3450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heward, S. J. & Widrich, J. Coronary perfusion pressure. In StatPearls (StatPearls Publishing, 2024). [PubMed]
- 41.Tsukamoto, S. et al. Cardiovascular and kidney outcomes of combination therapy with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: A systematic review and network meta-analysis. Diabetes Res. Clin. Pract.194, 110161 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Wang, C.-C. et al. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol.20, 351 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima Posada, I. et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone improves diastolic dysfunction in preclinical nondiabetic chronic kidney disease. J. Am. Heart Assoc.13, e032971 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanai, H., Adachi, H., Hakoshima, M. & Katsuyama, H. Significance of endothelial dysfunction amelioration for sodium-glucose cotransporter 2 inhibitor-induced improvements in heart failure and chronic kidney disease in diabetic patients. Metabolites13, 736 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolkhof, P. et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am. J. Nephrol.52, 642–652 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, Z. et al. Finerenone Added to RAS/SGLT2 blockade for CKD in alport syndrome. Results of a randomized controlled trial with Col4a3−/− mice. J. Am. Soc. Nephrol. JASN34, 1513–1520 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green, J. B. et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc.38, 894–903 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayour, A. A. et al. Effect of pharmacological selectivity of SGLT2 inhibitors on cardiovascular outcomes in patients with type 2 diabetes: A meta-analysis. Sci. Rep.14, 2188 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolkhof, P. et al. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: Comparison at bench and bedside. Handb. Exp. Pharmacol.243, 271–305 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Barnett, A. H. et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol.2, 369–384 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.