Abstract
Combinations of cytokines are known to reactivate transcription and replication of latent human immunodeficiency virus type 1 (HIV-1) proviruses in resting CD4+ T lymphocytes isolated from infected individuals. Transcription of the HIV-1 provirus by RNA polymerase II is strongly stimulated by the viral Tat protein. Tat function is mediated by a cellular protein kinase known as TAK (cyclin T1/P-TEFb) that is composed of Cdk9 and cyclin T1. We have found that treatment of peripheral blood lymphocytes and purified resting CD4+ T lymphocytes with the combination of interleukin-2 (IL-2), IL-6, and tumor necrosis factor alpha resulted in an increase in Cdk9 and cyclin T1 protein levels and an increase in TAK enzymatic activity. The cytokine induction of TAK in resting CD4+ T lymphocytes did not appear to require proliferation of lymphocytes. These results suggest that induction of TAK by cytokines secreted in the microenvironment of lymphoid tissue may be involved in the reactivation of HIV-1 in CD4+ T lymphocytes harboring a latent provirus.
The introduction of drug combinations known as highly active antiretroviral therapy (HAART) has been a major advance in the treatment of human immunodeficiency virus type 1 (HIV-1) infection and AIDS. HAART inhibits key steps in the virus life cycle by targeting the viral reverse transcriptase and protease, and plasma virus levels become undetectable in many patients after therapy (reviewed in references 5 and 10). However, there appear to be anatomical reservoirs of HIV-1, such as the central nervous system, male urogenital tract, and rectal mucosa, that may allow low levels of viral replication and contribute to the long-term persistence of HIV-1 (1, 21, 43). Additionally, an extremely stable reservoir of latently infected, resting memory CD4+ T lymphocytes is present in patients undergoing HAART therapy (7, 11). Recently, latently HIV-infected naive CD4+ T lymphocytes have also been observed (33).
The presence of this latent reservoir of HIV-infected cells is of concern, since these cells remain as a potential source of reactivation of viral replication. These cells reside predominantly in the microenvironment of lymphoid tissue, where endogenous cytokine secretion regularly occurs in response to normal antigenic stimuli. It has been demonstrated that in vitro combinations of the immunoregulatory cytokine interleukin-2 (IL-2) and the proinflammatory cytokines IL-6 and tumor necrosis factor alpha (TNF-α) reactivate HIV-1 replication in latently infected resting CD4+ T lymphocytes isolated both from antiretroviral-naive patients and from patients receiving HAART and in whom plasma viremia is below detectable levels (9). These findings suggest that combinations of cytokines secreted in response to nonspecific stimuli or as a result of a specific antigenic stimulus could be involved in the reappearance of plasma viremia in patients receiving HAART in whom HIV-1 replication was successfully contained initially but in whom therapy was interrupted.
Expression of the integrated HIV-1 provirus by RNA polymerase II is greatly enhanced by the viral Tat transactivator protein. Tat stimulates elongation by RNA polymerase II through the recruitment of a cellular kinase, TAK (Tat-associated kinase), to the TAR RNA element present at the 5′ ends of all nascent HIV-1 transcripts (reviewed in references 17 and 36). TAK is composed of the catalytic subunit Cdk9 and the regulatory subunit cyclin T1 (41, 45), and it is one of several distinct P-TEFb complexes. P-TEFb is a positive-acting elongation factor originally isolated from Drosophila melanogaster nuclear extracts (reviewed in reference 34). Multiple P-TEFb complexes are present in human cells that contain Cdk9 and differ by the regulatory cyclin subunit; cyclins T1, T2a, and T2b; and possibly cyclin K (15, 32). P-TEFb/TAK is believed to activate transcriptional elongation by hyperphosphorylation of the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase II and perhaps other components of the transcription complex, thereby relieving repression of elongation by negative factors (38, 40). Several independent lines of investigation have established that TAK (cyclin T1/P-TEFb) plays a critical role in Tat transactivation (2, 16, 19, 22, 27, 30, 31, 35, 39, 44, 45).
Regulation of TAK function in CD4+ T lymphocytes and monocytes/macrophages is potentially an important issue with regard to HIV-1 replication in infected individuals. TAK is induced in peripheral blood mononuclear cells (PBMCs) and peripheral blood lymphocytes (PBLs) stimulated with either phorbol 12-myristate 13-acetate (PMA) or phytohemagglutinin (PHA) (20, 23, 41). TAK is also induced in purified CD4+ T lymphocytes upon activation by a number of methods, including PHA, PMA plus ionomycin, and antibodies against CD3 and CD28 (23). The induction of TAK in lymphocytes involves an increase in both the mRNA levels and protein levels of both Cdk9 and cyclin T1 (20, 23). TAK is also induced when the promonocytic cell lines HL-60 and U937 are stimulated by PMA to differentiate to macrophage-like cells (41). The induction in promonocytic cell lines involves an increase in previously limiting amounts of cyclin T1 protein levels by a posttranscriptional mechanism (23).
Because latently infected resting CD4+ T lymphocytes carrying integrated HIV-1 proviruses can be reactivated in vitro by combinations of IL-2, IL-6, and TNF-α, we were interested in investigating whether TAK could be induced by these cytokines. In this study, we found that TAK is induced in PBLs and resting CD4+ T lymphocytes by the combination of these cytokines. These results suggest that induction of TAK by cytokines may be involved in the mechanism of reactivation of latent HIV-1 proviruses.
MATERIALS AND METHODS
Isolation of PBLs and resting CD4+ T lymphocytes.
PBLs were from heparinized blood drawn from hepatitis B virus-, hepatitis C virus-, and HIV-1-negative healthy blood donors (obtained from the Gulf Coast Regional Blood Center). As described previously (23), PBLs were purified by centrifugation through Isolymph (Gallard/Schlesinger) or Ficoll-Paque (Pharmacia), followed by two rounds of depletion of monocytes by adherence to plastic tissue culture dishes. PBLs were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin.
CD4+ T lymphocytes were isolated from PBMCs by indirect magnetic labeling using a MACS cell isolation kit according to the protocol of the manufacturer (Miltenyi Biotec). Non-CD4+ T lymphocytes, i.e., B lymphocytes, monocytes, NK cells, CD8+ T lymphocytes, dendritic cells, early erythroid cells, platelets, and basophils, were indirectly magnetically labeled using a cocktail of hapten-conjugated CD8, CD11b, CD16, CD19, CD36, and CD56 antibodies and MACS MicroBeads coupled to an antihapten monoclonal antibody. The CD4+ T lymphocytes were purified by depletion of the magnetically labeled cells by retaining them on a MACS column in the magnetic field of the MidiMACS. The resting CD4+ T lymphocytes were further purified by magnetically labeling the activated population with CD30 MicroBeads and retaining them on a column that was placed in the magnetic field of a MACS separator. The purity of cells was evaluated by flow cytometry (Beckman-Coulter XL-MCL) with phycoerythrin-conjugated anti-CD4 antibodies; CD4+ T lymphocytes were 95 to 98% pure.
Activation of cells.
For activation, cells were adjusted to 106 cells/ml. Resting CD4+ T lymphocytes were activated by incubation with 5 μg of PHA (Sigma) per ml and 100 U of IL-2 (Hoffmann-La Roche) per ml or with a combination of cytokines (200 U of IL-2 per ml, 400 U of IL-6 [Sigma] per ml, and 2.5 ng of TNF-α [Sigma] per ml) at 37°C for 72 h. PBLs were activated by incubation with either 200 U of IL-2 per ml, 400 U of IL-6 per ml, or 2.5 ng of TNF-α per ml at 37°C for 72 h. For analysis of activation markers, cells were collected and washed twice with phosphate-buffered saline (PBS) containing 2% FBS. Cells were then stained with phycoerythrin-conjugated anti-CD69 and fluorescein isothiocyanate-conjugated anti-CD25 antibodies on ice for 30 min. Samples were analyzed by flow cytometry using a Beckman-Coulter XL-MCL cytometer.
Propidium iodide staining of the cells was done using the Cellular DNA Flow Cytometric Analysis Kit according to the protocol of the manufacturer (Boehringer Mannheim). Briefly, cells were collected and washed with cold PBS containing 2% FBS. Cells were fixed with ethanol for 30 min at 4°C. After permeabilization, cells were washed in PBS and resuspended at 106 cells/ml in PBS. Cells were then treated with 1 U of RNase for 30 min at 37°C, followed by addition of 50 μg of propidium iodide per ml. Cells were then analyzed by flow cytometry as described above.
Immunoblots.
Cells were washed in PBS and lysed in EBCD buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% Nonidet P-40, 5 mM dithiothreitol) containing protease inhibitors (aprotinin, leupeptin, and phenylmethylsulfonyl fluoride) as described previously (26). Protein concentrations were determined by the Bio-Rad protein assay, and 25 μg of total protein was analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Immunoblotting was performed by standard procedures using enhanced chemiluminescence for detection as described previously (24). Antibodies for detection of Cdk9, Cdk7, cyclin T2b, and cyclin T1 were obtained from Santa Cruz Biotechnology.
Kinase assays.
Kinase reactions were performed as described previously (26). Briefly, recombinant glutathione S-transferase (GST)–Tat-2 (HIV-2 Tat protein) fusion proteins attached to glutathione-Sepharose beads were incubated with 50 μg of extracts prepared from PBLs or CD4+ T lymphocytes. The complexes were then washed extensively and incubated for 60 min at room temperature in 50 mM Tris-HCl (pH 7.4)–5 mM MgCl2–2.5 mM MnCl2–5 mM dithiothreitol–200 ng of GST-CTD–5 μCi of [γ-32P]ATP (3,000 Ci/mmol). The reaction products were analyzed by electrophoresis on SDS–9% polyacrylamide gels, and phosphorylation of the hyperphosphorylated CTDo form of CTD was quantified with a Molecular Dynamics PhosphorImager.
RESULTS
Activation status of PBLs treated with cytokines.
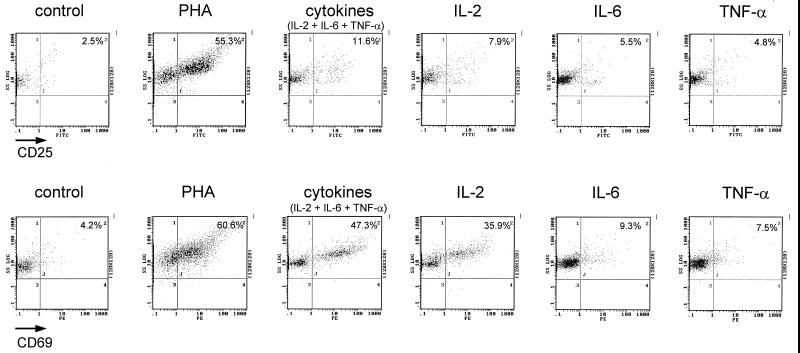
Previous work has shown that a combination of the cytokines IL-2, IL-6, and TNF-α is a potent inducer of viral replication in resting CD4+ T lymphocytes that harbor transcriptionally silent HIV-1 provirus (9). To investigate whether induction of TAK by the cytokines might contribute to the reactivation of HIV-1, we first examined TAK regulation in PBLs treated with cytokines. PBLs from healthy HIV-seronegative donors were isolated and incubated for 72 h in the presence of either medium alone, PHA, individual cytokines (IL-2, IL-6, or TNF-α), or a combination of the three cytokines. PHA was used in this study as a positive control, as we have previously shown that PHA induces TAK in PBLs and purified CD4+ T lymphocytes (23, 41). To monitor activation of cells, expression of CD25 (IL-2 receptor α subunit) and CD69 was measured by flow cytometry (Fig. 1). Both CD25 and CD69 are induced early during T-cell activation and are commonly evaluated as activation markers. When incubation was with medium alone, only low levels of CD25 or CD69 expression were observed. After incubation in the presence of PHA, there were large increases in expression of both markers. With IL-2 alone, CD69 expression was increased significantly and CD25 expression was increased moderately. With IL-6 or TNF-α alone, only small increases in CD25 and CD69 expression were observed. With the combination of IL-2, IL-6, and TNF-α, expression of both CD25 and CD69 was significantly increased. However, the induction of CD25 and CD69 by the combination of cytokines was less than that observed for PHA, suggesting that the combination of cytokines was less potent than PHA in activating the PBL cultures.
FIG. 1.
CD25 and CD69 expression in cytokine-treated PBLs. PBLs purified from donor 1 were cultured for 72 h and analyzed by flow cytometry.
Cdk9 and cyclin T1 levels and TAK activity are induced in PBLs treated with the combination of cytokines.
To determine whether cytokine treatment of PBLs induces TAK, cell extracts were prepared from cultures of PBLs from two donors and immunoblotting was performed to measure the protein levels of Cdk9 and cyclin T1, the two known subunits of TAK (Fig. 2A). The expression of cyclin T1 was significantly induced in PBLs treated with PHA or the combination of cytokines. We carried out immunoblotting with serial dilutions of cellular extracts to quantify the inductions of Cdk9, cyclin T1, and Cdk7 (data not shown). We estimate that cyclin T1 levels were induced sixfold and four- to sixfold following treatment with PHA or cytokines, respectively. With individual cytokines (IL-2, IL-6, or TNF-α) the level of cyclin T1 was equivalent to that in the control nonactivated PBLs. The expression of Cdk9 was moderately induced upon treatment of PBLs with PHA or the combination of cytokines. We estimate that Cdk9 levels increased two- to fourfold following treatment with PHA or with the combination of cytokines. IL-2 alone induced Cdk9 in donor 1 but had only a marginal effect in donor 2. IL-6 or TNF-α alone had a minimal effect to no effect on Cdk9 levels. To evaluate the specificity of TAK induction in the PBLs, the level of Cdk7, the catalytic subunit of the TFIIH complex, was examined. The level of Cdk7 was altered to a lesser extent than that of either cyclin T1 or Cdk9 upon treatment with PHA or cytokines. Cdk7 levels were about twofold higher in PHA- or cytokine-treated cells. We were unable to detect an induction of levels of cyclin T2b, another cyclin partner of Cdk9, in both donors 1 and 2 with PHA or the cytokine combination (data not shown). We carried out similar immunoblot analyses for cyclin T1 and Cdk9 in PHA- and cytokine-treated PBLs isolated from five other donors and observed results similar to those shown in Fig. 2A.
FIG. 2.
Cdk9 and cyclin T1 protein levels and TAK kinase activity are increased in PBLs following treatment with a combination of cytokines. PBLs purified from two healthy donors were cultured in media as indicated for 72 h; CD25 and CD69 expression of donor 1 was analyzed in Fig. 1. (A) Equal amounts of protein from cell extracts were analyzed for Cdk9, cyclin T1, and Cdk7 by immunoblotting. (B) TAK kinase assays were performed with recombinant CTD as a substrate, and products were analyzed on an SDS–9% polyacrylamide gel. CTDo is the hyperphosphorylated form of CTD; CTDa is the underphosphorylated form of CTD.
We performed in vitro kinase assays to determine whether the induction of cyclin T1 and Cdk9 protein levels in PBLs resulted in an increase in TAK enzymatic activity. In this kinase assay, a GST fusion to the wild-type HIV-2 Tat (Tat-2) protein is used to specifically bind TAK from cell extracts (26). TAK bound to the Tat-2 protein hyperphosphorylates a recombinant CTD substrate to generate the CTDo form, which is detected by slower migration in SDS-polyacrylamide gels compared to the underphosphorylated CTDa form. TAK levels were significantly elevated in extracts from PBLs treated with PHA and the combination of cytokines in both donor 1 and donor 2 (Fig. 2B). As quantified by generation of the CTDo form of the CTD substrate, PHA treatment induced TAK activity sevenfold in both donors 1 and 2. The combination of cytokines induced TAK activity nine- and threefold in donors 1 and 2, respectively. There was no significant induction of TAK activity in extracts from PBLs treated with individual cytokines IL-2, IL-6, or TNF-α. We conclude from these experiments that the combination of cytokines induces Cdk9 and cyclin T1 protein levels and TAK enzymatic activity in PBLs.
Activation status of purified, resting CD4+ T lymphocytes treated with cytokines.
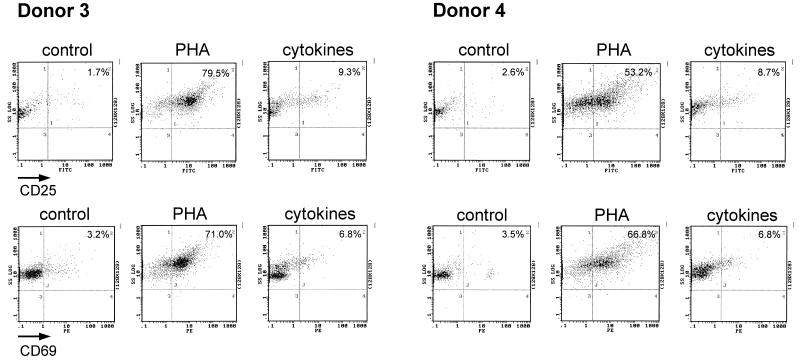
To examine TAK regulation in CD4+ T lymphocytes, resting CD4+ T lymphocytes were purified from healthy HIV-seronegative donors by negative selection and incubated for 3 days with medium alone, PHA, or a combination of IL-2, IL-6, and TNF-α. As with PBLs, the activation status of the cytokine-treated CD4+ T lymphocytes was monitored by examining CD25 and CD69 expression (Fig. 3). With PHA treatment, there was a large increase in CD25 and CD69 expression in both donors. Upon treatment with the combination of cytokines, CD25 expression and CD69 expression were only modestly increased in both donors. These data indicate that similar to the case for PBL cultures, the cytokine combination is less potent than PHA in activation of resting CD4+ T lymphocytes.
FIG. 3.
CD25 and CD69 expression in cytokine-treated CD4+ T lymphocytes. Resting CD4+ T lymphocytes purified from donors 3 and 4 were cultured for 72 h as indicated and analyzed by flow cytometry.
Cdk9 and cyclin T1 levels and TAK activity are induced by cytokine treatment of resting CD4+ T lymphocytes.
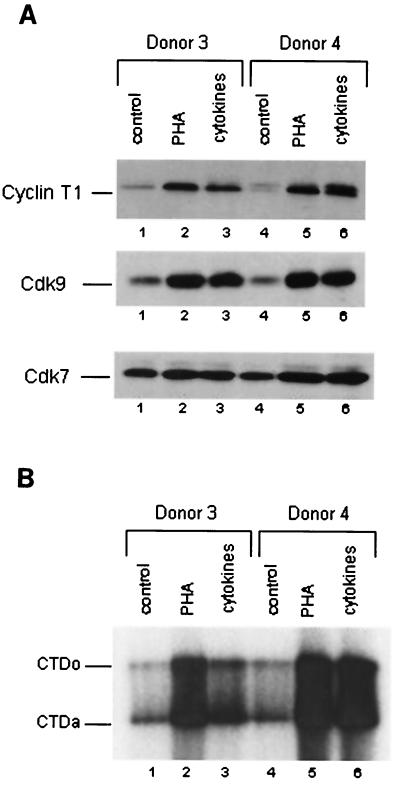
To examine Cdk9 and cyclin T1 levels in cytokine-treated resting CD4+ T lymphocytes from donors 3 and 4 (analyzed in Fig. 3), immunoblotting was performed with extracts prepared from cultures that were incubated for 72 h in medium alone, PHA, or the combination of cytokines (Fig. 4A). There was a strong induction of Cdk9 and cyclin T1 following incubation with PHA or the combination of cytokines with both donors. There were only modest inductions of Cdk7 by either PHA or the cytokine combination. By quantitative Western blotting (data not shown), we estimate that Cdk9 levels were induced four- to sixfold by PHA or cytokines, cyclin T1 levels were increased sixfold by PHA or cytokines, and Cdk7 levels were induced less than twofold by PHA or cytokines. Induction of Cdk9 and cyclin T1 in cytokine-treated resting CD4+ T lymphocytes was examined for four additional donors, and results similar to those presented in Fig. 4A were observed for all donors.
FIG. 4.
Cdk9 and cyclin T1 protein levels and TAK activity are increased in resting CD4+ T lymphocytes following treatment with a combination of cytokines. Purified resting CD4+ T cells from two healthy donors were cultured in media as indicated for 72 h. Expression of CD25 and CD69 is shown in Fig. 3. (A) Equal amounts of protein from cell extracts were analyzed for Cdk9, cyclin T1, and Cdk7 by immunoblotting. (B) TAK kinase assays were performed with recombinant CTD as a substrate, and products were analyzed on an SDS–9% polyacrylamide gel. CTDo is the hyperphosphorylated form of CTD; CTDa is the underphosphorylated form of CTD.
We performed in vitro kinase assays to determine whether the induction of cyclin T1 and Cdk9 protein levels in resting CD4+ T lymphocytes also resulted in an increase in TAK activity (Fig. 4B). An induction of TAK activity upon cytokine treatment was observed with both PHA and the combination of cytokines in both donor 3 and donor 4. PHA treatment induced TAK activity 7- and 10-fold in donors 3 and 4, respectively. The cytokine combination induced TAK activity three- and sevenfold in donors 3 and 4, respectively. We conclude from these results that the combination of IL-2, IL-6, and TNF-α induces Cdk9 and cyclin T1 protein levels and TAK activity in resting CD4+ T lymphocytes.
Induction of DNA synthesis in PBLs and resting CD4+ T lymphocytes.
To determine whether combination cytokine treatment was able to induce cellular proliferation under these experimental conditions, the percentages of cells in the S phase of the cell cycle in 72-h cultures of PBLs and resting CD4+ T lymphocytes were determined by propidium iodide staining and flow cytometry (Table 1). In the case of PBLs incubated with medium alone, 0.4 and 2.1% of cells were in the S phase in donors 5 and 6, respectively. Upon treatment with PHA or the combination of cytokines, about 10 to 14% of the cells were in S phase at 72 h. Treatment of PBLs with IL-2 caused about 3 to 5% of the cells to enter the S phase at 72 h, while fewer than 2% of the cells were in S phase at 72 h following treatment with IL-6 or TNF-α. The results of immunoblotting with cell extracts prepared from donors 5 and 6 showed the expected induction in protein levels of both Cdk9 and cyclin T1 (data not shown).
TABLE 1.
S-phase cells in PBLs and resting CD4+ T-lymphocytes culturesa
| Donor | Activator(s) | % S-phase cells |
|---|---|---|
| PBL donor 5 | None | 0.4 |
| PHA | 14.2 | |
| IL-2 | 3.5 | |
| IL-6 | 0.4 | |
| TNF-α | 0.9 | |
| IL-2 + IL-6 + TNF-α | 10.4 | |
| PBL donor 6 | None | 2.1 |
| PHA | 11.7 | |
| IL-2 | 4.9 | |
| IL-6 | 2.1 | |
| TNF-α | 1.2 | |
| IL-2 + IL-6 + TNF-α | 13.8 | |
| CD4+ T-lymphocyte donor 7 | None | 1.7 |
| PHA | 23.4 | |
| IL-2 + IL-6 + TNF-α | 1.0 | |
| CD4+ T-lymphocyte donor 8 | None | 0.5 |
| PHA | 15.7 | |
| IL-2 + IL-6 + TNF-α | 0.8 |
PBLs or CD4+ T lymphocytes were cultured for 72 h.
For purified resting CD4+ T lymphocytes, PHA treatment caused 16 to 23% of cells to enter S phase after 72 h. However, <1% of the purified CD4+ T lymphocytes entered the S phase of the cell cycle upon treatment with the combination of cytokines, similar to the case for the nonactivated control cultures. The results of immunoblotting showed the expected induction of protein levels for both Cdk9 and cyclin T1 upon PMA and cytokine treatment of CD4+ T lymphocytes from donors 7 and 8 (data not shown). These results indicate that PHA and the combination of cytokines mediate T-cell activation to different extents in PBLs and resting CD4+ T lymphocytes. The combinations of cytokines appear to induce DNA synthesis in PBLs but not in purified resting CD4+ T lymphocytes. The combination of cytokines induced TAK but not DNA synthesis in the CD4+ T-lymphocyte cultures, suggesting that TAK can be induced without a requirement for cellular proliferation.
DISCUSSION
In this study, we investigated whether TAK can be induced by cytokines that are known to reactivate latent HIV-1 proviruses in CD4+ T lymphocytes isolated from infected individuals (9). We found that the combination of IL-2, IL-6, and TNF-α induces TAK in PBLs and resting CD4+ T lymphocytes, suggesting that TAK induction may be involved in reactivation of latent proviruses. Additional support for the proposal that TAK induction may be involved in reactivation of latent provirus comes from findings that the combination of cytokines used here allows infection and expression of HIV-1 vectors in resting CD4+ lymphocytes (37).
Cytokine induction of TAK may have important consequences for viral replication in the microenvironment of lymphoid tissue, where the majority of viral replication takes place. Cytokines secreted in response to nonspecific or antigenic stimulation may induce TAK in resting CD4+ T lymphocytes, providing a setting in which high levels of Tat transactivation and viral long terminal repeat-directed transcription would be expected to occur. This may contribute to both reactivation of proviral transcription in latently infected cells and increased viral replication in newly infected cells. Our analysis of cell cycle progression in cytokine-treated CD4+ T lymphocytes indicates that cellular proliferation need not occur for TAK to be induced (Table 1). However, cytokine treatment does activate resting CD4+ T lymphocytes to some extent, as in addition to TAK, CD69 and CD25 were also induced (Fig. 3). In light of these observations that TAK can be induced in minimally activated T cells, it is of interest that in situ hybridization and immunohistochemical analyses have detected SIV and HIV replication in CD4+ T cells that lack detectable activation markers (43a).
It should be noted that it is not established that TAK is actually limiting for HIV-1 replication under conditions where TAK function is low. Indeed, we are not aware of data that demonstrate that any cellular transcription factor thought to regulate transcription of the HIV-1 provirus is limiting for viral replication in primary cells, including perhaps most notably NF-κB. There are, however, a number of findings that suggest that TAK can be limiting for viral replication. Pharmacological inhibitors of Cdk9 kinase activity or overexpression of a dominant negative Cdk9 mutant protein inhibits HIV-1 replication in Jurkat T-cell lines, demonstrating that TAK is required for viral replication in vitro (6, 12, 14, 30). Correlative studies further suggest that an increase in TAK results in increased viral replication, as induction of TAK correlates with increased HIV-1 replication in activated PBMCs, PBLs, and resting CD4+ T lymphocytes (20, 23, 41). There is also a correlation between induction of TAK and reactivation of latent HIV-1 proviruses in promonocytic cell lines after PMA treatment (23, 41). To address whether TAK levels can be limiting for HIV-1 replication, lentiviral vectors can be used to transduce and overexpress Cdk9 and cyclin T1 in primary CD4+ T lymphocytes, and the effects on viral replication can be evaluated.
We performed reverse transcription-PCR assays to monitor changes in RNA levels for Cdk9 and cyclin T1 in PHA- and cytokine-treated cultures. The results suggested a small induction (<2.5-fold) by PHA and cytokines for both Cdk9 and cyclin T1 mRNAs in PBLs and resting CD4+ T cells (data not shown). Plasmid transfection studies with Jurkat T cells have shown a small increase in Cdk9 and cyclin T1 promoter function in response to T-cell activation (28, 29). It is therefore possible that cytokine induction of Cdk9 and cyclin T1 protein levels involves a transcriptional increase in the Cdk9 and cyclin T1 genes. It is also possible that posttranscriptional mechanisms contribute to the increase in protein levels, especially in light of the observation that cyclin T1 protein levels increase by a posttranscriptional mechanism in PMA-treated promonocytic cell lines (23).
It is not clear from our data whether the PHA- or cytokine-induced increase in Cdk9 and cyclin T1 protein levels can fully account for the increase in TAK enzymatic activity. For both activated PBLs and resting CD4+ T lymphocytes, the increase in TAK activity was greater than the increase in the protein levels of either subunit (Fig. 2 and 4), suggesting that mechanisms in addition to an increase in protein levels contribute to the magnitude of TAK induction. Cdk9 function can be regulated by autophosphorylation (18); it is possible that activation results in changes in the phosphorylation state of Cdk9 and/or cyclin T1, and this might lead to an increase in TAK activity (13). It is also possible that activation might lead to inactivation of an inhibitor of Cdk9, analogous to the p21 protein that represses Cdks involved in cell cycle regulation. Alternatively, it is possible that changes in subcellular localization of Cdk9 and cyclin T1 might regulate TAK function. In HeLa cells, both Cdk9 and cyclin T1 are predominantly nuclear and are concentrated in “speckle” structures that contain splicing factors (25). It is possible that subcellular localization in resting CD4+ T lymphocytes may negatively regulate TAK function, by sequestration of one or more protein subunits either in the cytoplasm or in a nonfunctional nuclear compartment.
Regulation of TAK function during the establishment of latent proviruses is an important issue. The latently infected CD4+ T-cell population is established as early as 10 days after the onset of symptoms of primary HIV-1 infection (8). However, mechanisms involved in the creation of latent infection in memory and naive CD4+ T lymphocytes are largely unknown. Early events in the HIV-1 life cycle in T lymphocytes, i.e., reverse transcription and proviral integration, are dependent upon cell activation (4, 42). Latently infected memory CD4+ T cells are likely to arise when an infected activated T lymphocyte somehow exits the cell cycle and enters a quiescent state. Latently infected naive cells may be generated during thymopoiesis of CD4+ thymocytes (3). For establishment of latent proviruses, T-cell activation may occur to the extent that reverse transcription and proviral integration occur, but TAK may fail to be induced. TAK may not be required for many T-cell activation events, as a dominant negative Cdk9 protein did not inhibit CD69, CD25, and IL-2 induction in activated Jurkat T cells (14). Alternatively, TAK may be required for some crucial T-cell activation events, but it might be down-regulated after initial T-cell activation. Identification of mechanisms whereby TAK function can be down-regulated might provide clues into how latent proviruses are established.
ACKNOWLEDGMENTS
This work was supported by grants AI42558 (to C.H.H.), AI35381 (to A.P.R.), and AI45374 (to A.P.R.) from the National Institutes of Health. Flow cytometry was supported by the Center for AIDS Research at Baylor College of Medicine (grant AI36211).
REFERENCES
- 1.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Tawadros R, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bieniasz P D, Grdina T A, Bogerd H P, Cullen B R. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc Natl Acad Sci USA. 1999;96:7791–7796. doi: 10.1073/pnas.96.14.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks D G, Kitchen S G, Kitchen C M, Scripture-Adams D D, Zack J A. Generation of HIV latency during thymopoiesis. Nat Med. 2001;7:459–464. doi: 10.1038/86531. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butera S T. Therapeutic targeting of human immunodeficiency virus type-1 latency: current clinical realities and future scientific possibilities. Antiviral Res. 2000;48:143–176. doi: 10.1016/s0166-3542(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 6.Chao S H, Fujinaga K, Marion J E, Taube R, Sausville E A, Senderowicz A M, Peterlin B M, Price D H. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem. 2000;275:28345–28348. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 7.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y H, Brookmeyer R, Zeiger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 8.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun T W, Engel D, Mizell S B, Ehler L A, Fauci A S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun T W, Fauci A S. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc Natl Acad Sci USA. 1999;96:10958–10961. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun T W, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano R F. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 12.Flores O, Lee G, Kessler J, Miller M, Schlief W, Tomassini J, Hazuda D. Host-cell positive transcription elongation factor b kinase activity is essential and limiting for HIV type 1 replication. Proc Natl Acad Sci USA. 1999;96:7208–7213. doi: 10.1073/pnas.96.13.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong Y W, Zhou Q. Relief of two built-in autoinhibitory mechanisms in P-TEFb is required for assembly of a multicomponent transcription elongation complex at the human immunodeficiency virus type 1 promoter. Mol Cell Biol. 2000;20:5897–5907. doi: 10.1128/mcb.20.16.5897-5907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foskett S M, Ghose R, Tang D N, Lewis D E, Rice A P. Antiapoptotic function of Cdk9 (TAK/P-TEFb) in U937 promonocytic cells. J Virol. 2001;75:1220–1228. doi: 10.1128/JVI.75.3.1220-1228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu T J, Peng J, Lee G, Price D H, Flores O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J Biol Chem. 1999;274:34527–34530. doi: 10.1074/jbc.274.49.34527. [DOI] [PubMed] [Google Scholar]
- 16.Fujinaga K, Cujec T P, Peng J, Garriga J, Price D H, Grana X, Peterlin B M. The ability of positive transcription elongation factor b to transactivate human immunodeficiency virus transcription depends on a functional kinase domain, cyclin T1, and Tat. J Virol. 1998;72:7154–7159. doi: 10.1128/jvi.72.9.7154-7159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber M E, Jones K A. HIV-1 Tat: coping with negative elongation factors. Curr Opin Immunol. 1999;11:460–465. doi: 10.1016/S0952-7915(99)80077-6. [DOI] [PubMed] [Google Scholar]
- 18.Garber M E, Mayall T P, Suess E M, Meisenhelder J, Thompson N E, Jones K A. CDK9 autophosphorylation regulates high-affinity binding of the human immunodeficiency virus type 1 tat-P-TEFb complex to TAR RNA. Mol Cell Biol. 2000;20:6958–6969. doi: 10.1128/mcb.20.18.6958-6969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber M E, Wei P, KewalRamani V N, Mayall T P, Herrmann C H, Rice A P, Littman D R, Jones K A. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998;12:3512–3527. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garriga J, Peng J, Parreno M, Price D H, Henderson E E, Grana X. Upregulation of cyclin T1/CDK9 complexes during T cell activation. Oncogene. 1998;17:3093–3102. doi: 10.1038/sj.onc.1202548. [DOI] [PubMed] [Google Scholar]
- 21.Glass J D, Johnson R T. Human immunodeficiency virus and the brain. Annu Rev Neurosci. 1996;19:1–26. doi: 10.1146/annurev.ne.19.030196.000245. [DOI] [PubMed] [Google Scholar]
- 22.Gold M O, Yang X, Herrmann C H, Rice A P. PITALRE, the catalytic subunit of TAK, is required for human immunodeficiency virus Tat transactivation in vivo. J Virol. 1998;72:4448–4453. doi: 10.1128/jvi.72.5.4448-4453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann C H, Carroll R G, Wei P, Jones K A, Rice A P. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J Virol. 1998;72:9881–9888. doi: 10.1128/jvi.72.12.9881-9888.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann C H, Gold M O, Rice A P. Viral transactivators specifically target distinct cellular protein kinases that phosphorylate the RNA polymerase II C-terminal domain. Nucleic Acids Res. 1996;24:501–509. doi: 10.1093/nar/24.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann C H, Mancini M A. The Cdk9 and cyclin T subunits of TAK/P-TEFb localize to splicing factor-rich nuclear speckle regions. J Cell Sci. 2001;114:1491–1503. doi: 10.1242/jcs.114.8.1491. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov D, Kwak Y T, Nee E, Guo J, Garcia-Martinez L F, Gaynor R B. Cyclin T1 domains involved in complex formation with Tat and TAR RNA are critical for tat-activation. J Mol Biol. 1999;288:41–56. doi: 10.1006/jmbi.1999.2663. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Rice A P. Genomic organization and characterization of promoter function of the human CDK9 gene. Gene. 2000;252:51–59. doi: 10.1016/s0378-1119(00)00215-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Rice A P. Isolation and characterization of the human cyclin T1 promoter. Gene. 2000;252:39–49. doi: 10.1016/s0378-1119(00)00214-6. [DOI] [PubMed] [Google Scholar]
- 30.Mancebo H S Y, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D H, Flores O. P-TEFb kinase is required for HIV Tat transactivation in vivo and in vitro. Genes Dev. 1997;11:2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napolitano G, Licciardo P, Gallo P, Majello B, Giordano A, Lania L. The CDK9-associated cyclins T1 and T2 exert opposite effects on HIV-1 Tat activity. AIDS. 1999;13:1453–1459. doi: 10.1097/00002030-199908200-00003. [DOI] [PubMed] [Google Scholar]
- 32.Peng J, Zhu Y, Milton J T, Price D H. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierson T, Hoffman T L, Blankson J, Finzi D, Chadwick K, Margolick J B, Buck C, Siliciano J D, Doms R W, Siliciano R F. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J Virol. 2000;74:7824–7833. doi: 10.1128/jvi.74.17.7824-7833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price D H. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol Cell Biol. 2000;20:2629–2634. doi: 10.1128/mcb.20.8.2629-2634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanathan Y, Reza S M, Young T M, Mathews M B, Pe'ery T. Human and rodent transcription elongation factor P-TEFb: interactions with human immunodeficiency virus type 1 tat and carboxy-terminal domain substrate. J Virol. 1999;73:5448–5458. doi: 10.1128/jvi.73.7.5448-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taube R, Fujinaga K, Wimmer J, Barboric M, Peterlin B M. Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology. 1999;264:245–253. doi: 10.1006/viro.1999.9944. [DOI] [PubMed] [Google Scholar]
- 37.Unutmaz D, KewalRamani V N, Marmon S, Littman D R. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog G A, Winston F, Buratowski S, Handa H. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97:41–51. doi: 10.1016/s0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Gold M O, Tang D N, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Dornadula G, Beumont M, Livornese L, Van Uitert B, Henning K, Pomerantz R J. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 43a.Zhang Z Q, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Chen D, Pierstorff E, Luo K. Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J. 1998;17:3681–3691. doi: 10.1093/emboj/17.13.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Pe'ery T, Peng J, Ramanathan Y, Marshall N F, Marshall T A B, Amendt B, Mathews M B, Price D H. Transcriptional elongation factor p-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]