Abstract
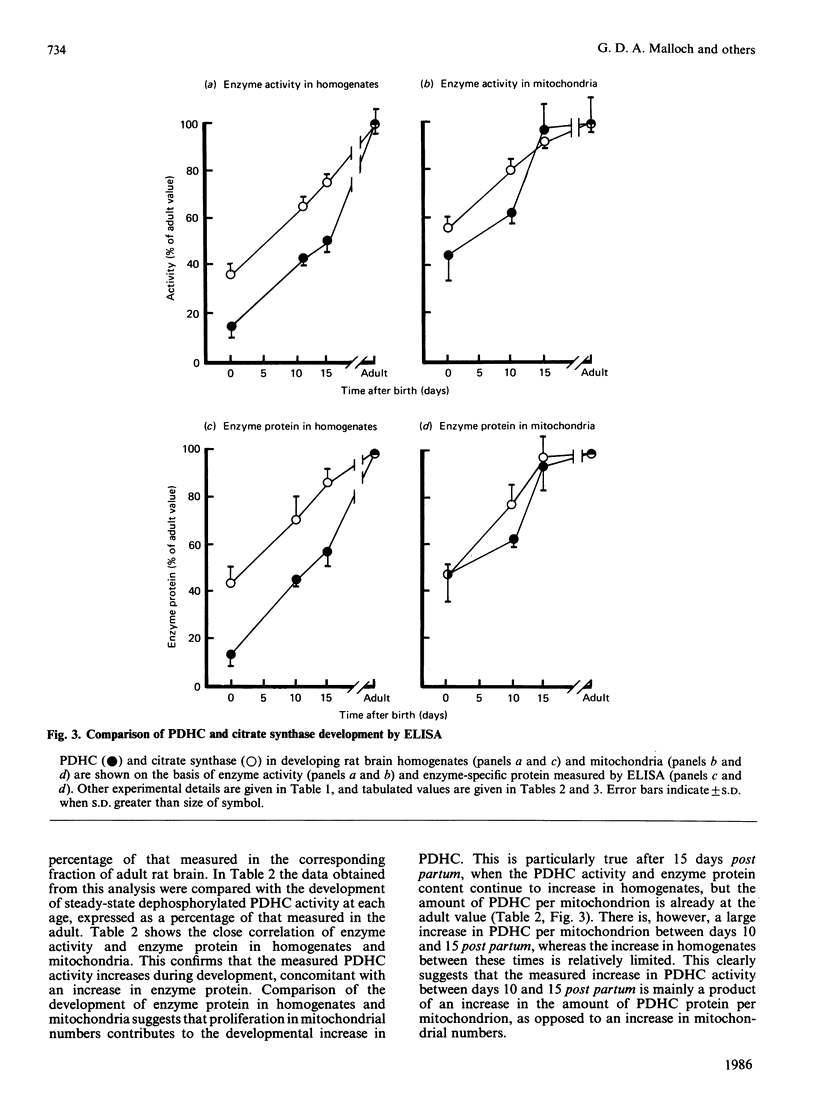
The enzyme activity of the pyruvate dehydrogenase complex (PDHC) was measured in mitochondria prepared from developing rat brain, before and after steady-state dephosphorylation of the E1 alpha subunit. A marked increase in dephosphorylated (fully activated) PDHC activity occurred between days 10 and 15 post partum, which represented approx. 60% of the difference in fully activated PDHC activity measured in foetal and adult rat brain mitochondria. There was no detectable change in the active proportion of the enzyme during mitochondrial preparation nor any qualitative alteration in the detectable catalytic and regulatory components of the complex, which might account for developmental changes in PDHC activity. The PDHC protein content of developing rat brain mitochondria and homogenates was measured by an enzyme-linked immunoadsorbent assay. The development of PDHC protein in both fractions agreed closely with the development of the PDHC activity. The results suggest that the developmental increase in PDHC activity is due to increased synthesis of PDHC protein, which is partly a consequence of an increase in mitochondrial numbers. However, the marked increase in PDHC activity measured between days 10 and 15 post partum is mainly due to an increase in the amount of PDHC per mitochondrion. The development of citrate synthase enzyme activity and protein was measured in rat brain homogenates and mitochondria. As only a small increase in citrate synthase activity and protein was detected in mitochondria between days 10 and 15 post partum, the marked increase in PDHC protein and enzyme activity may represent specific PDHC synthesis. As several indicators of acquired neurological competence become apparent during this period, it is proposed that preferential synthesis of PDHC may be crucial to this process. The results are discussed with respect to the possible roles played by PDHC in changes of respiratory-substrate utilization and the acquisition of neurological competence occurring during the development of the brain of a non-precocial species such as the rat.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. F., Clark J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978 Nov 15;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. Energy metabolism in rat brain: inhibition of pyruvate decarboxylation by 3-hydroxybutyrate in neonatal mitochondria. J Neurochem. 1981 Jul;37(1):179–185. doi: 10.1111/j.1471-4159.1981.tb05306.x. [DOI] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. The control of pyruvate dehydrogenase in isolated brain mitochondria. J Neurochem. 1978 May;30(5):1003–1008. doi: 10.1111/j.1471-4159.1978.tb12392.x. [DOI] [PubMed] [Google Scholar]
- Booth R. F., Patel T. B., Clark J. B. The development of enzymes of energy metabolism in the brain of a precocial (guinea pig) and non-precocial (rat) species. J Neurochem. 1980 Jan;34(1):17–25. doi: 10.1111/j.1471-4159.1980.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Roche T. E. A unifying mechanism for stimulation of mammalian pyruvate dehydrogenase(a) kinase by reduced nicotinamide adenine dinucleotide, dihydrolipoamide, acetyl coenzyme A, or pyruvate. J Biol Chem. 1978 Jan 25;253(2):496–503. [PubMed] [Google Scholar]
- Clark J. B., Land J. M. Differential effects of 2-oxo acids on pyruvate utilization and fatty acid synthesis in rat brain. Biochem J. 1974 Apr;140(1):25–29. doi: 10.1042/bj1400025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Cremer J. E., Heath D. F. The estimation of rates of utilization of glucose and ketone bodies in the brain of the suckling rat using compartmental analysis of isotopic data. Biochem J. 1974 Sep;142(3):527–544. doi: 10.1042/bj1420527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcucci O. L., Hunter A., Lindsay J. G. Low immunogenicity of the common lipoamide dehydrogenase subunit (E3) of mammalian pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase multienzyme complexes. Biochem J. 1985 Mar 1;226(2):509–517. doi: 10.1042/bj2260509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. A simple modification to an enzymic method for the preparation of [gamma-32P]ATP free of salt and buffer. Anal Biochem. 1979 Mar;93(2):272–274. doi: 10.1016/s0003-2697(79)80151-7. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R., Blass J. P. The regulation of pyruvate dehydrogenase in brain in vivo. J Neurochem. 1976 Apr;26(4):709–714. doi: 10.1111/j.1471-4159.1976.tb04441.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai J. C., Walsh J. M., Dennis S. C., Clark J. B. Synaptic and non-synaptic mitochondria from rat brain: isolation and characterization. J Neurochem. 1977 Mar;28(3):625–631. doi: 10.1111/j.1471-4159.1977.tb10434.x. [DOI] [PubMed] [Google Scholar]
- Leong S. F., Clark J. B. Regional enzyme development in rat brain. Enzymes of energy metabolism. Biochem J. 1984 Feb 15;218(1):139–145. doi: 10.1042/bj2180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLICHAP J. G. Development of seizure patterns in newborn animals; significance of brain carbonic anhydrase. Proc Soc Exp Biol Med. 1957 Oct;96(1):125–129. doi: 10.3181/00379727-96-23411. [DOI] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. B., Olson M. S. Evidence for the regulation of the branched chain alpha-keto acid dehydrogenase multienzyme complex by a phosphorylation/dephosphorylation mechanism. Biochemistry. 1982 Aug 31;21(18):4259–4265. doi: 10.1021/bi00261a012. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Phosphorylation-dephosphorylation cycles and the regulation of fuel selection in mammals. Curr Top Cell Regul. 1981;18:107–129. doi: 10.1016/b978-0-12-152818-8.50013-x. [DOI] [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- SAMSON F. E., Jr, DAHL N. A. Cerebral energy requirement of neonatal rats. Am J Physiol. 1957 Feb;188(2):277–280. doi: 10.1152/ajplegacy.1957.188.2.277. [DOI] [PubMed] [Google Scholar]
- Schapiro S., Salas M., Vukovich K. Hormonal effects on ontogeny of swimming ability in the rat: assessment of central nervous system development. Science. 1970 Apr 3;168(3927):147–150. doi: 10.1126/science.168.3927.147. [DOI] [PubMed] [Google Scholar]
- Sheu K. F., Kim Y. T. Studies on the bovine brain pyruvate dehydrogenase complex using the antibodies against kidney enzyme complex. J Neurochem. 1984 Nov;43(5):1352–1358. doi: 10.1111/j.1471-4159.1984.tb05394.x. [DOI] [PubMed] [Google Scholar]
- Stanley C. J., Perham R. N. Purification of 2-oxo acid dehydrogenase multienzyme complexes from ox heart by a new method. Biochem J. 1980 Oct 1;191(1):147–154. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucek S., Cheng S. C. Provenance of the acetyl group of acetylcholine and compartmentation of acetyl-CoA and Krebs cycle intermediates in the brain in vivo. J Neurochem. 1974 Jun;22(6):893–914. doi: 10.1111/j.1471-4159.1974.tb04314.x. [DOI] [PubMed] [Google Scholar]