Abstract
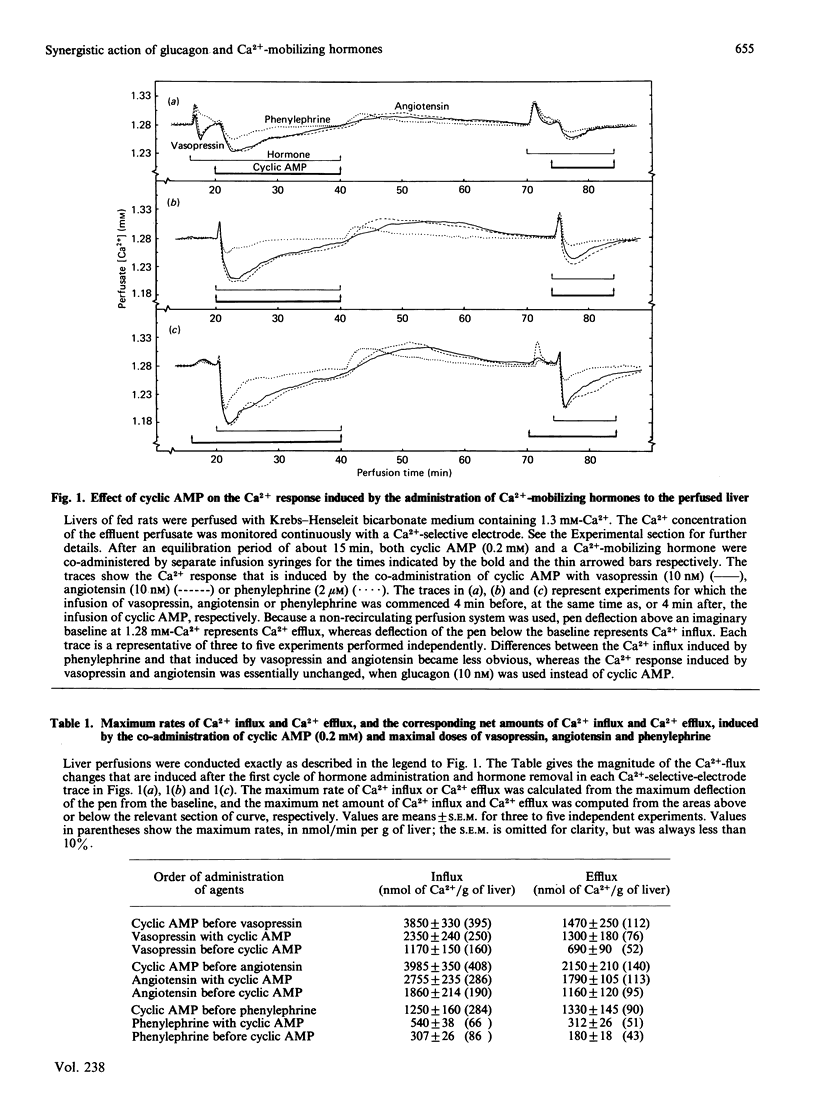
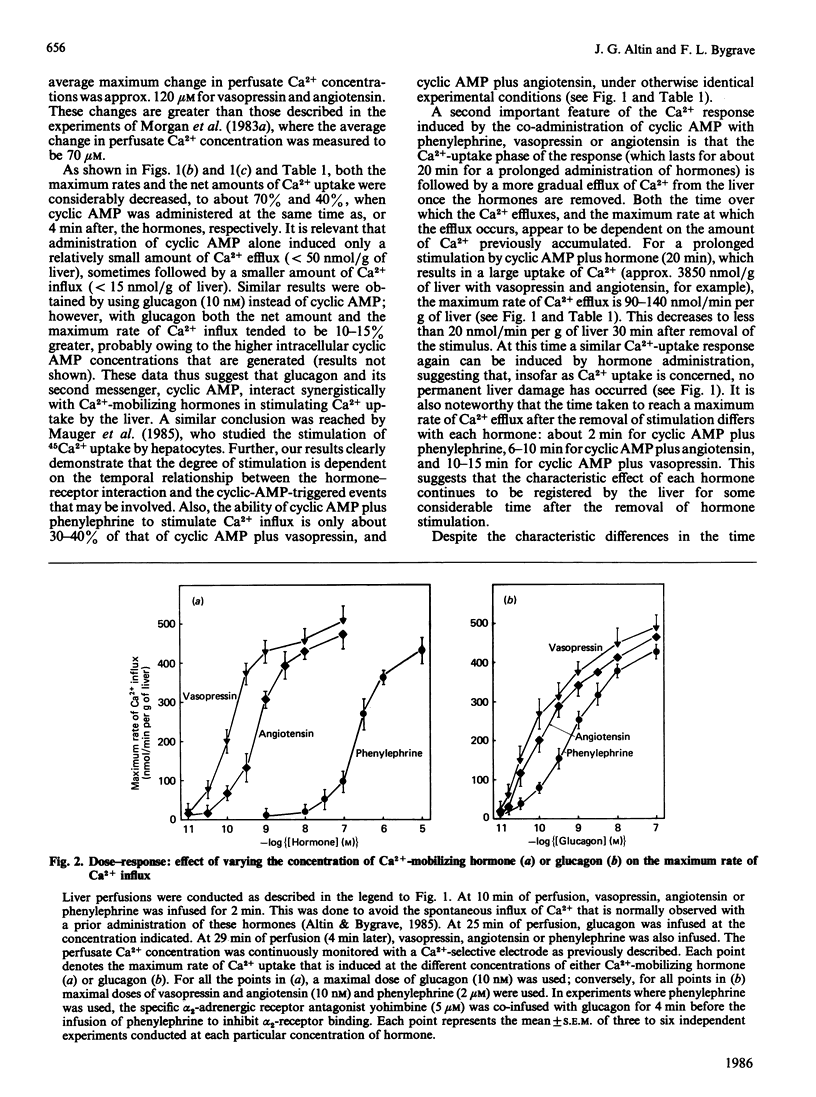
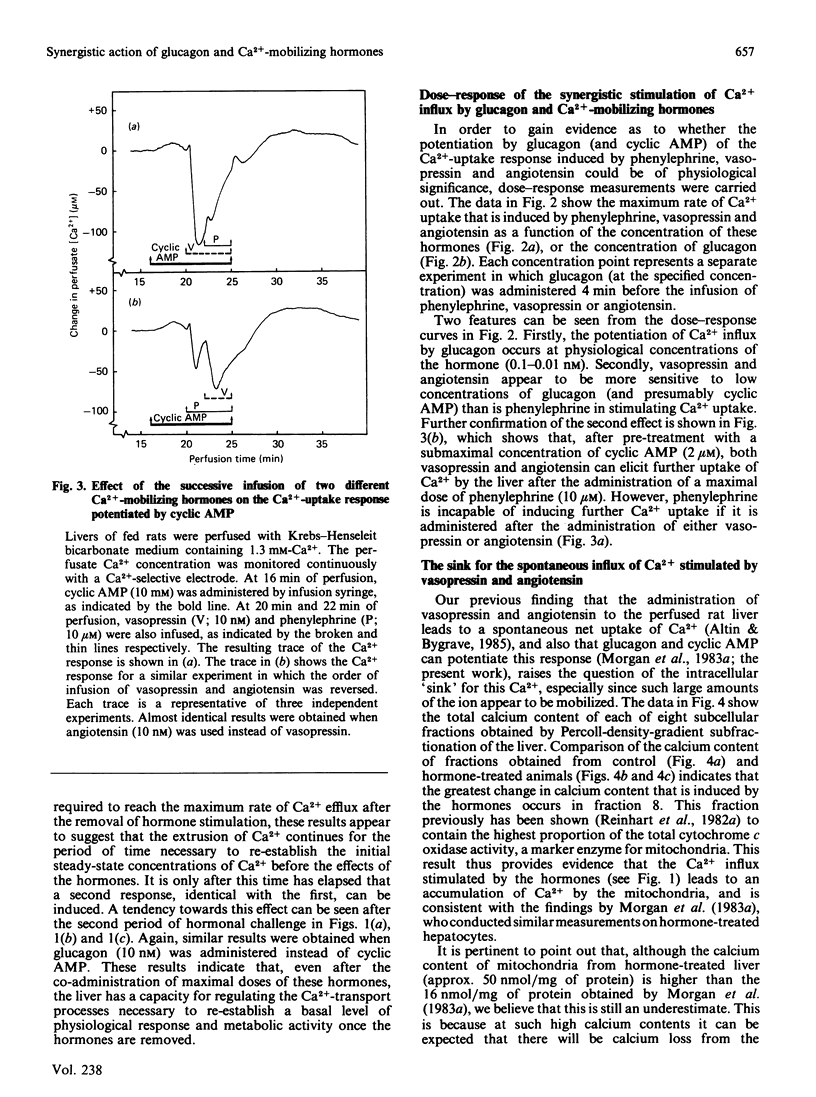
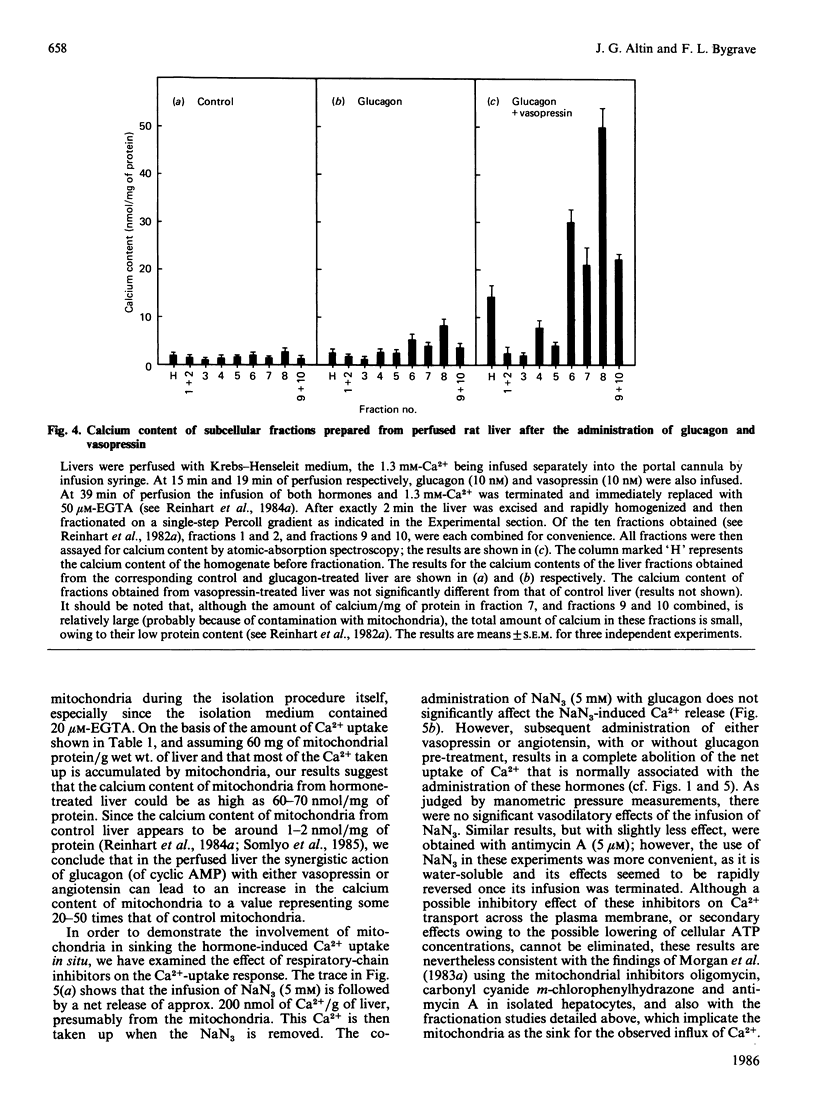
A perfused liver system incorporating a Ca2+-sensitive electrode was used to study the long-term effects of glucagon and cyclic AMP on the mobilization of Ca2+ induced by phenylephrine, vasopressin and angiotensin. At 1.3 mM extracellular Ca2+ the co-administration of glucagon (10 nM) or cyclic AMP (0.2 mM) and a Ca2+-mobilizing hormone led to a synergistic potentiation of Ca2+ uptake by the liver, to a degree which was dependent on the order of hormone administration. A maximum net amount of Ca2+ influx, corresponding to approx. 3800 nmol/g of liver (the maximum rate of influx was 400 nmol/min per g of liver), was induced when cyclic AMP or glucagon was administered about 4 min before vasopressin and angiotensin. These changes are over an order of magnitude greater than those induced by Ca2+-mobilizing hormones alone [Altin & Bygrave (1985) Biochem. J. 232, 911-917]. For a maximal response the influx of Ca2+ was transient and was essentially complete after about 20 min. Removal of the hormones was followed by a gradual efflux of Ca2+ from the liver over a period of 30-50 min; thereafter, a similar response could be obtained by a second administration of hormones. Dose-response measurements indicate that the potentiation of Ca2+ influx by glucagon occurs even at low (physiological) concentrations of the hormone. By comparison with phenylephrine, the stimulation of Ca2+ influx by vasopressin and angiotensin is more sensitive to low concentrations of glucagon and cyclic AMP, and can be correlated with a 20-50-fold increase in the calcium content of mitochondria. The reversible uptake of such large quantities of Ca2+ implicates the mitochondria in long-term cellular Ca2+ regulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. The Ca2+-mobilizing actions of vasopressin and angiotensin differ from those of the alpha-adrenergic agonist phenylephrine in the perfused rat liver. Biochem J. 1985 Dec 15;232(3):911–917. doi: 10.1042/bj2320911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andia-Waltenbaugh A. M., Kimura S., Wood J., Divakaran P., Friedmann N. Effects of glucagon, insulin and cyclic-AMP on mitochondrial calcium uptake in the liver. Life Sci. 1978 Dec 11;23(24):2437–2443. doi: 10.1016/0024-3205(78)90303-x. [DOI] [PubMed] [Google Scholar]
- Andia-Waltenbaugh A. M., Tate C. A., Friedmann N. K. The effect of glucagon on the kinetics of hepatic mitochondrial calcium uptake. Mol Cell Biochem. 1981 May 26;36(3):177–184. doi: 10.1007/BF02357035. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet A., Berthon B., Claret M. Hormone-induced increase in free cytosolic calcium and glycogen phosphorylase activation in rat hepatocytes incubated in normal and low-calcium media. Biochem J. 1985 Jun 15;228(3):565–574. doi: 10.1042/bj2280565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Bühler H. U., da Prada M., Haefely W., Picotti G. B. Plasma adrenaline, noradrenaline and dopamine in man and different animal species. J Physiol. 1978 Mar;276:311–320. doi: 10.1113/jphysiol.1978.sp012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Jenkinson D. H., Koller K. Interactions between receptors that increase cytosolic calcium and cyclic AMP in guinea-pig liver cells. Br J Pharmacol. 1984 Sep;83(1):281–291. doi: 10.1111/j.1476-5381.1984.tb10144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Friedmann N., Park C. R. Early effects of 3',5'-adenosine monophosphate on the fluxes of calcium end potassium in the perfused liver of normal and adrenalectomized rats. Proc Natl Acad Sci U S A. 1968 Oct;61(2):504–508. doi: 10.1073/pnas.61.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Effects of glucagon and N6O2'-dibutyryladenosine 3':5'-cyclic monophosphate on calcium transport in isolated rat liver mitochondria. Biochem J. 1978 Oct 15;176(1):295–304. doi: 10.1042/bj1760295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch C. J., Blackmore P. F., Charest R., Exton J. H. The relationships between receptor binding capacity for norepinephrine, angiotensin II, and vasopressin and release of inositol trisphosphate, Ca2+ mobilization, and phosphorylase activation in rat liver. Mol Pharmacol. 1985 Aug;28(2):93–99. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem J. 1985 Nov 1;231(3):581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. Studies on the activation of rat liver pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase by adrenaline and glucagon. Role of increases in intramitochondrial Ca2+ concentration. Biochem J. 1985 Nov 1;231(3):597–608. doi: 10.1042/bj2310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Modulation of the alpha 1-adrenergic control of hepatocyte calcium redistribution by increases in cyclic AMP. J Biol Chem. 1983 Apr 25;258(8):5110–5116. [PubMed] [Google Scholar]
- Morgan N. G., Charest R., Blackmore P. F., Exton J. H. Potentiation of alpha 1-adrenergic responses in rat liver by a cAMP-dependent mechanism. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4208–4212. doi: 10.1073/pnas.81.13.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Shipp C. C., Exton J. H. Studies on the mechanism of inhibition of hepatic cAMP accumulation by vasopressin. FEBS Lett. 1983 Nov 14;163(2):277–281. doi: 10.1016/0014-5793(83)80835-7. [DOI] [PubMed] [Google Scholar]
- Prpić V., Bygrave F. L. On the inter-relationship between glucagon action, the oxidation-reduction state of pyridine nucleotides, and calcium retention by rat liver mitochondria. J Biol Chem. 1980 Jul 10;255(13):6193–6199. [PubMed] [Google Scholar]
- Prpić V., Spencer T. L., Bygrave F. L. Stable enhancement of calcium retention in mitochondria isolated from rat liver after the administration of glucagon to the intact animal. Biochem J. 1978 Dec 15;176(3):705–714. doi: 10.1042/bj1760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The action of alpha-adrenergic agonists on plasma-membrane calcium fluxes in perfused rat liver. Biochem J. 1984 May 15;220(1):43–50. doi: 10.1042/bj2200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., van de Pol E., Taylor W. M., Bygrave F. L. An assessment of the calcium content of rat liver mitochondria in vivo. Biochem J. 1984 Mar 1;218(2):415–420. doi: 10.1042/bj2180415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Reinhart P., Hunt N. H., Bygrave F. L. Role of 3',5'-cyclic AMP in glucagon-induced stimulation of ruthenium red-insensitive calcium transport in an endoplasmic reticulum-rich fraction of rat liver. FEBS Lett. 1980 Mar 24;112(1):92–96. doi: 10.1016/0014-5793(80)80136-0. [DOI] [PubMed] [Google Scholar]
- Vargas A. M., Halestrap A. P., Denton R. M. The effects of glucagon, phenylephrine and insulin on the phosphorylation of cytoplasmic, mitochondrial and membrane-bound proteins of intact liver cells from starved rats. Biochem J. 1982 Oct 15;208(1):221–229. doi: 10.1042/bj2080221. [DOI] [PMC free article] [PubMed] [Google Scholar]


