Abstract
Background
Sepsis, a life-threatening condition characterized by a dysregulated immune response to infection, remains a significant clinical challenge globally. This study aims to enhance the predictive accuracy of existing sepsis severity scores by developing augmented versions of the SOFA and SAPS-III models, termed Pro-SOFA and Pro-SAPS, through the integration of biomarkers procalcitonin (PCT), neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP).
Methods
This prospective observational study was conducted in the medical ICU of a tertiary care hospital in southern India from August 2022 to December 2023. A total of 301 adult patients suspected or confirmed to have sepsis were assessed for eligibility, with 171 patients completing the study. Demographic and clinical data were collected; SOFA and SAPS-III scores were calculated and augmented with PCT, NLR, and CRP to develop Pro-SOFA and Pro-SAPS models. The performance of these models was evaluated using Brier scores, AUC, and net reclassification index (NRI).
Results
The augmented Pro-SOFA and Pro-SAPS models demonstrated superior predictive accuracy compared to their original counterparts. The Brier scores for Pro-SOFA and Pro-SAPS were 0.181 and 0.165, respectively, indicating better calibration than the original scores. The Pro-SAPS showed significant improvement over the original SAPS-III score (NRI = 0.50, SE = 0.14, p < 0.01). Similarly, Pro-SOFA outperformed the original SOFA (NRI = 0.49, SE = 0.13, p < 0.01).
Conclusion and clinical significance
Integrating PCT, CRP, and NLR with SOFA and SAPS-III scores to develop Pro-SOFA and Pro-SAPS significantly improves the predictive accuracy for sepsis mortality and can thus potentially improve sepsis outcomes.
How to cite this article
Nandakumar A, Sudeep S, Sreemohan AC, Vijayakumar S, Sudhakaran GJ, Gutjahr G, et al. Developing Augmented Pro-SOFA and Pro-SAPS Models by Integrating Biomarkers PCT, NLR, and CRP with SOFA and SAPS-III Scores. Indian J Crit Care Med 2024;28(10):935–941.
Keywords: Augmented models, Biomarkers, C-reactive protein, Intensive care unit, Neutrophil-to-lymphocyte ratio, Procalcitonin, Simplified acute physiology score III, Sequential organ failure assessment
Highlights
This is the first study to develop augmented Pro-SOFA and Pro-SAPS models by integrating biomarkers procalcitonin (PCT), neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP) with SOFA and SAPS-III scores.
Augmented Pro-SOFA and Pro-SAPS models demonstrated superior predictive accuracy compared with existing conventional SOFA and SAPS-III scores in sepsis patients.
Introduction
Sepsis, characterized by a dysregulated host immune response to infection, represents a global health crisis posing significant challenges to healthcare systems worldwide, more so in low- and middle-income countries (LMICs) with limited resources.1 Despite the broad array of prognostic tools available, the sequential organ failure assessment (SOFA) and the simplified acute physiology score (SAPS) have emerged as leading risk scores due to their practical applicability and accuracy in predicting hospital mortality.2–5 When comparing SOFA and SAPS-II models, the SAPS-III model demonstrated superior performance in discriminating 28-day mortality.6 These risk scores primarily assess organ dysfunction and physiological instability, overlooking the laboratory indicators of inflammation – a critical driver of the sepsis process.
The clinical biomarkers of sepsis that are commonly used and are readily available in the clinical setting include C-reactive protein (CRP), Neutrophil-to-lymphocyte ratio, and procalcitonin (PCT).6–8 The rationale behind selecting these particular scores and biomarkers stems from their widespread availability and established clinical utility, especially in settings with limited resources. Studies have shown that PCT levels are associated with mortality and SOFA scores in sepsis patients.9 The PCT measurement is effective for bacterial sepsis detection, and integration of PCT with SOFA score outperforms CRP in predicting in-hospital mortality, paving precedence for further exploration of this concept.9,10
In previous studies, researchers have attempted to enhance existing clinical risk scores by incorporating various biomarkers, including pentraxin-related protein (PTX3), PCT, IL-6, and lactate.11,12 However, these efforts have either yielded unpromising results or involved biomarkers not applicable for the Indian clinical setting. Although some attempts were made to integrate biomarkers like CRP and PCT with the qSOFA score, we contend that such integrations are counterproductive as quick scores are designed for rapid assessment and do not traditionally include integration of blood parameters.13 Hence, the integration of the commonly used biomarkers including PCT, CRP, and NLR with the SOFA and SAPS-III scores aims to create augmented versions, Pro-SOFA and Pro-SAPS. This could enhance the scores’ predictive accuracy by incorporating an estimate of the patient's inflammatory status in conjunction with organ dysfunction and physiological stress.
This study hypothesizes that incorporating direct measures of inflammation in sepsis with risk scores focusing on organ failure and physiological parameters can significantly improve sepsis mortality prediction. By doing so, we seek to offer a more comprehensive tool for sepsis prognosis, capable of guiding clinical triaging more effectively and aligning with the resource constraints of LMIC healthcare facilities, but at the same time contributing meaningfully to the global fight against sepsis.
Methods
Study Design and Setting
This was a prospective observational study conducted in a medical ICU at a multi-speciality tertiary care academic hospital in South India. The Institutional Ethics Committee approved the study. The study was conducted from August 2022 to December 2023.
Study Population
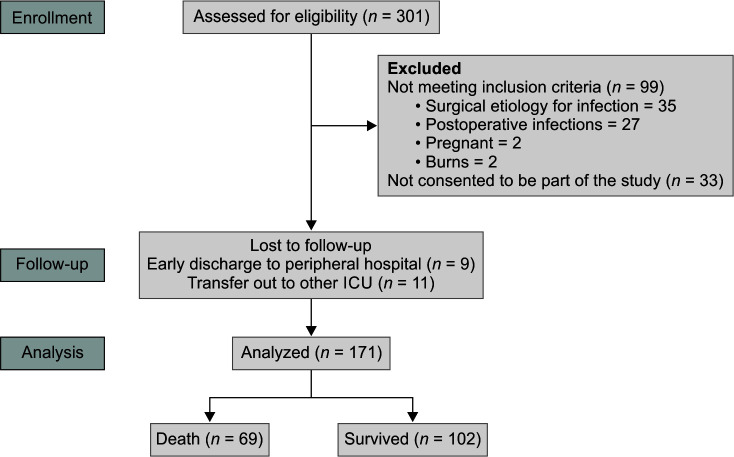
The study included all adult patients over the age of 18 who were enrolled at the time of suspicion or confirmation of sepsis in the medical ICU as part of the prospective sepsis registry, OASIS*. Pregnant and post-operative sepsis patients were excluded. Informed written consent was obtained from all participants. Among the septic patients admitted to the ICU, 301 were assessed for eligibility. Of these, 110 individuals were excluded: 99 did not meet the inclusion criteria, and 11 did not consent to participate in the study. Additionally, 20 patients dropped out during the follow-up period. Finally, 171 patients completed the study (Fig. 1).
Fig. 1.
Patient disposition diagram
Data Collection
Demographic data, vital parameters, lab parameters indicative of organ dysfunction and biomarkers were systematically gathered on a daily basis. The patient's clinical condition was monitored and recorded on a daily basis until they were discharged from ICU or deceased. The initial values of CRP, NLR, and PCT, measured at the time of study enrollment, were used to assess sepsis severity and calculate sepsis-related scores.
Development of Pro-SOFA and Pro-SAPS
The SOFA and SAPS-III scores were computed from the collected data. These scores were augmented by integrating inflammatory biomarkers PCT, NLR, and CRP.
Statistical Analysis
The continuous variables were summarized by mean and standard deviation and the discrete variables by frequency and proportion. The missing values in the dataset were imputed using Multiple Imputation with the aregImpute algorithm.14 For the augmented scores Pro-SAPS and Pro-SOFA, multiple logistic regression analysis was employed to investigate the relationship between the predictor variables (existing risk scores, PCT, NLR, and CRP) and the binary outcome variable (in-hospital mortality). After visual inspection of the relationships between the predictor variables and the outcome, restricted cubic splines with three degrees of freedom were used to transform PCT and NLR, and a factor variable was created to represent the interaction between NLR and CRP. A nomogram was used to create the augmented scores from the fitted logistic regression models.
Bootstrapping was used to evaluate the predictive performance of the scores in terms of three measures.15 First, the overall accuracy was assessed using the Brier score.16 The Brier score is a measure used to assess the accuracy of probabilistic predictions. It is calculated as the mean-squared difference between the predicted probability and the actual outcome. A Brier score ranges from 0 to 1, where 0 indicates perfect accuracy and 1 indicates the worst possible prediction. A lower Brier score signifies better predictive accuracy. Secondly, the area under the receiver operating characteristic curve (AUC) with a 95% confidence interval (CI) was used to evaluate the discrimination performance of each score. Thirdly, the goodness-of-fit of each model was assessed using the Nagelkerke R2 score.17 Additionally, calibration was evaluated using the bias-corrected method.18 We also performed a graphical assessment of the calibration graph with actual probability on the y-axis and the bias-corrected predicted probability on the x-axis. Finally, we compared the predictive performance of the augmented scores to the original scores by calculating the net reclassification index (NRI).19 The NRI is a metric used to evaluate the improvement in risk prediction using a new model compared with an existing one. It quantifies how many subjects are correctly reclassified into higher or lower categories of risk with the new model. The NRI is mostly used when comparing the performance of different models in classifying subjects into risk categories. A positive NRI indicates that the new model improves classification, while a negative NRI suggests worse all performance. All statistical analyses were conducted using R programming software (version 4.3.0).
Results
The study involved 171 patients who were admitted to ICU due to sepsis from August 2022 to December 2023. The average age of the patients is 62.9 years (Table 1). There were 66 (32.7%) female patients and 115 (67.3%) male patients (Table 2). The length of stay in the ICU ranges between 1 and 45 days with an average of 8.9 days.
Table 1.
Means and standard deviations of patient characteristics at the time of admission for the overall sample and subgroups stratified by in hospital mortality
| Parameter | Overall | Survived | Died | p-value | |||
|---|---|---|---|---|---|---|---|
| Mean (Overall) | SD (Overall) | Mean (Survived) | SD (Survived) | Mean (Died) | SD (Died) | ||
| ICU stay (days) | 8.9 | 6.8 | 8.6 | 6.7 | 9.4 | 6.9 | 0.479 |
| Hospital stay (days) | 18.6 | 13.7 | 20.8 | 14.9 | 15.4 | 10.9 | 0.007 |
| Age | 62.9 | 13.9 | 62.3 | 12.9 | 63.8 | 15.3 | 0.484 |
| Pulse (bpm) | 89.7 | 17.5 | 88.1 | 14.6 | 92.1 | 21.0 | 0.171 |
| Systolic blood pressure (mm Hg) | 131.6 | 25.1 | 137.3 | 23.3 | 123.3 | 25.3 | 0.000 |
| Diastolic blood pressure (mm Hg) | 66.3 | 14.6 | 68.3 | 13.9 | 63.2 | 15.2 | 0.031 |
| Respiratory rate (BR.PM) | 21.7 | 9.0 | 22.3 | 10.1 | 20.7 | 7.0 | 0.221 |
| SpO2 (%) | 96.9 | 3.8 | 97.0 | 3.9 | 96.9 | 3.5 | 0.948 |
| Temperature (°F) | 98.1 | 2.2 | 97.9 | 2.1 | 98.4 | 2.4 | 0.228 |
| pH | 7.3 | 0.6 | 7.3 | 0.8 | 7.4 | 0.1 | 0.341 |
| PaCO2 (mm Hg) | 33.8 | 9.0 | 35.4 | 8.6 | 31.5 | 9.2 | 0.006 |
| PO2 (mm Hg) | 92.7 | 44.3 | 89.6 | 44.5 | 97.3 | 44.0 | 0.265 |
| HCO3 (mEq/L) | 23.9 | 21.2 | 25.3 | 21.7 | 22.0 | 20.3 | 0.313 |
| Lactate (mmol/L) | 1.7 | 1.4 | 1.3 | 0.6 | 2.3 | 1.9 | 0.000 |
| GRES (mg/dL) | 173.4 | 88.7 | 174.2 | 95.1 | 172.2 | 79.2 | 0.881 |
| White blood cells (cell/µL) | 14.5 | 9.3 | 14.2 | 7.4 | 15.0 | 11.7 | 0.620 |
| Neutrophils (cells/µL) | 81.0 | 12.0 | 80.0 | 11.2 | 82.4 | 13.1 | 0.237 |
| Lymphocytes (cells/µL) | 9.3 | 6.8 | 10.1 | 6.4 | 8.2 | 7.2 | 0.081 |
| Eosinophils (cells/µL) | 1.0 | 2.0 | 1.2 | 2.0 | 0.7 | 1.9 | 0.124 |
| Monocytes (cells/µL) | 6.1 | 3.1 | 7.5 | 3.1 | 4.2 | 2.2 | 0.041 |
| NLR | 15.9 | 14.8 | 13.4 | 12.3 | 19.5 | 17.3 | 0.013 |
| PCT | 17.8 | 30.2 | 13.3 | 26.4 | 24.5 | 34.3 | 0.063 |
| CRP | 122.4 | 98.0 | 121.6 | 98.2 | 123.5 | 98.3 | 0.907 |
| Platelets (cells/µL) | 171.3 | 115.1 | 197.0 | 115.3 | 133.5 | 104.6 | 0.000 |
| Urea (mmol/L) | 90.6 | 60.6 | 83.9 | 57.2 | 100.3 | 64.4 | 0.110 |
| BUN (mmol/L) | 45.3 | 30.3 | 42.0 | 28.6 | 50.2 | 32.2 | 0.110 |
| CR (mg/dL) | 2.7 | 3.8 | 2.5 | 2.3 | 3.1 | 5.3 | 0.353 |
| Bilirubin (µm/L) | 2.2 | 4.5 | 1.2 | 1.8 | 3.7 | 6.5 | 0.003 |
| INR | 1.8 | 3.3 | 1.3 | 0.5 | 2.5 | 4.8 | 0.252 |
Table 2.
Counts and percentages of patient characteristics at baseline for the overall sample and subgroups stratified by in-hospital mortality
| Parameter | Overall | Survived | Died | p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Female | 56 | 32.7 | 35 | 34.3 | 21 | 30.4 | 0.716 |
| Male | 115 | 67.3 | 67 | 65.7 | 48 | 69.6 | 0.716 |
| HT | 77 | 45.0 | 45 | 44.1 | 32 | 46.4 | 0.893 |
| CLD | 22 | 12.9 | 12 | 11.8 | 10 | 14.5 | 0.772 |
| T-2DM | 86 | 50.3 | 52 | 51.0 | 34 | 49.3 | 0.950 |
| CAD | 23 | 13.5 | 13 | 12.7 | 10 | 14.5 | 0.920 |
| COPD | 10 | 5.0 | 7 | 6.9 | 3 | 4.3 | 0.722 |
| CKD | 29 | 17.0 | 14 | 13.7 | 15 | 21.7 | 0.245 |
There were 50 (29.2%) missing values for PCT, 17 (9.9%) for CRP and 5 (2.9%) for NLR.
Augmented SAPS-III and SOFA scores were developed by integrating the inflammatory biomarkers CRP, PCT, and NLR to the existing scores. The CRP was found to have a smaller effect on the risk for mortality prediction but to significantly modify the effect of the NLR. Therefore, for patients with low NLR the risk score includes CRP whereas in patients with high NLR the risk prediction is independent of CRP. Figures 2 and 3 show the influence of PCT and NLR on in-hospital mortality adjusted for the SAPS-III score and CRP.
Fig. 2.
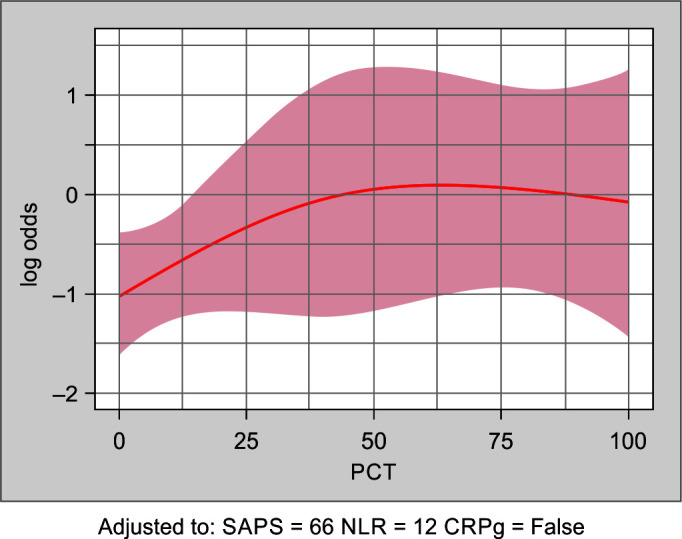
Shows the estimated log-odds for in-hospital mortality as a function of PCT, together with a 95% confidence band, when adjusting for the CRP, NLR, and SAPS-III score
Fig. 3.
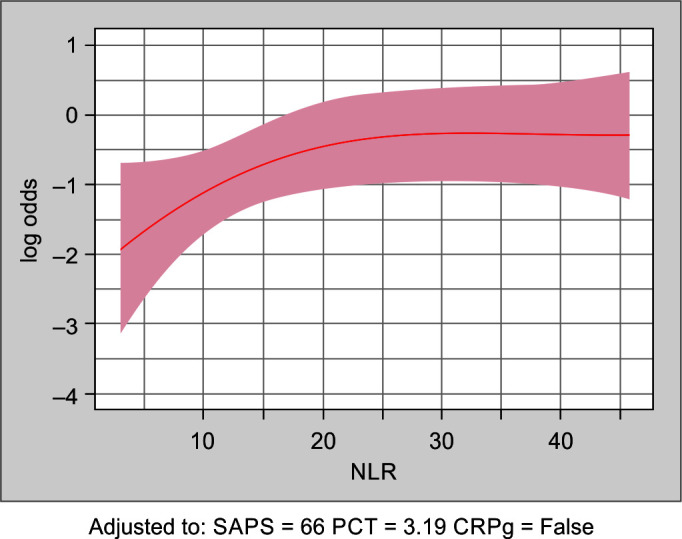
Shows the estimated log-odds for in-hospital mortality as a function of NLR, together with a 95% confidence band, when adjusting for PCT, CRP, and SAPS-III
The augmented risk scores are shown in Tables 3 and 4. The augmented score is calculated by adding specific points, according to the level of PCT, NLR, and CRP, to the existing scores. The NLR below 10 had significance only when there is a synergistic CRP of more than 100.
Table 3.
The additions to be made to the existing SAPS-III score based on the values of three predictor variables, using a nomogram-based approach
| Marker | Category | Points |
|---|---|---|
| PCT | 0–9 | 0 |
| 10–19 | 2 | |
| 20–29 | 5 | |
| 30–39 | 7 | |
| 40–49 | 8 | |
| ≤50 | 9 | |
| NLR | 0–9 and CRP < 100 | 0 |
| 0–9 and CRP > 100 | 7 | |
| 10–19 | 10 | |
| 20–29 | 15 | |
| ≤30 | 17 |
Table 4.
The additions to be made to the existing SOFA score based on the values of three predictor variables, using a nomogram-based approach
| Marker | Category | Points |
|---|---|---|
| PCT | 0–9 | 0 |
| 10–19 | 1 | |
| 20–29 | 2 | |
| 30–39 | 3 | |
| ≤40 | 4 | |
| NLR | 0–9 and CRP > 100 | 3 |
| 10–19 | 4 | |
| ≤20 | 7 |
The scores to augment the existing SAPS-III and SOFA scores were obtained from the nomograms of the logistic regression models.
Each variable is categorized into intervals, and the corresponding points are specified for each interval. These points are added to the original SAPS-III score (Table 3).
Each variable is categorized into intervals, and the corresponding points are specified for each interval. These points are added to the original SOFA score (Table 4).
Next, existing scores, quick scores, and augmented scores will be compared in the performance.
Table 5 shows the AUC, Brier, and R2 values of the augmented, existing and quick scores. The Brier scores for newly developed models such as Pro-SAPS and pro-SOFA indicate superior accuracy or calibration compared to other models. Pro-SAPS significantly improved the prediction over the original SAPS3 score (NRI = 0.50, SE = 0.14, p < 0.01). Similarly, Pro-SOFA significantly improved the prediction over the original SOFA score (NRI = 0.49, SE = 0.13, p < 0.01). The Brier score for q-SOFA is the highest (0.231) and it denotes the least accuracy among other models.
Table 5.
Comparison of the performance measures AUC value, Brier score, and R2 score for augmented, and existing SAPS and SOFA scores
| Score | AUC | Brier | R2 |
|---|---|---|---|
| pro-SAPS | 0.831 | 0.165 | 0.403 |
| SAPS-III | 0.797 | 0.179 | 0.337 |
| pro-SOFA | 0.793 | 0.181 | 0.329 |
| SOFA | 0.762 | 0.193 | 0.257 |
| qSOFA | 0.597 | 0.231 | 0.050 |
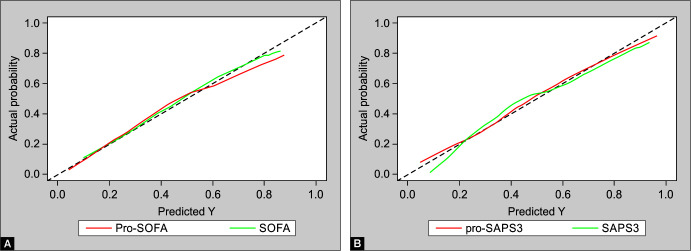
The calibration graph in Figure 4 shows the bias-corrected calibrated curves of each score, with pro-SAPS being the well-calibrated one among the SAPS-III variants and SOFA being the well-calibrated one among the SOFA variants.
Figs 4A and B.
(A) Calibration curve of augmented and existing SAPS; (B) Calibration curve of augmented and existing SOFA score
Discussion
This prospective study identified an approach to enhance the prediction of in hospital sepsis mortality by integrating weighted risk scores of commonly used clinical biomarkers of inflammation—PCT, NLR, and CRP—with established risk scores SOFA and SAPS-III, resulting in the development of augmented risk scores Pro-SOFA and Pro-SAPS. The primary innovation of our research is the superior predictive accuracy of Pro-SOFA and Pro-SAPS when compared with SOFA and SAPS-III scores.
The existing literature indicates that SAPS-III is a better prediction model than SOFA for in-hospital mortality among sepsis patients. Our study not only confirms the same, but also finds that Pro-SAPS outperforms Pro-SOFA.20–22
The inability to decrease PCT by more than 80% has been used as an independent predictor of mortality in sepsis patients (MOSES study).23 Our study focused on the quantitative value of PCT on the day of sepsis diagnosis and found that PCT levels significantly correlate with in-hospital mortality, indicating its usefulness as a marker of sepsis severity.24 Though, PCT has been demonstrated as a mortality predictor in sepsis (citation), its clinical application has largely focused on differentiating infectious from non-infectious fevers and guiding antibiotic de-escalation, rather than predicting sepsis severity or mortality.25 Although some studies suggest that PCT's predictive value in sepsis severity may be limited, there has been little exploration into integrating it—and other biomarkers—into sepsis severity scores like SOFA and SAPS.4 While PCT may not independently predict mortality as strongly as once anticipated, our findings suggest that when used alongside established scores, it adds significant value, potentially refining risk stratification and improving outcomes in sepsis management. We selected PCT, NLR, and CRP for integration because they are commonly used in clinical practice. While numerous other biomarkers—such as neutrophil bands and cytokines—might offer superior predictive accuracy, their integration into routine risk scores poses challenges.26,27 Specifically, many healthcare settings do not universally measure these biomarkers, and their inclusion could hinder the widespread applicability of the scoring systems. For instance, automated systems often do not report neutrophil band forms, limiting the generalizability of their use. Additionally, the high cost of cytokine assays further restricts their routine application.28 By focusing on widely accessible and routinely used biomarkers, we aim to enhance the practicality and adoption of these integrated scores in diverse clinical settings.23,27 While CRP was not significantly associated with mortality by itself it was found to be a significant effect modifier for NLR. We observed that the NLR ranging up to 9 correlated with sepsis mortality in the subset of patients with CRP more than 100. The NLR >9 was observed to be an independent mortality predictor in sepsis, as previously stated in the literature.29 Hence, we found that for sepsis patients with high NLR, there is no added benefit of integrating CRP into the risk score whereas in patients with NLR <9, CRP was useful in predicting the presence of sepsis severity.
The increasing weightage across the scores from the quick scores to the existing risk scores to the augmented scores illustrates a methodical enhancement in predictive accuracy by elucidating the significance of various parameter combinations in sepsis prognostication.
Our findings align with previous research that highlights the significance of PCT, NLR, and CRP in sepsis, reinforcing the concept that a multifaceted approach to sepsis prognostication, which integrates both clinical risk scores and biomarkers, can provide a more accurate reflection of the severity of the patient's condition.30 The successful integration of these biomarkers with the SOFA and SAPS-III scores to develop Pro-SOFA and Pro-SAPS models fills a critical gap in sepsis prognosis, particularly in the Indian context where the burden of sepsis is substantial, yet access to advanced diagnostic tools may be limited. These findings highlight the importance of considering not only individual biomarkers in isolation but also their combined impact when integrated into the existing risk scores in providing a nuanced understanding of sepsis severity.
Despite these promising results, our study acknowledges certain limitations. The single-center observational nature of the research confines its findings to the study population and setting, potentially limiting the generalizability of the augmented scores to other clinical environments or demographic groups. Additionally, while the augmented scores demonstrate improved predictive accuracy, their implementation in clinical practice requires validation in diverse healthcare settings, across different healthcare settings including in other LMICs and HICs where the variability in healthcare infrastructure and patient demographics may influence their effectiveness.
Conclusion
In conclusion, the integration of PCT, CRP, and NLR with SOFA and SAPS-III scores to create augmented models represents a significant step forward in mortality prediction in sepsis. The Pro-SOFA and Pro-SAPS models promise to enhance clinical decision-making, enabling earlier and accurate identification of patients at high risk for mortality. Our work contributes to the ongoing effort to improve sepsis outcomes worldwide, underscoring the need for continued innovation in the development of diagnostic and prognostic tools tailored to the unique challenges of sepsis in the global context.
Clinical Significance
This study introduces Pro-SOFA and Pro-SAPS models, integrating commonly used biomarkers in clinical practice in ICU settings, such as PCT, NLR, and CRP, with existing SOFA and SAPS-III scores. These augmented models demonstrate significantly improved predictive accuracy for sepsis mortality, and potentially improving sepsis management.
Authors' Contribution
Dipu TS made substantial contributions to the conception or design of the work, manuscript writing; supervised, reviewed and edited the work. Anandakrishnan N and Georg Gutjahr contributed in manuscript writing, statistical analysis, and interpretation of data for the work; Shashank, Aryalakshmi CS, Gayathri JS, and Sreedhar Vijayakumar contributed in data acquisition and drafting of the manuscript. Rahul Krishnan Pathinaruporthi, Sabarish B Nair, Subhash Chandra, Shyam Sudhar, Zubair Umar Mohammed, Niranjan Nair, and Merlin Moni contributed in revising it critically for important intellectual content and final approval of the version to be published.
Orcid
Anandakrishnan Nandakumar https://orcid.org/0009-0008-3428-8774
Shashank Sudeep https://orcid.org/0009-0003-7087-1346
Aryalakshmi Chakkalamparambath Sreemohan https://orcid.org/0009-0004-6960-4627
Sreedhar Vijayakumar https://orcid.org/0009-0001-2602-978X
Gayathri Jayasree Sudhakaran https://orcid.org/0009-0009-1006-7288
Georg Gutjahr https://orcid.org/0000-0002-1925-8349
Rahul K Pathinaruporthi https://orcid.org/0000-0002-0435-6085
Sabarish Balachandran https://orcid.org/0000-0001-6333-4479
Subash Chandra https://orcid.org/0000-0003-4557-7295
Shyam Sundar Purushothaman https://orcid.org/0009-0002-0225-7523
Zubair U Mohamed https://orcid.org/0000-0002-0409-9066
Sashi N Nair https://orcid.org/0000-0002-9507-1113
Merlin Moni https://orcid.org/0000-0003-1873-1488
Dipu T Sathyapalan https://orcid.org/0000-0003-1098-9900
Footnotes
Source of support: Financial support was provided by ICMR grant number BMI/12/(15)/2022 for OASIS sepsis registry database creation and also received Institutional support - Amrita seed grant number ASG2022026.
Conflict of interest: None
Patient consent statement: The author(s) have obtained written informed consent from the patient for publication of the case report details and related images.
References
- 1.Machado FR, Nsutebu E, AbDulaziz S, Daniels R, Finfer S, Kissoon N, et al. Sepsis 3 from the perspective of clinicians and quality improvement initiatives. J Crit Care. 2017;40(4):315–317. doi: 10.1016/j.jcrc.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 2.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinde VV, Jha A, Natarajan MS, Vijayakumari V, Govindaswamy G, Sivaasubramani S, et al. Serum PCT vs SOFA score in predicting outcome in sepsis patients in medical intensive care unit. Indian J Crit Care Med. 2023;27(5):348. doi: 10.5005/jp-journals-10071-24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagliani A, Fasolino A, Quilico F, Gentile FR, Ambrosini E, Baldi E, et al. Performance of APACHE, SOFA and SAPS-2 score in predicting good neurological outcome at discharge from ICU in patient admitted after an out-of-hospital cardiac arrest. Eur Heart J. 2023;12(Supplement_1):zuad036-017. doi: 10.1093/ehjacc/zuad036.017. [DOI] [Google Scholar]
- 6.Zhu Y, Zhang R, Ye X, Liu H, Wei J. SAPS III is superior to SOFA for predicting 28-day mortality in sepsis patients based on Sepsis 3.0 criteria. Int J Infectious Dis. 2022;114:135–141. doi: 10.1016/j.ijid.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Hegazy MA, Omar AS, Samir N, Moharram A, Weber S, Radwan WA. Amalgamation of PCT, C-reactive protein, and sequential organ failure scoring system in predicting sepsis survival. Anesth Essays Res. 2014;8(3):296–301. doi: 10.4103/0259-1162.143115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Donadello K, Schmit X. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin. 2011;27(2):241–251. doi: 10.1016/j.ccc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care. 1999;3(1):45–50. doi: 10.1186/cc306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11(1):464. doi: 10.1038/s41598-020-79431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer ZC, Schreinemakers JM, Mulder PG, de Waal RA, Ermens AA, van der Laan L. The role of C-reactive protein and the SOFA score as parameter for clinical decision making in surgical patients during the intensive care unit course. PLoS One. 2013;8(2):e55964. doi: 10.1371/journal.pone.0055964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Moon S, Park DW, Cho HJ, Kim JY, Park J, et al. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock: A prospective observational study according to the Sepsis-3 definitions. Medicine. 2020;99(22):e20495. doi: 10.1097/MD.0000000000020495. [DOI] [PubMed] [Google Scholar]
- 13.Rijhwani P, Jain SS, Goel P. Prediction of mortality in sepsis with biomarkers and qSOFA score combination: An observational study in a tertiary care centre of western India. J Res Clin Med. 2023;11(1):21. doi: 10.34172/jrcm.2023.33404. [DOI] [Google Scholar]
- 14.Cortiñas Abrahantes J, Sotto C, Molenberghs G, Vromman G, Bierinckx B. A comparison of various software tools for dealing with missing data via imputation. Stat Comput Simul. 2011;81(11):1653–1675. doi: 10.1080/00949655.2010.498788. [DOI] [Google Scholar]
- 15.Bertolini R, Finch SJ, Nehm RH. Quantifying variability in predictions of student performance: Examining the impact of bootstrap resampling in data pipelines. Comp Educ: Artif Intell. 2022;3:100067. doi: 10.1016/j.caeai.2022.100067. [DOI] [Google Scholar]
- 16.Brier GW. Verification of forecasts expressed in terms of probability. Monthly Weather Rev. 1950;78(1):1–3. Corpus ID:122906757. [Google Scholar]
- 17.Cohen J, Cohen P, West SG, Aiken LS. (3rd ed.). Routledge; 2002. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; p. 502. ISBN 978-0-8058-2223-6. [Google Scholar]
- 18.Harrell FE, Jr,, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Abe M, Soma M, Takeda Y, Kurihara I, Itoh H, et al. Development and validation of subtype prediction scores for the workup of primary aldosteronism. J Hypertens. 2018;36(11):2269–2276. doi: 10.1097/HJH.0000000000001855. [DOI] [PubMed] [Google Scholar]
- 20.Farhan A, Ali SM, Bin V, Mansoor RC, Rehman HU. Comparison of SAPS III and SOFA score for the prediction of mortality among ICU patients. Int J Endorsing Health Sci Res. 2021;9(4):437–442. doi: 10.29052/IJEHSR.v9.i4.2021. [DOI] [Google Scholar]
- 21.Mbongo CL, Monedero P, Guillen-Grima F, Yepes MJ, Vives M, Echarri G. Performance of SAPS3, compared with APACHE II and SOFA, to predict hospital mortality in a general ICU in Southern Europe. Eur J Anaesthesiol. 2009;26(11):940–945. doi: 10.1097/EJA.0b013e32832edadf. [DOI] [PubMed] [Google Scholar]
- 22.Roepke RM, Besen BA, Daltro-Oliveira R, Guazzelli RM, Bassi E, Salluh JI, et al. Predictive performance for hospital mortality of SAPS 3, SOFA, ISS, and new ISS in critically ill trauma patients: A validation cohort study. J Intensive Care Med. 2024;1:44–51. doi: 10.1177/08850666231188051. [DOI] [PubMed] [Google Scholar]
- 23.Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. Serial procalcitonin predicts mortality in severe sepsis patients: Results from the multicenter procalcitonin MOnitoring SEpsis (MOSES) Crit Care Med. 2017;45(5):781–789. doi: 10.1128/JCM.01851-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan A, Shrestha P, Guleria R, Pandey RM, Wig N. Development of a mortality prediction formula due to sepsis/severe sepsis in a medical intensive care unit. Lung India. 2015;32(4):313–319. doi: 10.4103/0970-2113.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldirà J, Ruiz-Rodríguez JC, Wilson DC, Ruiz-Sanmartin A, Cortes A, Chiscano L, et al. Biomarkers and clinical scores to aid the identification of disease severity and intensive care requirement following activation of an in-hospital sepsis code. Ann Intensive Care. 2020;10(1):7. doi: 10.1186/s13613-020-0625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins EC, Silveira LD, Viegas K, Beck AD, Fioravantti G, Cremonese RV, et al. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: A case-control study. Rev Bras Ter Intensiva. 2019;31(1):64–70. doi: 10.5935/0103-507X.20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davoudian S, Piovani D, Desai A, Mapelli SN, Leone R, Sironi M, et al. A cytokine/PTX3 prognostic index as a predictor of mortality in sepsis. Front Immunol. 2022;13:979232. doi: 10.3389/fimmu.2022.979232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streimish I, Bizzarro M, Northrup V, Wang C, Renna S, Koval N, et al. Neutrophil CD64 as a diagnostic marker in neonatal sepsis. Pediatr Infect Dis J. 2012;31(7):777–781. doi: 10.1097/INF.0b013e318256fb07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Luo Q, Su Z, Li Y, Wang H, Liu Q, et al. Neutrophil-to-lymphocyte ratio as a predictor of mortality in intensive care unit patients: A retrospective analysis of the Medical Information Mart for Intensive Care III Database. BMJ Open. 2021;11(11):e053548. doi: 10.1136/bmjopen-2021-053548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miro M, del Valle GS, Agámez G, García PP, Martínez HE, Olivas E. Correlation between SOFA score and procalcitonin blood levels in peritonitis patients: 12AP3-4. Eur J Anaesthesiol. 2011;28:173. doi: 10.1097/00003643-201106001-00556. [DOI] [Google Scholar]