Abstract
BACKGROUND
Inhibitors of the Na+-coupled glucose transporter SGLT2 (SGLT2i) primarily shift the reabsorption of large amounts of glucose from the kidney’s early proximal tubule to downstream tubular segments expressing SGLT1, and the non-reabsorbed glucose is spilled into the urine together with some osmotic diuresis. How can this protect the kidneys and heart from failing as observed in individuals with and without type 2 diabetes?
GOAL
Mediation analyses identified clinical phenotypes of SGLT2i associated with improved kidney and heart outcome, including a reduction of plasma volume or increase in hematocrit, and lowering of serum urate levels and albuminuria. This review outlines how primary effects of SGLT2i on the early proximal tubule can explain these phenotypes.
RESULTS
The physiology of tubule-glomerular communication provides the basis for acute lowering of GFR and glomerular capillary pressure, which contributes to lowering of albuminuria but also to long term preservation of GFR, at least in part by reducing kidney cortex oxygen demand. Functional co-regulation of SGLT2 with other sodium and metabolite transporters in the early proximal tubule explains why SGLT2i initially excrete more sodium than expected and are uricosuric, thereby reducing plasma volume and serum urate. Inhibition of SGLT2 reduces early proximal tubule gluco-toxicity and by shifting transport downstream may simulate “systemic hypoxia”, and the resulting increase in erythropoiesis, together with the osmotic diuresis, enhances hematocrit and improves blood oxygen delivery. Cardio-renal protection by SGLT2i is also provided by a fasting-like and insulin-sparing metabolic phenotype and, potentially, by off-target effects on the heart and microbiotic formation of uremic toxins.
Keywords: blood pressure, fasting, GFR, gliflozin, hypertension, natriuresis, proximal tubule, uremic toxins
The sodium-dependent glucose transporter SGLT2 in the early proximal tubule of the kidney is the primary mechanism for the reabsorption of glucose filtered by the kidney glomeruli (see ref. 1 for review). As a consequence, inhibitors of SGLT2 (SGLT2i) spill glucose into the urine. This formed the primary rationale for the development of these compounds as blood glucose-lowering drugs in individuals with type 2 diabetes mellitus (T2DM). However, large placebo-controlled trials demonstrated that SGLT2i not only improve blood glucose control but, as a class effect, can protect the kidneys and heart from failing. When given on top of standard of care (including renin–angiotensin system blockade) in people at high cardiovascular (CV) risk with T2DM or chronic kidney disease (CKD), these drugs safely reduce the risk of acute kidney injury by ~25% and of kidney disease progression by ~40%.2 Notably, similar kidney protective effects were reported for SGLT2i independent of T2DM,2 but the latter and/or hyperglycemia may further enhance the efficacy.3 In addition, SGLT2i improve CV outcomes, particularly hospitalization for heart failure.2 These CV benefits are most prominent in patients with preexisting heart failure, including heart failure with reduced or preserved ejection, and, likewise, occur largely independent of T2DM.2,4
What is the magic of inhibiting a glucose transporter in the early proximal tubule of the kidney? Due to a high glomerular filtration rate (GFR), the kidneys filter large amounts of glucose (~180 g/day or ~1/3 of the body caloric expenditure).1 The kidneys are programmed to retain this valuable energy substrate, which is maladaptive and maintains hyperglycemia in individuals with diabetes mellitus. In euglycemia, most of the filtered glucose (~97%) is being reabsorbed by high-capacity SGLT2 in the “early” proximal tubule (S1/S2 segments).1,5 Glucose is typically not metabolized in these segments and exits passively through basolateral GLUT21 (Figure 1). The glucose that escapes SGLT2 is taken up downstream by SGLT1 in the “late” proximal tubule (S2/S3 segments) and thick ascending limb (Figure 2).1 Under physiological conditions, tubular SGLT1 only reabsorbs ~3% of filtered glucose.6 This changes dramatically, however, when SGLT2i enhance the glucose delivered to the late proximal tubule and unmask a glucose transport capacity of SGLT1 of ~40%–50% of the filtered glucose under euglycemic conditions.7 In a nutshell, SGLT2i shift the reabsorption of large amounts of glucose from the early proximal tubule to the downstream late proximal tubule and thick ascending limb, and spill glucose into the urine which modestly lowers hyperglycemia. Moreover, since SGLT2 transports 1 Na+ per glucose and SGLT1 transports 2 Na+ per glucose,1 this shift, viewed in isolation, may even cause Na+ retention. No obvious magic detected so far.
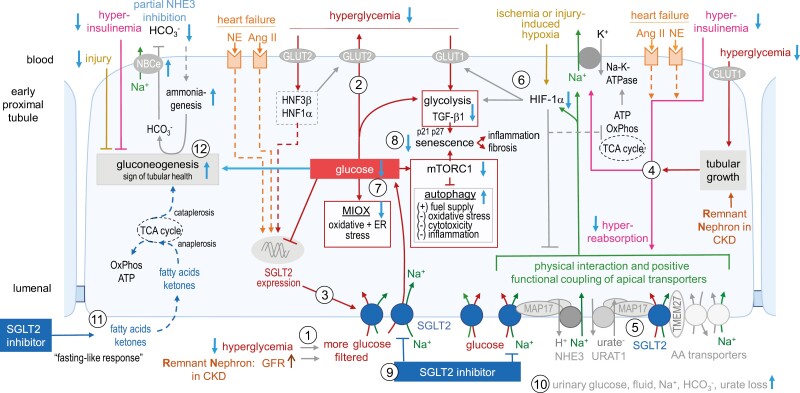
Figure 1.
SGLT2i reduce early proximal tubule hyperreabsorption and glucotoxicity. (1) Glycemia and glomerular filtration rate (GFR) determine the amount of filtered glucose that is reabsorbed together with Na+ by SGLT2, and exits the cells through basolateral GLUT2 (2). (3, 4) In diabetes or heart failure, or in hyperfiltering remnant nephrons (RN) in CKD, SGLT2 expression and activity can be increased by tubular growth, hyperglycemia, hyperinsulinemia, or increased angiotensin II (Ang II) and sympathetic tone (norepinephrine, NE). These settings also stimulate basolateral Na-K-ATPase, which is physiologically energized by adenosine triphosphate (ATP)-generating tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OxPhos), and drives enhanced apical transport via SGLT2, NHE3, and URAT1, which are functionally coupled via neuro-hormonal regulation and scaffolding proteins (4, 5). (6) Hypoxia due to ischemia and injury, potentiated by excessive hyperreabsorption, activates HIF-1α, which limits hyperreabsorption and induces a metabolic shift to glycolysis in order to reduce reactive oxygen species formation from oxidative phosphorylation, but the involved basolateral glucose uptake (via GLUT1) is also linked to induction of TGF-β1 and tubular growth. (7) Excessive cytosolic glucose stimulates oxidative stress via myo-inositol oxygenase (MIOX) and inhibits autophagy through mammalian target of rapamycin complex 1 (mTORC1) activation (8). TGF-β1 enhances cyclin-dependent kinase inhibitors p21 and p27 and together with mTORC1 promotes senescence, which is linked to inflammation and fibrosis. (9) SGLT2i attenuate the described deleterious effects (see blue arrows) by reducing hyperinsulinemia, hyperglycemia, excessive intracellular glucose, and tubular hyperreabsorption (10). (11) SGLT2i enhance the delivery of fatty acids and ketone bodies as energy substrates. (12) SGLT2i may enhance gluconeogenesis (GNG) by lowering hyperinsulinemia, cytosolic glucose, and tubular injury, or due to a mild reduction in HCO3− following partial NHE3 inhibition). GNG is linked to tubular health, maybe in part by removing TCA cycle intermediates (cataplerosis) thereby facilitating the feeding of fatty acids and ketones into the TCA cycle (anaplerosis). Abbreviations: AA, amino acid; CKD, chronic kidney disease; HIF-1α, hypoxia-inducible factor 1 alpha; HNF, hepatic nuclear factor; NHE3, Na-H-exchanger 3; TGF-β1, transforming growth factor β1; TMEM27, scaffolding protein collectrin; URAT1, urate transporter 1. Adapted with permission from ref. 92.
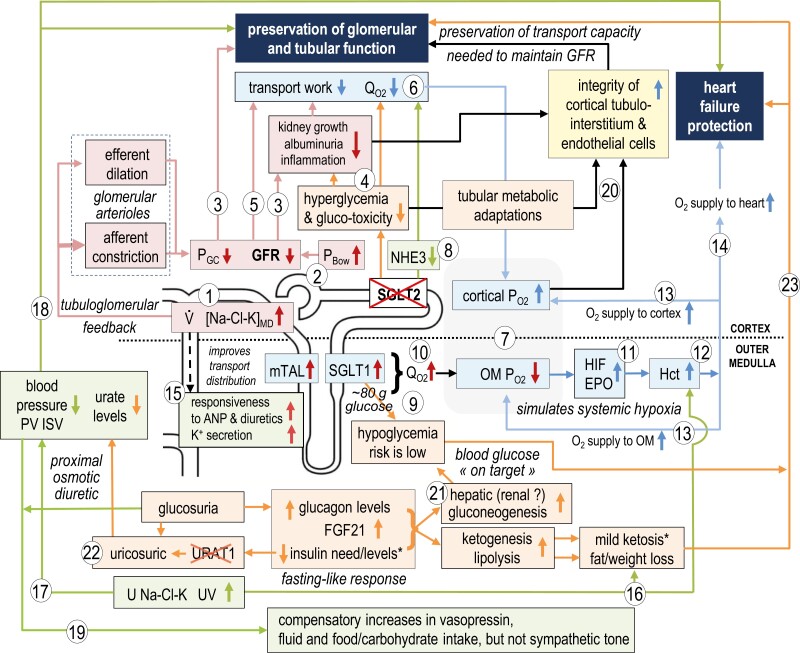
Figure 2.
Primary effects of SGLT2i on the early proximal tubule can explain phenotypes associated with kidney and heart protection. The protective phenotypes identified in mediation analyses include a reduction of plasma volume (PV) or increase in hematocrit (Hct), and a lowering of serum urate levels and albuminuria. SGLT2i increase delivery of NaCl and K ([Na-Cl-K]MD) and fluid (V) to the macula densa, which lowers GFR through the physiology of tubuloglomerular feedback (TGF) (1) and by increasing hydrostatic pressure in Bowman’s space (PBow) (2). SGLT2i lower GFR and glomerular capillary pressure (PGC) by afferent arteriole constriction and by efferent arteriole dilation. Lowering PGC and GFR (3) and hyperglycemia (4) protect glomerular and tubular function and reduce albuminuria by lessening physical stress and filtration of albumin and other tubule-toxic compounds, by reducing tubular growth and inflammation, and by lowering tubular transport work (5). The latter reduces cortical oxygen demand () (6) and increases cortical oxygen availability () (7). Early proximal tubule transport work is further reduced by cellular SGLT2 blockade and the associated partial inhibition of other apical transporters including Na-H-exchanger NHE3 (8). SGLT2i shift glucose reabsorption to downstream SGLT1, the latter limiting glucosuria and hypoglycemia risk (9). Shifting the reabsorption of Na+, glucose and other metabolites to late proximal tubule and medullary thick ascending limb (mTAL) improves distribution of transport along the nephron but can increase (10) and lower in the outer medulla (OM) (7), where it may activate hypoxia-inducible factor (HIF) and enhance erythropoietin (EPO) release (11). The increase in Hct (12) improves O2 delivery to the kidney medulla and cortex (13) and other organs (14). More delivery of NaCl and fluid downstream of early proximal tubules facilitates responsiveness to an atrial natriuretic peptide (ANP) and diuretics as well as kaliuresis (15). The induced diuresis further increases Hct (16) and reduces PV and interstitial (ISV) volume and blood pressure (17). These effects can help protect the failing heart (18), while excessive fluid loss is prevented by compensatory transport upregulation downstream of the early proximal tubule, in part via vasopressin (19). Reduced cellular stress and improved metabolism in the early proximal tubule promote the integrity of the tubular and endothelial system and preserve tubular transport capacity and thereby GFR in the long term (20). The glucosuric effect induces a fasting-like response including lower therapeutic and/or endogenous insulin levels and increases in glucagon and FGF21 (21). This induces compensatory lipolysis, ketogenesis, and gluconeogenesis. SGLT2 inhibitors are uricosuric; involving URAT1 inhibition, potentially in part by lowering insulin (22). These metabolic adaptations reduce urate levels, the hypoglycemia risk, and body and organ fat mass, which together with the resulting mild ketosis have the potential to further protect the kidney and heart (18, 23). Abbreviations: U Na-Cl-K, urinary salt, and K excretion; UV, urinary flow rate. Adapted with permission from ref. 92.
To learn more about SGLT2i, mediation analyses asked which clinical phenotypes of SGLT2i are associated with improved kidney and heart outcomes? This kind of analysis does not prove cause and effect, but it is remarkable that the strongest predictors of organ protection are basically identical for the kidney and heart, and include a reduction of plasma volume or increase in hematocrit, and lowering of serum urate levels and albuminuria.8–11 As discussed in the following, all these clinical phenotypes can be explained directly or indirectly by the primary effect of these drugs on their target in the early proximal tubule of the kidney (Figure 2). This review outlines the primary effects and consequences of inhibiting SGLT2 in the proximal tubule and how this can protect the kidney and the heart, but also considers the emerging evidence for potential off-target effects of SGLT2i.
SGLT2I INDUCE A FASTING-LIKE METABOLIC PHENOTYPE THAT IS INDEPENDENT OF DIABETES AND INSULIN SPARING
By spilling glucose and calories into the urine, SGLT2i induce a compensatory and fasting-like metabolic response1,12 that, due to extensive refinement during evolution, should be for the benefit of the organism and independent of diabetes.1 This affects a key aspect and sequala of Western lifestyle: the urinary glucose loss by SGLT2i improves insulin resistance and lowers hyperinsulinemia and/or therapeutic insulin dosing. This promotes a shift in substrate utilization from carbohydrates to lipids, thereby reducing body fat, including visceral and subcutaneous fat13 (Figure 2). The released free fatty acids are in part used for the hepatic formation of ketone bodies, which provide additional energy for many organs, including the heart and kidneys14,15 (Figures 1 and 2). On the flip side, excess ketone formation in response to SGLT2i is a concern in conditions of low food intake and insulin levels, including in patients with type 1 diabetes mellitus (T1DM).16
In contrast to insulin therapy, SGLT2i largely leave the hypoglycemia risk unchanged and lower body weight,13 and thus do not carry these deleterious effects of insulin that negatively affect CV risk.17 Exogenous insulin and hyperinsulinemia also enhance the CV risk by promoting kidney salt and urate retention (Figure 1). In other words, SGLT2i carry organ-protective potential in part by lowering insulin dosing or hyperinsulinemia, the latter being often found in obesity and outside of T2DM.
With regard to the hypoglycemia risk, SGLT2i stop lowering blood glucose once the drop in blood glucose (~60–70 mg/dl) allows all the filtered glucose to be recovered by downstream SGLT1 (~80 g/day)1 (Figure 2). In addition, compensatory upregulation of gluconeogenesis in response to SGLT2i in the liver, and potentially the kidney, provide critical glucose for organs, like the brain, and also keep the hypoglycemia risk low13 (Figures 1 and 2). Thus, in individuals with T2DM, SGLT2i protect the renal and CV system from dangerous blood glucose highs and lows, i.e., blood glucose remains more on target, with only modest net effects expected on HbA1C.
SGLT2I LOWER GLUCOTOXICITY IN THE EARLY PROXIMAL TUBULE
SGLT2i reduce the apical glucose load into the cells of the early proximal tubule. In addition and as suggested by data in nondiabetic mice, SGLT2i can reduce kidney GLUT2 protein expression, which may attenuate basolateral back leak of glucose when apical SGLT2 is blocked.18 This is associated with reduced protein expression of proximal tubular enzymes involved in the handling of glucose, oxidative stress, and cellular detoxification, including myo-inositol oxygenase (MIOX, Figure 1) and glutathione peroxidase 3 (GPX3).18 In rodent proximal tubules, SGLT2i induce starvation-like responses including sirtuin (SIRT)/5' AMP-activated protein kinase (AMPK) activation and inhibition of the protein kinase B (AKT)/mTORC1 pathway, thereby counteracting the primary pathophysiology of diabetes and overnutrition and inducing protective autophagy while inhibiting deleterious senescence, which is linked to inflammation and fibrosis1 (Figure 1). Suppression of proximal tubular mTORC1 by SGLT2i has been confirmed by single-cell RNA sequencing studies in kidney biopsies of young persons with T2DM.19
In patients with T2DM and albuminuria, SGLT2i increased urinary metabolites linked to mitochondrial metabolism, potentially indicating improvement of mitochondrial function and activity.20 Similarly, single-cell RNA sequencing of proximal tubules in db/db mice indicated that SGLT2i restored mitochondrial function and fatty acid oxidation in proximal tubules.21 In nondiabetic mice SGLT2i caused distinct effects on kidney metabolism reflecting responses to partial NHE3 inhibition (see below) as well as urinary loss of glucose and NaCl; this included upregulated renal gluconeogenesis and using tubular secretion of the tricarboxylic acid cycle intermediate, alpha-ketoglutarate, to communicate to the distal nephron the need for compensatory NaCl reabsorption.22 Upregulation of gluconeogenesis could be of particular interest, because its activity is positively linked to proximal tubule mitochondrial function and kidney protection23,24 (for more details see Figure 1).
The clinical observation that SGLT2i have a phosphate-sparing effect25 may also relate to the interference with early proximal tubule SGLT2. An increase in luminal Na+ availability as a consequence of SGLT2i may facilitate proximal tubule Na+-coupled phosphate reabsorption. Moreover, SGLT2-dependent glucose uptake is needed for phosphate sensing by the proximal tubule, which involves glycolysis-driven formation and subsequent release of glycerol-3-phosphate to stimulate bone release of phosphaturic FGF23.26 While the SGLT2i-induced increase in serum phosphate is associated with an increase in circulating FGF23,27,28 the rise in FGF23 could be attenuated and insufficient due to the blunted glycerol-3-phosphate response.
SGLT2I CAUSE A BROAD RECONFIGURATION OF METABOLITE TRANSPORTERS IN THE EARLY PROXIMAL TUBULE
Recent data indicate an extensive physical and functional coupling of SGLT2 with other sodium and metabolite transporters in the luminal membrane of the early proximal tubule (Figure 1). The inhibitory interaction of SGLT2i with these transporters is thought to involve scaffolding proteins like MAP17, but may also relate to changes in transporter phosphorylation and the insulin-lowering effect of SGLT2i1 (Figures 1 and 2). Insulin co-stimulates SGLT2, NHE3, the urate transporter URAT1, and potentially more transporters in the early proximal tubule1 (Figure 1). This co-stimulation and interaction make sense in the post-prandial phase when the rise in GFR enhances the amounts of filtered sodium, glucose, bicarbonate, urate, and other substrates that are reabsorbed in the early proximal tubule. In hyperinsulinemic states like T2DM and obesity, co-stimulation facilitates renal glucose, NaCl, and urate retention. Finally and most importantly for this review, this co-stimulation and interaction make an array of transport processes in the early proximal tubule sensitive to SGLT2i1 (Figures 1 and 2).
Studies in rodents first described that the inhibitory effect of SGLT2i on NHE3 contributes to both the acute natriuretic and the chronic volume depletion effect.22,29 A single dose of empagliflozin, titrated to cause minimal glucosuria [i.e., shifting glucose reabsorption from SGLT2 (transporting 1 Na+/glucose) to SGLT1 (transporting 2 Na+/glucose)], increased urinary excretion of Na+ and bicarbonate and raised urine pH in wild-type mice but had an antinatriuretic effect in mice lacking tubular NHE3.22 In accordance, application of empagliflozin in patients with diabetes and heart failure resulted in a larger than expected decrease in proximal tubule Na+ reabsorption with several observations suggesting inhibition of NHE3,30 and similar findings were made in healthy volunteers.31 Studies in knockout mice32 and pharmacological studies in humans33 implicated URAT1 inhibition in the serum urate lowering effect of SGLT2i (Figures 1 and 2).
Recent mouse and human kidney SGLT2 interactome studies and kidney cortex proteomics/phospho-proteomics in nondiabetic mice confirmed the interaction of SGLT2 with multiple NHE-scaffolding proteins. In both mouse and human kidney tissue, SGLT2 interacted with MAP17, NHE-RF2, NHE-RF3 as well as the G-protein-coupled receptor GPRC5C,18 which has likewise been implicated in NHE3 regulation.34 Dapagliflozin actually reduced MAP17 protein expression in mouse kidneys.18 Furthermore, evidence was presented that SGLT2 can physically interact with URAT1 as well as with other apical urate transporters (incl. GLUT9 and ABCG2), and dapagliflozin reduced URAT1 protein expression and phosphorylation in the mouse kidney cortex. Beyond these interactions, the studies demonstrated direct or indirect physical interactions of SGLT2 with multiple other apical metabolite transport proteins in the early proximal tubule, many of which are Na coupled, including potential interactions with early proximal tubule amino acid transport18 (Figure 1).
In summary, inhibition of SGLT2i appears to lower the early proximal tubule transport burden not only by inhibiting the cotransport of Na+ and glucose but, as a consequence of insulin co-stimulation and/or physical interaction, by partially inhibiting multiple other transporters at the same time, many of which are also Na+ coupled.18
SGLT2I SHIFT TRANSPORT LOAD AND TRANSPORT WORK DOWNSTREAM: DELETERIOUS OR BENEFICIAL?
The early proximal tubule has evolved in a way such that it reabsorbs a large fraction of the glomerular filtrate, which comes with a large demand for oxygen. This setup works fine in a healthy kidney, but may make the early proximal tubule particularly vulnerable during kidney injury. As discussed above, SGLT2i shifts some of the glucose, NaCl, urate, amino acid, and fluid reabsorption downstream, and thereby more equally distributes the transport burden along the tubular system, which may help to preserve tubular function in the long term.1 This transport shift in response to SGLT2i is associated with a shift in energy and oxygen demand from early to late proximal tubule and thick ascending limb as proposed by mathematical modeling35–37 and, more recently, by changes in tricarboxylic acid and glycolysis-related gene expression in single-cell RNA sequencing of kidney biopsies of young persons with T2D.19 In the nondiabetic setting, the transport shift by SGLT2i is associated with an increase in kidney weight38 including hypertrophy of late proximal tubules/S3 segments39 as well as downregulation of renal membrane SGLT1 protein expression, potentially to limit tubular glucose overload in those segments.38 Thus, SGLT2i more equally distribute the transport burden along the nephron but put stress on these downstream segments and the vulnerable region of the outer medulla, which may dampen the overall kidney benefits of SGLT2i. The transport shift-induced stress by SGLT2i is attenuated by the associated early reduction in GFR (see below) and potentially by metabolic use of the delivered glucose by downstream segments.1
The transport shift may explain the upregulation of stress markers, like hemoxygenase-138,40; on the flip side, this way SGLT2i may drive a tubular pre-conditioning-like adaptation1 that could contribute to the clinically observed protection from acute kidney injury.38,40–42 Moreover and based on mathematical modeling, the transport shift has been proposed to “simulate” systemic hypoxia at the oxygen sensor in the deep cortex and outer medulla of the kidney37 (Figure 2): by shifting transport work from the early proximal tubule to tubular segments in the deep cortex and outer medulla, including proximal tubule S3 segments and medullary thick ascending limbs, SGLT2i increase the oxygen consumption at these sites, which is then sensed as reduced oxygen availability by the erythropoietin secreting interstitial cells found primarily in this region of the kidney. Thus, independent of systemic hypoxia, SGLT2i trick these cells to sense hypoxia.37 Supporting this hypothesis, treatment of individuals with T2DM with an SGLT2i for 32 weeks reduced kidney medullary oxygenation and this was associated with increased erythropoietin levels and hematocrit.43 The latter is further enhanced by the diuretic effect of SGLT2i (see below). The resulting improvement in oxygenation of the kidney (particular importance of kidney cortex oxygenation for CKD progression44,45) and heart may explain why the increase in hematocrit and hemoglobin are among the strongest predictors of improved kidney and CV outcome by SGLT2i8–11 (Figure 2).
THE BIPHASIC EFFECT OF SGLT2I ON GFR
The difficulty to protect the kidneys is in part due to their structural complexity. Are some structures, segments, or cells of the kidney more important to protect than others in order to conserve kidney function? Does this include the early proximal tubule? A more general approach could be to manipulate the kidneys’ energy and O2 needs. Since basically all filtered NaCl and fluid (and many other filtered solutes) need to be reabsorbed along the tubular system to match urinary excretion to daily intake, and since this transport work costs energy and oxygen, the GFR is the primary determinant of kidney energy and oxygen need. Metabolic control of heart function includes workload-induced coronary vasodilation to adjust oxygen supply to demand; this involves cellular adenosine release and activation of adenosine A2 receptors. Metabolic control of kidney function also involves adenosine release in response to excessive transport work or energy imbalance, and adenosine primarily reduces GFR.46 Also the diseased kidney may in part reduce GFR to limit the kidneys’ energy and oxygen need.47 These responses point to a general strategy of kidney protection, namely a functional and modest lowering of GFR. A functional reduction in GFR and glomerular capillary pressure (PGC) has been implicated in kidney protection by renin–angiotensin system blockade48,49 and now by SGLT2 inhibition,50 and the clinical trials provided strong evidence that the 2 strategies are additive.50
Considering that GFR is typically ~100-fold greater than urine flow rate, there is a need for mechanisms that coordinate glomerular filtration and tubular transport capacity. SGLT2i functionally lower GFR by manipulating one of these mechanisms, namely the tubuloglomerular feedback (TGF). The TGF describes an inverse relationship between the NaCl and fluid delivery to the macula densa (MD) and the GFR of the same nephron (Figure 3), which serves to stabilize fluid and electrolyte delivery to the MD thereby facilitating fine regulation of excretion in downstream distal segments.50 SGLT2i reduce proximal tubule reabsorption and enhance the delivery of NaCl and fluid to the MD; the physiology of the TGF then aims to normalize MD NaCl and fluid delivery by reducing GFR (Figures 2 and 3). This has been demonstrated by micropuncture studies in hyperfiltering diabetic rats,51,52 and the GFR lowering effect of short-term SGLT2 inhibition was subsequently confirmed in diabetic mice and humans with T1DM and T2DM.50 Some GFR reduction was also observed in nondiabetic mice and humans.50
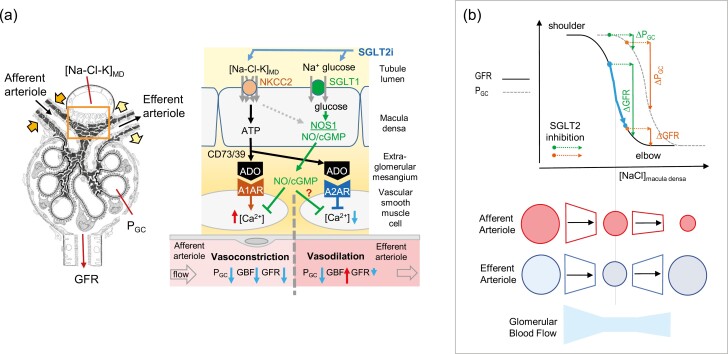
Figure 3.
Glomerular hemodynamic response to SGLT2i. (a) SGLT2i increase Na-Cl-K delivery to the macula densa (MD), which is sensed by an NKCC2-dependent mechanism and enhances basolateral release of ATP. ATP is converted by endonucleotidases CD73/39 to adenosine (ADO), which activates adenosine A1 receptor (A1AR) on vascular smooth muscle cells of the afferent arteriole to increase cytosolic Ca2+ and induce vasoconstriction. ADO can also activate adenosine A2 receptors (A2AR) on the efferent arteriole to reduce cytosolic Ca2+ and induce vasodilation. Both effects contribute to the TGF response and lower GFR and PGC. Efferent dilation can enhance GFR by increasing renal blood flow (RBF), thus causing a stronger effect on PGC than GFR. SGLT2i increase glucose delivery to the MD, which is sensed by MD SGLT1 and triggers NOS1-mediated NO formation, which can dilate the afferent arteriole but potentially also the efferent arteriole. Volume depletion (e.g., following SGLT2i) can inhibit MD NOS1 activity. (b) Glomerular hemodynamic effects of SGLT2i superimposed on a model of TGF, which incorporates effects on afferent and efferent arteriolar resistances. Idealized TGF curves are shown for GFR and PGC. The PGC curve lies to the right of the GFR curve owing to differences in the way that pre- and postglomerular resistances react across the range of inputs, as shown in the bottom portion of the figure. Note the efferent arteriolar dilation for high [NaCl] at the macula densa. Depending on the position of the operating point, identical increases in macula densa delivery by SGLT2i can induce different relative decreases in GFR vs. PGC; e.g., in CKD and due to hyperfiltration of remnant nephrons, the TGF may operate closer to the elbow of the TGF curve (blue arrow), resulting in a smaller decline in GFR but a larger decline in PGC. Abbreviations: CKD, chronic kidney disease; NO, nitric oxide; NOS1, NO synthase; PGC, glomerular capillary pressure. Adapted with permission from ref. 53,92.
When MD cells sense an increase in luminal NaCl, the resulting TGF-induced basolateral release of ATP promotes local formation of adenosine, which primarily constricts the afferent arteriole (via adenosine A1 receptors) but can also dilate the efferent arteriole (via adenosine A2 receptors)50 (Figures 2 and 3). Both effects lower PGC as was directly demonstrated in response to SGLT2i by micropuncture in rats with T1DM.53 Those studies further indicated that SGLT2 inhibition can induce a robust reduction in PGC even when GFR decreases only slightly and vice versa. This argued against a sole effect of SGLT2i on the afferent arteriole, and implied combined constriction of the afferent arteriole and dilation of the efferent arteriole53 (Figure 3), which could explain the observed reduction in filtration fraction by SGLT2i in some of the clinical studies.54,55 This may have implications in advanced CKD (GFR <30 ml/(min × 1.73 m2)), where the initial GFR drop in response to SGLT2 inhibition can be small, but the kidney protective effect is preserved.56 We hypothesize that compensatory hyperfiltration in remaining nephrons shifts the operating point along the TGF curve, such that the increase in MD NaCl by SGLT2i (and/or glucose, see below) induces a predominant reduction in PGC53 (Figure 3).
The MD expresses SGLT1 in its apical membrane and thereby senses luminal glucose to stimulate local nitric oxide (NO) formation via neuronal NO synthase (NOS1).57–59 The SGLT2i-induced rise in MD glucose delivery activates the MD–SGLT1–NOS1 pathway58 (Figure 3). Considering the close proximity of the MD to its afferent and efferent arteriole, it seems possible that the SGLT2i-induced activation of the MD–SGLT1–NOS1 pathway dilates not only the afferent but also the efferent arteriole (the latter effect reducing PGC), particularly in settings of CKD where endothelial integrity and efferent arteriolar NO tone may be reduced.
Most importantly, SGLT2i induce a biphasic GFR response in patients with T2DM and normal or reduced kidney function: the described initial reduction in GFR is followed by long-term GFR preservation.50 Moreover, eGFR recovered to baseline when long-term SGLT2i treatment was discontinued,50 indicating that the early rise in plasma creatinine in response to an SGLT2 inhibitor reflects a “functional and reversible” reduction in GFR, and is part of the therapeutic effect. Indeed, SGLT2i decreased urinary excretion of markers of tubular injury in patients with T2DM,60 and meta-analyses of clinical studies showed a ~25% reduced incidence of acute kidney injury.41,42 Furthermore, the application of SGLT2i in individuals with T2DM and acute kidney injury was associated with a lower risk of mortality (by 31%), and major kidney (by 38%) and adverse cardiovascular events (by 25%) compared with nonuse.61
By reducing GFR and PGC (and increasing hydrostatic back pressure in Bowman space due to delivering more fluid to the high resistance distal tubule), SGLT2i reduce the physical stress on glomerular capillaries and diminish glomerular filtration of tubulo-toxic factors (e.g., albumin with or without fatty acids bound to it, growth hormones, advance glycation end products).50 The interaction of these factors with the tubular system requires energy and promotes hypoxia, impairs autophagy, and triggers tubular remodeling, dedifferentiation, senescence, renal oxidative stress, inflammation, and fibrosis, and thereby the development and progression of diabetic and nondiabetic kidney disease50,62 (Figure 2).
Preservation of cortical oxygenation is associated with the preservation of kidney function in patients with CKD.45 By lowering GFR and SGLT2-dependent transport processes, SGLT2i reduce oxygen-consuming transport work in the early proximal tubule as predicted by mathematical modeling37 and confirmed on the kidney cortex level in albuminuric patients with T1DM.44 Particularly in the more chronic setting, not all clinical studies find an increase in cortical O2 pressure with SGLT2i, which may reflect improved oxidative phosphorylation and oxygen utilization by the kidney cortex. Thus, by lowering GFR and the oxygen-consuming transport work in the kidney cortex, SGLT2i have the potential to preserve the integrity of the remaining nephrons and overall kidney function in the long term50 (Figure 2).
SGLT2I REDUCE FLUID OVERLOAD AND IMPROVE HEART FAILURE OUTCOME
The improvement in heart failure outcome by SGLT2i occurs within weeks of treatment and is independent of hyperglycemia.2,4 The immediate diuresis and natriuresis, the described kidney protective mechanisms, as well as the diabetes-independent metabolic resetting and the improvement of insulin sensitivity, all have the potential for SGLT2i-mediated heart protection.13,63 This includes reducing volume overload and hypertension, preserving or increasing hematocrit and hemoglobin and thereby oxygen supply, inducing mild ketosis and thereby providing more energy substrate, lowering urate levels, inhibition of the sympathetic nervous system and NLRP3 inflammasome, and preserving GFR and limiting hyperkalemia, thereby improving tolerance to renin–angiotensin-aldosterone system (RAAS) blockade13 (Figure 2).
SGLT2i promote mild osmotic diuresis and natriuresis that reduce fluid overload, whole-body sodium content, and blood pressure, which may afford rapid protection against heart failure.13,64 In heart failure, upregulation of proximal tubule NHE3, driven by angiotensin II or sympathetic nervous system, may further sensitize early proximal tubule Na+ reabsorption to SGLT2i.22,29,30
The SGLT2i-induced natriuresis is immediately limited and increasingly attenuated by downstream SGLT1, by NKCC2-mediated NaCl reabsorption in the thick ascending limb52,65 being stimulated by greater NaCl delivery37 and vasopressin levels,66,67 as well as by the modest lowering in arterial blood pressure. Additional compensation may include growth of the S3 segment and collecting duct39 and α-ketoglutarate-driven NaCl transport in intercalated cells.22 Thus, the compensation reduces the natriuretic response within several days to weeks of continuous SGLT2i application. On the flip side, the compensation lowers the risk of severe volume depletion by SGLT2i and the clinically observed Mg2+ retention25 may be due to increased thick ascending limb reabsorption.
The SGLT2i-induced reduction in fluid overload is independent of diabetes or kidney function,68,69 and has been implicated in the initial reduction in systolic blood pressure, while the (mechanistically less clear) reduction in sympathetic nervous system activity may contribute to the long-term reduction.68 The natriuretic effect of SGLT2i, which is associated with lowering plasma volume and increasing hematocrit,70 appears more sustained and induces a relatively greater reduction in interstitial vs. intravascular volume than the loop diuretic bumetanide, potentially due to the osmotic diuresis causing a greater electrolyte-free water clearance71 (Figure 2). Moreover, the SGLT2i-induced increase in NaCl and fluid delivery downstream of the early proximal tubule is expected to enhance the responsiveness to loop and thiazide diuretics and potentially also endogenous atrial natriuretic peptide (Figure 2).
SGLT2 gene knockout or inhibition facilitates urinary K+ excretion.5,13 The above-described adaptations reduce kidney K+ loss in response to SGLT2i by limiting the rise in distal tubule fluid and Na+ delivery and thereby flow-induced K+ secretion and principal cell Na+/K+ exchange. As a net effect, SGLT2i either cause no effect or a slight reduction in plasma K+ concentration in individuals with normal kidney function, but lower the hyperkalemia risk due to RAAS blockade and protect against hyperkalemia in individuals with CKD13,72 (Figure 2).
SGLT2i have clinically documented antiarrhythmic effects on the heart73–75 but the involved mechanisms remain unclear. Potentially relevant in this regard is evidence for SGLT2-independent cardiac effects of SGLT2i. These “off-target effects” of SGLT2i have been proposed to affect pro-inflammatory and oxidative stress processes, ion transport mechanisms (like NHE1) controlling cellular sodium and calcium homeostasis, and cardiac metabolic and mitochondrial pathways76 but more studies are needed.77 In addition, SGLT2i have been proposed to directly interact with and inhibit the intestinal microbiota fermentation of amino acids to uremic toxins (Figure 4). This lowers circulating levels of the uremic toxin p-cresol sulfate, as documented in mice (including Sglt2 KO mice), rats as well as patients with diabetes or decompensated heart failure, and bears potential to lower cardiac formation and release of GDF15 and thereby improve cardiac contractility and rhythm.18 GDF15 has been linked to cardiac atrophy78 as well as higher mortality risk in hemodialysis patients.79 Uremic toxins positively regulate their own kidney secretion and excretion by stimulating basolateral organic anion transporters (OAT1/OAT3) in the proximal tubule. As a consequence, by reducing the formation and circulating concentrations of uremic toxins, SGLT2i can lower the activity of these kidney transporters, which has 2 consequences: first, transporter substrates can be retained in circulation, including amino acid metabolism-derived compounds with CV benefits (e.g., lowering blood pressure or inflammation18,80) and, second, proximal tubular exposure to organic anion toxins can be attenuated by SGLT2i18 (Figure 4) as recently proposed for aristolochic acid.81
Figure 4.
SGLT2i may protect the kidney and heart by inhibition of microbiotic formation of uremic toxins. Chemical structures of SGLT2 inhibitors dapagliflozin and empagliflozin and p-cresol are shown high-lighting similarities in red, which may provide a mechanistic basis. SGLT2i lower circulating levels of uremic toxins including p-cresol/p-cresol sulfate, thereby lowering cardiac formation and deleterious effects of GDF15 on the heart and kidney. Uremic toxins positively regulate their own kidney excretion by stimulating basolateral organic anion transporters (OAT1/OAT3) in the proximal tubule. By reducing circulating levels of uremic toxins, SGLT2i can lower the activity of these kidney transporters which can retain body-derived OAT substrates, including amino acid metabolism-derived compounds with cardiovascular benefits. On the other hand, proximal tubular exposure to uremic and other organic anion toxins would be attenuated. As suggested by proteomics in mice and human kidney interactome studies, SGLT2i may enhance kidney expression and physically interact with the environment-susceptible P-glycoprotein (P-gp, ABCB1), a primary proximal tubule pathway involved in secretion of xenobiotics and toxins.18 Abbreviations: aKG, alpha-ketoglutarate; OAT1/3, organic anion transporters 1 and 3. Adapted with permission from ref. 18.
OUTLOOK
Insights from multi-omic studies are needed at the cellular level of the early proximal tubule, where SGLT2 is targeted, but also in downstream segments exposed to greater amounts of glucose, NaCl, and multiple other substrates. This includes the S3 segment of the proximal tubule but also the MD, which has been implicated in broader kidney remodeling, in part through glucose sensing.50,58,82 How do SGLT2i interfere with the efferent arteriole as well as the erythropoietin system? Why is sympathetic nervous system activation attenuated in response to SGLT2i,13 and what is the quantitative relevance of off-target effects of SGLT2i on the heart, kidney, and other organs. Is there therapeutic potential for SGLT2i in type 1 diabetes83,84? What is the role of SGLT1 in dual SGLT2/SGLT1 inhibitors85,86? Are SGLT2i synergistic with other kidney protective strategies, beyond renin-angiotensin system blockade, like GLP1 agonists87 and aldosterone antagonists88? Finally, can we use what we learn from SGLT2 and its inhibition about the inner workings of the kidney for the identification of new therapeutic targets? Table 1 summarizes the characteristics of SGLT2 and its inhibition. The Na-coupled neutral amino transporter SLC6A19 fulfills these characteristics, and the first studies document metabolic benefits89 and kidney protection90 of the knockout in mice and highlight a potential creatinine phenotype of human mutations in the gene.91
Table 1.
Characteristic of SGLT2 and its inhibition
|
|
|
|
|
|
|
FUNDING
The author was supported by National Institutes of Health (NIH) Grants R01 DK112042, RF1 AG061296, R01 DK132690, R01 DK 134616, and the University of Alabama at Birmingham/University of California-San Diego O’Brien Center of Acute Kidney Injury NIH Grant P54 DK137307, and the Department of Veterans Affairs.
CONFLICT OF INTEREST
Over the past 24 months, V.V. has served as a speaker and received honoraria from Astra-Zeneca and Boehringer-Ingelheim, and received grant support for investigator-initiated research from Boehringer-Ingelheim, Gilead, Lexicon, Novo-Nordisk, and Maze Therapeutics.
AUTHOR CONTRIBUTIONS
V.V. wrote review.
DATA AVAILABILITY
Not applicable.
REFERENCES
- 1. Vallon V. Glucose transporters in the kidney in health and disease. Pflugers Arch 2020; 472:1345–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nuffield Department of Population Health Renal Studies Group, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022; 400:1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heerspink HJL, Jongs N, Chertow GM, Langkilde AM, McMurray JJV, Correa-Rotter R, Rossing P, Sjostrom CD, Stefansson BV, Toto RD, Wheeler DC, Greene T; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021; 9:743–754. [DOI] [PubMed] [Google Scholar]
- 4. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, McMurray JJV, Solomon SD.. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022; 400:757–767. [DOI] [PubMed] [Google Scholar]
- 5. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T.. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011; 22:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H.. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012; 61:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V.. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 2014; 306:F188–F193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM.. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018; 41:356–363. [DOI] [PubMed] [Google Scholar]
- 9. Fitchett D, Inzucchi SE, Zinman B, Wanner C, Schumacher M, Schmoor C, Ohneberg K, Ofstad AP, Salsali A, George JT, Hantel S, Bluhmki E, Lachin JM, Zannad F.. Mediators of the improvement in heart failure outcomes with empagliflozin in the EMPA-REG OUTCOME trial. ESC Heart Fail 2021; 8:4517–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Neal B, Perkovic V, de Zeeuw D, Neuen BL, Arnott C, Simpson R, Oh R, Mahaffey KW, Heerspink HJL.. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int 2020; 98:769–777. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, Neal B.. Mediators of the effects of Canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 2020; 8:57–66. [DOI] [PubMed] [Google Scholar]
- 12. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 2020; 43:508–511. [DOI] [PubMed] [Google Scholar]
- 13. Vallon V, Verma S.. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 2021; 83:503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mudaliar S, Alloju S, Henry RR.. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016; 39:1115–1122. [DOI] [PubMed] [Google Scholar]
- 15. Ferrannini E, Mark M, Mayoux E.. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016; 39:1108–1114. [DOI] [PubMed] [Google Scholar]
- 16. Qiu H, Novikov A, Vallon V.. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 2017; 33:5. [DOI] [PubMed] [Google Scholar]
- 17. Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK.. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 2015; 38:316–322. [DOI] [PubMed] [Google Scholar]
- 18. Billing AM, Kim YC, Gullaksen S, Schrage B, Raabe J, Hutzfeldt A, Demir F, Kovalenko E, Lasse M, Dugourd A, Fallegger R, Klampe B, Jaegers J, Li Q, Kravtsova O, Crespo-Masip M, Palermo A, Fenton RA, Hoxha E, Blankenberg S, Kirchhof P, Huber TB, Laugesen E, Zeller T, Chrysopoulou M, Saez-Rodriguez J, Magnussen C, Eschenhagen T, Staruschenko A, Siuzdak G, Poulsen PL, Schwab C, Cuello F, Vallon V, Rinschen MM.. Metabolic communication by SGLT2 inhibition. Circulation 2024; 149:860–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaub JA, AlAkwaa FM, McCown PJ, Naik AS, Nair V, Eddy S, Menon R, Otto EA, Demeke D, Hartman J, Fermin D, O’Connor C, Subramanian L, Bitzer M, Harned R, Ladd P, Pyle L, Pennathur S, Inoki K, Hodgin JB, Brosius FC, Nelson RG, Kretzler M, Bjornstad P.. SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth onset type 2 diabetes. J Clin Invest 2023; 133:e164486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mulder S, Heerspink HJL, Darshi M, Kim JJ, Laverman GD, Sharma K, Pena MJ.. Effects of dapagliflozin on urinary metabolites in people with type 2 diabetes. Diabetes Obes Metab 2019; 21:2422–2428. [DOI] [PubMed] [Google Scholar]
- 21. Wu J, Sun Z, Yang S, Fu J, Fan Y, Wang N, Hu J, Ma L, Peng C, Wang Z, Lee K, He JC, Li Q.. Kidney single-cell transcriptome profile reveals distinct response of proximal tubule cells to SGLT2i and ARB treatment in diabetic mice. Mol Ther 2021; 30:1741–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, Sharma K, Thomson SC, Vallon V.. A role for tubular Na(+)/H(+) exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 2020; 319:F712–F728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verissimo T, Dalga D, Arnoux G, Sakhi I, Faivre A, Auwerx H, Bourgeois S, Paolucci D, Gex Q, Rutkowski JM, Legouis D, Wagner CA, Hall AM, de Seigneux S.. PCK1 is a key regulator of metabolic and mitochondrial functions in renal tubular cells. Am J Physiol Renal Physiol 2023; 324:F532–F543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verissimo T, Faivre A, Rinaldi A, Lindenmeyer M, Delitsikou V, Veyrat-Durebex C, Heckenmeyer C, Fernandez M, Berchtold L, Dalga D, Cohen C, Naesens M, Ricksten SE, Martin PY, Pugin J, Merlier F, Haupt K, Rutkowski JM, Moll S, Cippa PE, Legouis D, de Seigneux S.. Decreased renal gluconeogenesis is a hallmark of chronic kidney disease. J Am Soc Nephrol 2022; 33:810–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Huan Y, Leibensperger M, Seo B, Song Y.. Comparative effects of sodium-glucose cotransporter 2 inhibitors on serum electrolyte levels in patients with type 2 diabetes: a pairwise and network meta-analysis of randomized controlled trials. Kidney360 2022; 3:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou W, Simic P, Zhou IY, Caravan P, Vela Parada X, Wen D, Washington OL, Shvedova M, Pierce KA, Clish CB, Mannstadt M, Kobayashi T, Wein MN, Juppner H, Rhee EP.. Kidney glycolysis serves as a mammalian phosphate sensor that maintains phosphate homeostasis. J Clin Invest 2023; 133:e164610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rau M, Thiele K, Hartmann NK, Mollmann J, Wied S, Hohl M, Marx N, Lehrke M.. Effects of empagliflozin on markers of calcium and phosphate homeostasis in patients with type 2 diabetes—data from a randomized, placebo-controlled study. Bone Rep 2022; 16:101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Jong MA, Petrykiv SI, Laverman GD, van Herwaarden AE, de ZD, Bakker SJL, Heerspink HJL, de Borst MH.. Effects of dapagliflozin on circulating markers of phosphate homeostasis. Clin J Am Soc Nephrol 2019; 14:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borges-Junior FA, Silva Dos Santos D, Benetti A, Polidoro JZ, Wisnivesky ACT, Crajoinas RO, Antonio EL, Jensen L, Caramelli B, Malnic G, Tucci PJ, Girardi ACC.. Empagliflozin inhibits proximal tubule NHE3 activity, preserves GFR, and restores euvolemia in nondiabetic rats with induced heart failure. J Am Soc Nephrol 2021; 32:1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rao VS, Ivey-Miranda JB, Cox ZL, Moreno-Villagomez J, Maulion C, Bellumkonda L, Chang J, Field MP, Wiederin DR, Butler J, Collins SP, Turner JM, Wilson FP, Inzucchi SE, Wilcox CS, Ellison DH, Testani JM.. Empagliflozin in heart failure: regional nephron sodium handling effects. J Am Soc Nephrol 2024; 35:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biancalana E, Rossi C, Raggi F, Distaso M, Trico D, Baldi S, Ferrannini E, Solini A.. Empagliflozin and renal sodium-hydrogen exchange in healthy subjects. J Clin Endocrinol Metab 2023; 108:e567–e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Novikov A, Fu Y, Huang W, Freeman B, Patel R, van GC, Koepsell H, Busslinger M, Onishi A, Nespoux J, Vallon V.. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol 2019; 316:F173–F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suijk D, van Baar M, van Bommel E, Iqbal Z, Krebber M, Vallon V, Touw D, Hoorn E, Nieuwdorp M, Kramer M, Joles J, Bjornstad P, van Raalte D.. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol 2022; 17:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajkumar P, Cha B, Yin J, Arend LJ, Paunescu TG, Hirabayashi Y, Donowitz M, Pluznick JL.. Identifying the localization and exploring a functional role for Gprc5c in the kidney. FASEB J 2018; 32:2046–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Layton AT, Laghmani K, Vallon V, Edwards A.. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol 2016; 311:F1217–F1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Layton AT, Vallon V, Edwards A.. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 2015; 308:F1343–F1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Layton AT, Vallon V.. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 2018; 314:F969–F984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T.. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306:F194–F204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sinha F, Federlein A, Biesold A, Schwarzfischer M, Krieger K, Schweda F, Tauber P.. Empagliflozin increases kidney weight due to increased cell size in the proximal tubule S3 segment and the collecting duct. Front Pharmacol 2023; 14:1118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T.. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2013; 304:F156–F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilbert RE, Thorpe KE.. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab 2019; 21:1996–2000. [DOI] [PubMed] [Google Scholar]
- 42. Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ.. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7:845–854. [DOI] [PubMed] [Google Scholar]
- 43. Gullaksen S, Vernstrom L, Sorensen SS, Ringgaard S, Laustsen C, Funck KL, Poulsen PL, Laugesen E.. Separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: a randomised trial. Diabetologia 2023; 66:813–825. [DOI] [PubMed] [Google Scholar]
- 44. Laursen JC, Sondergaard-Heinrich N, de Melo JML, Haddock B, Rasmussen IKB, Safavimanesh F, Hansen CS, Storling J, Larsson HBW, Groop PH, Frimodt-Moller M, Andersen UB, Rossing P.. Acute effects of dapagliflozin on renal oxygenation and perfusion in type 1 diabetes with albuminuria: a randomised, double-blind, placebo-controlled crossover trial. EClinicalMedicine 2021; 37:100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pruijm M, Milani B, Pivin E, Podhajska A, Vogt B, Stuber M, Burnier M.. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 2018; 93:932–940. [DOI] [PubMed] [Google Scholar]
- 46. Vallon V, Muhlbauer B, Osswald H.. Adenosine and kidney function. Physiol Rev 2006; 86:901–940. [DOI] [PubMed] [Google Scholar]
- 47. Thurau K, Boylan JW.. Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med 1976; 61:308–315. [DOI] [PubMed] [Google Scholar]
- 48. Holtkamp FA, dZ D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ.. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011; 80:282–287. [DOI] [PubMed] [Google Scholar]
- 49. O’Bryan GT, Hostetter TH.. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 1997; 17:93–100. [PubMed] [Google Scholar]
- 50. Vallon V, Thomson SC.. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 2020; 16:317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallon V, Richter K, Blantz RC, Thomson S, Osswald H.. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 1999; 10:2569–2576. [DOI] [PubMed] [Google Scholar]
- 52. Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P.. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 2012; 302:R75–R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomson SC, Vallon V.. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am J Physiol Renal Physiol 2021; 320:F761–F771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, Touw DJ, Larsen EL, Poulsen HE, Kramer MHH, Nieuwdorp M, Joles JA, van Raalte DH.. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 2020; 97:202–212. [DOI] [PubMed] [Google Scholar]
- 55. Lytvyn Y, Kimura K, Peter N, Lai V, Tse J, Cham L, Perkins BA, Soleymanlou N, Cherney DZI.. Renal and vascular effects of combined SGLT2 and angiotensin-converting enzyme inhibition. Circulation 2022; 146:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bakris G, Oshima M, Mahaffey KW, Agarwal R, Cannon CP, Capuano G, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, Levin A, Neal B, Oh R, Pollock C, Rosenthal N, Wheeler DC, Zhang H, Zinman B, Jardine MJ, Perkovic V.. Effects of Canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m(2): subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol 2020; 15:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang J, Cai J, Cui Y, Jiang S, Wei J, Kim YC, Chan J, Thalakola A, Le T, Xu L, Wang L, Jiang K, Wang X, Wang H, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R.. Role of the macula densa sodium glucose cotransporter type 1-neuronal nitric oxide synthase-tubuloglomerular feedback pathway in diabetic hyperfiltration. Kidney Int 2022; 101:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson S, Liu R, Vallon V.. Knockout of Na(+)-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol 2019; 317:F207–F217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R.. Macula densa SGLT1-NOS1-TGF pathway—a new mechanism for glomerular hyperfiltration during hyperglycemia. J Am Soc Nephrol 2019; 30:578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL.. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 2018; 20:1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan HC, Chen JY, Chen HY, Yeh FY, Huang TT, Sun CY, Wang SI, Wei JC, Wu VC.. Sodium-glucose cotransport protein 2 inhibitors in patients with type 2 diabetes and acute kidney disease. JAMA Netw Open 2024; 7:e2350050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Faivre A, Bugarski M, Rinaldi A, Sakhi IB, Verissimo T, Legouis D, Correia S, Kaminska M, Dalga D, Malpetti D, Cippa PE, de Seigneux S, Hall AM.. Spatiotemporal landscape of kidney tubular responses to glomerular proteinuria. J Am Soc Nephrol 2024; 35:854–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verma S, Sharma A, Kanumilli N, Butler J.. Predictors of heart failure development in type 2 diabetes: a practical approach. Curr Opin Cardiol 2019; 34:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ.. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC.. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 2018; 7:e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Masuda T, Muto S, Fukuda K, Watanabe M, Ohara K, Koepsell H, Vallon V, Nagata D.. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep 2020; 8:e14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Masuda T, Ohara K, Vallon V, Nagata D.. SGLT2 inhibitor and loop diuretic induce different vasopressin and fluid homeostatic responses in nondiabetic rats. Am J Physiol Renal Physiol 2022; 323:F361–F369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Ruiten CC, Smits MM, Kok MD, Serne EH, van Raalte DH, Kramer MHH, Nieuwdorp M, RG IJ.. Mechanisms underlying the blood pressure lowering effects of dapagliflozin, exenatide, and their combination in people with type 2 diabetes: a secondary analysis of a randomized trial. Cardiovasc Diabetol 2022; 21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mayne KJ, Staplin N, Keane DF, Wanner C, Brenner S, Cejka V, Stegbauer J, Judge PK, Preiss D, Emberson J, Trinca D, Dayanandan R, Lee R, Nolan J, Omata A, Green JB, Cherney DZ, Hooi LS, Pontremoli R, Tuttle KR, Lees JS, Mark PB, Davies SJ, Hauske SJ, Steubl D, Bruckmann M, Landray MJ, Baigent FC, Haynes R, Herrington WG; centers E-KCGSSMfflotc, site staff from the E-KCGwcttS. Effects of empagliflozin on fluid overload, weight and blood pressure in chronic kidney disease. J Am Soc Nephrol 2024; 35:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lambers Heerspink HJ, de ZD, Wie L, Leslie B, List J.. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013; 15:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW.. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018; 20:479–487. [DOI] [PubMed] [Google Scholar]
- 72. Neuen BL, Oshima M, Agarwal R, Arnott C, Cherney DZ, Edwards R, Langkilde AM, Mahaffey KW, McGuire DK, Neal B, Perkovic V, Pong A, Sabatine MS, Raz I, Toyama T, Wanner C, Wheeler DC, Wiviott SD, Zinman B, Heerspink HJL.. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022; 145:1460–1470. [DOI] [PubMed] [Google Scholar]
- 73. Paasche A, Wiedmann F, Kraft M, Seibertz F, Herlt V, Blochberger PL, Javorszky N, Beck M, Weirauch L, Seeger T, Blank A, Haefeli WE, Arif R, Meyer AL, Warnecke G, Karck M, Voigt N, Frey N, Schmidt C.. Acute antiarrhythmic effects of SGLT2 inhibitors-dapagliflozin lowers the excitability of atrial cardiomyocytes. Basic Res Cardiol 2024; 119:93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liao J, Ebrahimi R, Ling Z, Meyer C, Martinek M, Sommer P, Futyma P, Di Vece D, Schratter A, Acou WJ, Zhu L, Kiuchi MG, Liu S, Yin Y, Purerfellner H, Templin C, Chen S.. Effect of SGLT-2 inhibitors on arrhythmia events: insight from an updated secondary analysis of >80,000 patients (the SGLT2i-Arrhythmias and Sudden Cardiac Death). Cardiovasc Diabetol 2024; 23:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fujiki S, Iijima K, Nakagawa Y, Takahashi K, Okabe M, Kusano K, Owada S, Kondo Y, Tsujita K, Shimizu W, Tomita H, Watanabe M, Shoda M, Watanabe M, Tokano T, Murohara T, Kaneshiro T, Kato T, Hayashi H, Maemura K, Niwano S, Umemoto T, Yoshida H, Ota K, Tanaka T, Kitamura N, Node K, Minamino T, investigators EI.. Effect of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes treated with an implantable cardioverter-defibrillator: the EMPA-ICD trial. Cardiovasc Diabetol 2024; 23:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dyck JRB, Sossalla S, Hamdani N, Coronel R, Weber NC, Light PE, Zuurbier CJ.. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: evidence for potential off-target effects. J Mol Cell Cardiol 2022; 167:17–31. [DOI] [PubMed] [Google Scholar]
- 77. Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, Swietach P, Shattock MJ.. Off-target effects of sodium-glucose co-transporter 2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart. Cardiovasc Res 2021; 117:2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ozcan M, Guo Z, Valenzuela Ripoll C, Diab A, Picataggi A, Rawnsley D, Lotfinaghsh A, Bergom C, Szymanski J, Hwang D, Asnani A, Kosiborod M, Zheng J, Hayashi RJ, Woodard PK, Kovacs A, Margulies KB, Schilling J, Razani B, Diwan A, Javaheri A.. Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity. Cell Metab 2023; 35:928–942.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. You AS, Kalantar-Zadeh K, Lerner L, Nakata T, Lopez N, Lou L, Veliz M, Soohoo M, Jing J, Zaldivar F, Gyuris J, Nguyen DV, Rhee CM.. Association of growth differentiation factor 15 with mortality in a prospective hemodialysis cohort. Cardiorenal Med 2017; 7:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vallon V, Eraly SA, Wikoff WR, Rieg T, Kaler G, Truong DM, Ahn SY, Mahapatra NR, Mahata SK, Gangoiti JA, Wu W, Barshop BA, Siuzdak G, Nigam SK.. Organic anion transporter 3 contributes to the regulation of blood pressure. J Am Soc Nephrol 2008; 19:1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oe Y, Kim YC, Sidorenko VS, Zhang H, Kanoo S, Lopez N, Goodluck HA, Crespo-Masip M, Vallon V.. SGLT2 inhibitor dapagliflozin protects the kidney in a murine model of Balkan nephropathy. Am J Physiol Renal Physiol 2024; 326:F227–F240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gyarmati G, Shroff UN, Riquier-Brison A, Desposito D, Ju W, Stocker SD, Izuhara A, Deepak S, Becerra Calderon A, Burford JL, Kadoya H, Moon JY, Chen Y, Rinschen MM, Ahmadi N, Lau L, Biemesderfer D, James AW, Minichiello L, Zlokovic BV, Gill IS, Kretzler M, Peti-Peterdi J.. Neuronally differentiated macula densa cells regulate tissue remodeling and regeneration in the kidney. J Clin Invest 2024; 134:e174558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fattah H, Vallon V.. The potential role of SGLT2 inhibitors in the treatment of type 1 diabetes mellitus. Drugs 2018; 78:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu H, Sridhar VS, Perkins BA, Rosenstock J, Cherney DZI.. SGLT2 inhibition in type 1 diabetes with diabetic kidney disease: potential cardiorenal benefits can outweigh preventable risk of diabetic ketoacidosis. Curr Diab Rep 2022; 22:317–332. [DOI] [PubMed] [Google Scholar]
- 85. Oe Y, Vallon V.. The pathophysiological basis of diabetic kidney protection by inhibition of SGLT2 and SGLT1. Kidney Dial 2022; 2:349–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pitt B, Bhatt DL.. Does SGLT1 inhibition add benefit to SGLT2 inhibition in type 2 diabetes? Circulation 2021; 144:4–6. [DOI] [PubMed] [Google Scholar]
- 87. Mann JFE, Rossing P, Bakris G, Belmar N, Bosch-Traberg H, Busch R, Charytan DM, Hadjadj S, Gillard P, Gorriz JL, Idorn T, Ji L, Mahaffey KW, Perkovic V, Rasmussen S, Schmieder RE, Pratley RE, Tuttle KR.. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat Med 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cohen S, Sternlicht H, Bakris GL.. Mineralocorticoid receptor antagonists in the treatment of diabetic kidney disease: their application in the era of SGLT2 inhibitors and GLP-1 receptor agonists. Curr Diab Rep 2022; 22:213–218. [DOI] [PubMed] [Google Scholar]
- 89. Jiang Y, Rose AJ, Sijmonsma TP, Broer A, Pfenninger A, Herzig S, Schmoll D, Broer S.. Mice lacking neutral amino acid transporter B(0)AT1 (Slc6a19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol Metab 2015; 4:406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Navarro Garrido A, Kim YC, Oe Y, Zhang H, Crespo-Masip M, Goodluck HA, Kanoo S, Sanders PW, Broer S, Vallon V.. Aristolochic acid-induced nephropathy is attenuated in mice lacking the neutral amino acid transporter B(0)AT1 (Slc6a19). Am J Physiol Renal Physiol 2022; 323:F455–F467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sveinbjornsson G, Mikaelsdottir E, Palsson R, Indridason OS, Holm H, Jonasdottir A, Helgason A, Sigurdsson S, Jonasdottir A, Sigurdsson A, Eyjolfsson GI, Sigurdardottir O, Magnusson OT, Kong A, Masson G, Sulem P, Olafsson I, Thorsteinsdottir U, Gudbjartsson DF, Stefansson K.. Rare mutations associating with serum creatinine and chronic kidney disease. Hum Mol Genet 2014; 23:6935–6943. [DOI] [PubMed] [Google Scholar]
- 92. Vallon V. How can inhibition of glucose and sodium transport in the early proximal tubule protect the cardiorenal system? Nephrol Dial Transplant 2024; e-pub ahead of print. doi: 10.1093/ndt/gfae060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.