Abstract
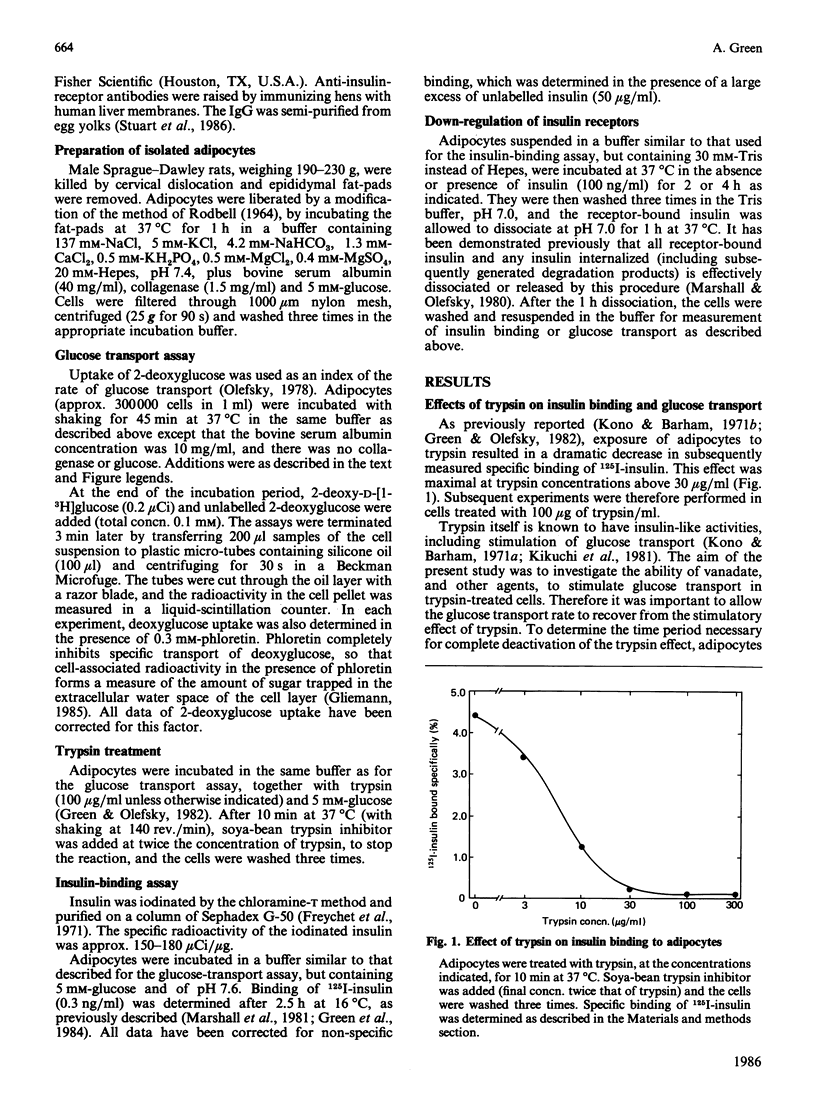
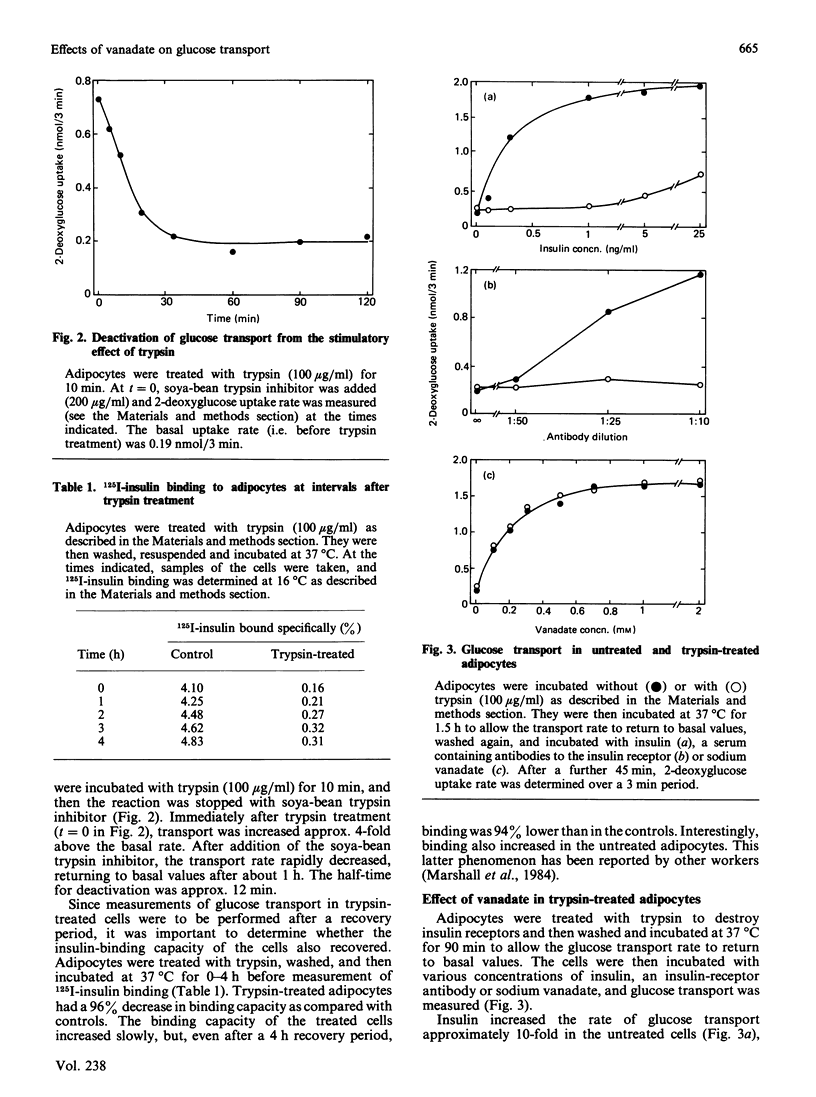
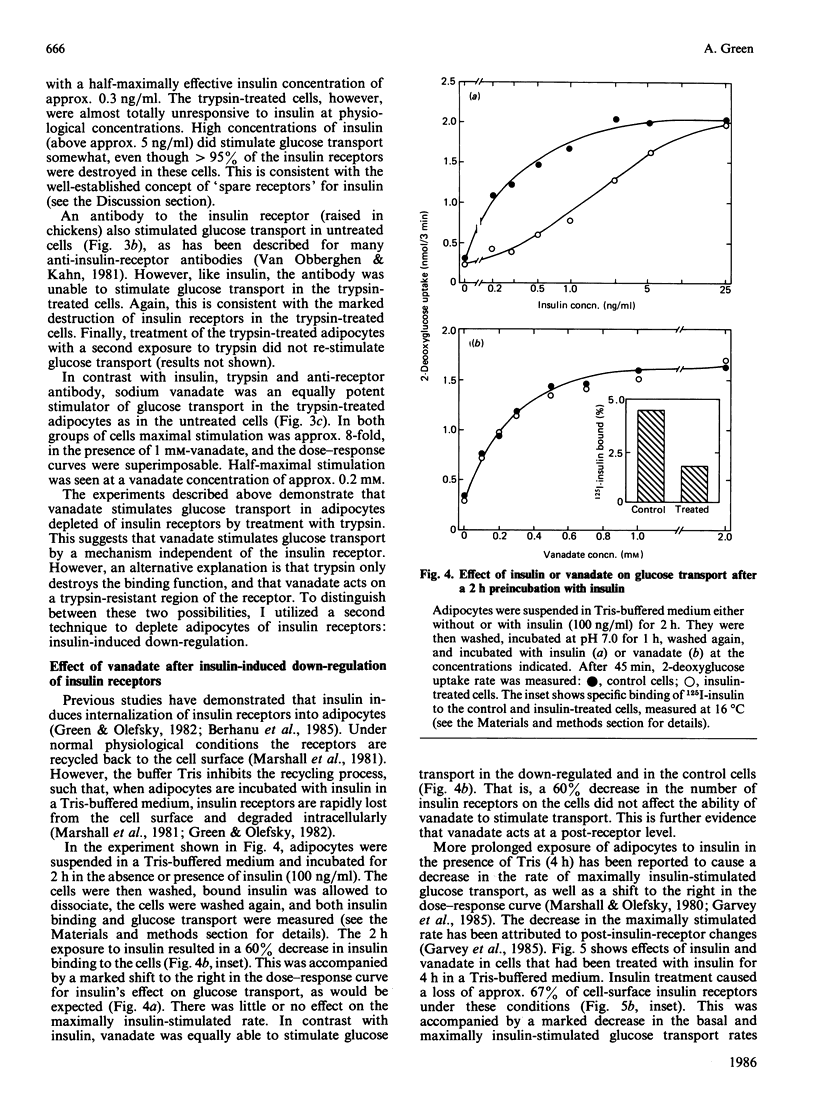
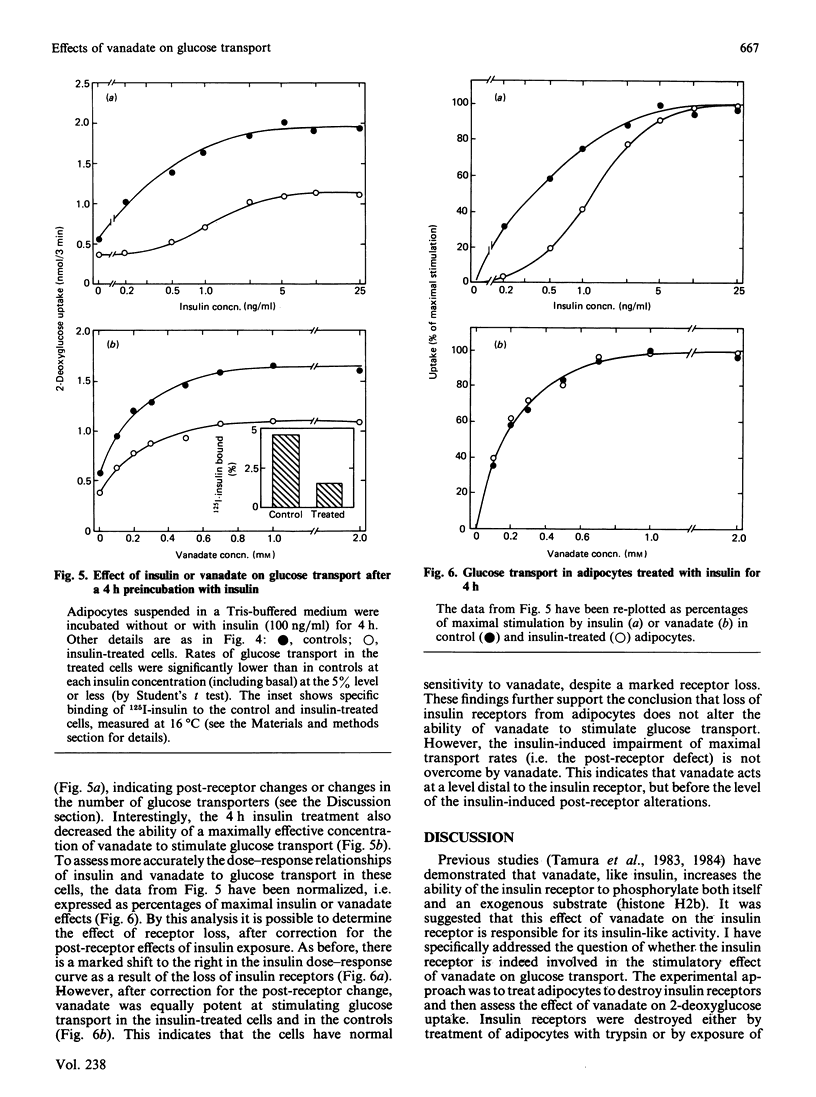
Sodium vanadate has several insulin-like effects. To determine whether vanadate acts via the insulin receptor, I investigated the effect of vanadate on glucose transport (2-deoxyglucose uptake) in adipocytes that had been treated to decrease the number of insulin receptors. Trypsin (100 micrograms/ml) caused greater than 95% loss of 125I-insulin binding and rendered glucose transport resistant to both insulin and an anti-insulin-receptor antibody. However, vanadate caused an 8-fold increase in the transport rate [EC50 (concn. giving 50% of maximum effect) 0.2 mM] in both control and trypsin-treated cells, demonstrating that the insulin receptor does not have to be intact for vanadate to stimulate glucose transport. Insulin receptors were depleted by treatment of adipocytes with insulin (100 ng/ml) in the presence of Tris (which blocks receptor recycling). A 2 h treatment caused 60% loss of receptors, and a shift to the right in the dose-response curve for insulin stimulation of glucose transport (EC50 0.3 ng of insulin/ml in controls, 1.2 ng/ml in treated cells). The response to vanadate was again unaffected. Treatment with insulin for 4 h caused a 67% decrease in insulin binding and, in addition to the rightward shift in the insulin dose-response curve, a decrease in basal and maximal transport rates (which cannot be explained by decreased insulin receptor number). The EC50 of vanadate was again equal in control and treated cells, but glucose transport in the presence of a maximally effective concentration of vanadate (1 mM) was decreased. I conclude that the effect of vanadate on glucose transport is independent of the insulin receptor. Induction of a post-receptor defect (which may be a decrease in the total number of cellular glucose transporters) by prolonged exposure to insulin decreases the potency of a maximally effective concentration of vanadate. The findings demonstrate that vanadate stimulates glucose transport by an effect at a level distal to the insulin receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clausen T., Andersen T. L., Stürup-Johansen M., Petkova O. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. XI. The effect of vanadate on 45Ca-efflux and sugar transport in adipose tissue and skeletal muscle. Biochim Biophys Acta. 1981 Aug 20;646(2):261–267. doi: 10.1016/0005-2736(81)90332-1. [DOI] [PubMed] [Google Scholar]
- Delfert D. M., McDonald J. M. Vanadyl and vanadate inhibit Ca2+ transport systems of the adipocyte plasma membrane and endoplasmic reticulum. Arch Biochem Biophys. 1985 Sep;241(2):665–672. doi: 10.1016/0003-9861(85)90593-4. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Kleinzeller A. The insulin-mimetic effects of vanadate in isolated rat adipocytes. Dissociation from effects of vanadate as a (Na+-K+)ATPase inhibitor. J Biol Chem. 1980 Jun 10;255(11):5306–5312. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Marshall S. Insulin receptor down-regulation is linked to an insulin-induced postreceptor defect in the glucose transport system in rat adipocytes. J Clin Invest. 1985 Jul;76(1):22–30. doi: 10.1172/JCI111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A., Bustillos D. P., Misbin R. I. beta-Hydroxybutyrate increases the insulin sensitivity of adipocyte glucose transport at a postreceptor level. Diabetes. 1984 Nov;33(11):1045–1050. doi: 10.2337/diab.33.11.1045. [DOI] [PubMed] [Google Scholar]
- Green A., Olefsky J. M. Evidence for insulin-induced internalization and degradation of insulin receptors in rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):427–431. doi: 10.1073/pnas.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedo J. A., Kasuga M., Van Obberghen E., Roth J., Kahn C. R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: evidence for heterogeneity. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4791–4795. doi: 10.1073/pnas.78.8.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H. U., Kasuga M., Kahn C. R. Insulin receptor phosphorylation in intact adipocytes and in a cell-free system. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1538–1545. doi: 10.1016/s0006-291x(82)80082-x. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Schwartz C., Creacy S., Larner J. Independent control of selected insulin-sensitive cell membrane and intracellular functions-the linkage of cell membrane and intracellular events controlled by insulin. III. The influence of trypsin on cell membrane hexose transport and on glycogen synthase and mitochondrial pyruvate dehydrogenase activation. Mol Cell Biochem. 1981 Jul 7;37(2):125–130. doi: 10.1007/BF02354936. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. Insulin-like effects of trypsin on fat cells. Localization of the metabolic steps and the cellular site affected by the enzyme. J Biol Chem. 1971 Oct 25;246(20):6204–6209. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Marshall S., Garvey W. T., Geller M. Primary culture of isolated adipocytes. A new model to study insulin receptor regulation and insulin action. J Biol Chem. 1984 May 25;259(10):6376–6384. [PubMed] [Google Scholar]
- Marshall S., Green A., Olefsky J. M. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981 Nov 25;256(22):11464–11470. [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978 Apr 15;172(1):137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Ramasarma T., Crane F. L. Does vanadium play a role in cellular regulation? Curr Top Cell Regul. 1981;20:247–301. doi: 10.1016/b978-0-12-152820-1.50011-0. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Dubler R. E., Larner J. Insulin-like effect of vanadate on adipocyte glycogen synthase and on phosphorylation of 95,000 dalton subunit of insulin receptor. Biochem Biophys Res Commun. 1983 May 31;113(1):80–86. doi: 10.1016/0006-291x(83)90434-5. [DOI] [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Whipple J. H., Fujita-Yamaguchi Y., Dubler R. E., Cheng K., Larner J. A novel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. J Biol Chem. 1984 May 25;259(10):6650–6658. [PubMed] [Google Scholar]
- Tolman E. L., Barris E., Burns M., Pansini A., Partridge R. Effects of vanadium on glucose metabolism in vitro. Life Sci. 1979 Sep 24;25(13):1159–1164. doi: 10.1016/0024-3205(79)90138-3. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Kahn C. R. Autoantibodies to insulin receptors. Mol Cell Endocrinol. 1981 Jun;22(3):277–293. doi: 10.1016/0303-7207(81)90037-x. [DOI] [PubMed] [Google Scholar]


