Abstract
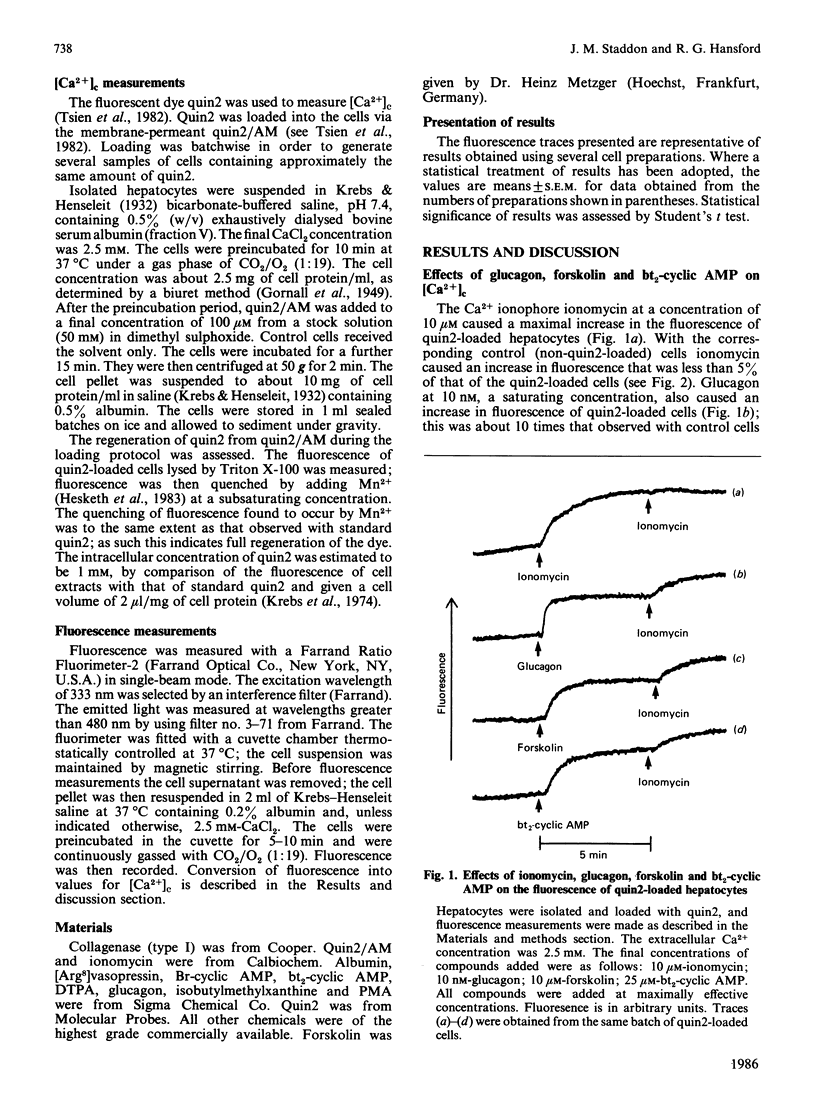
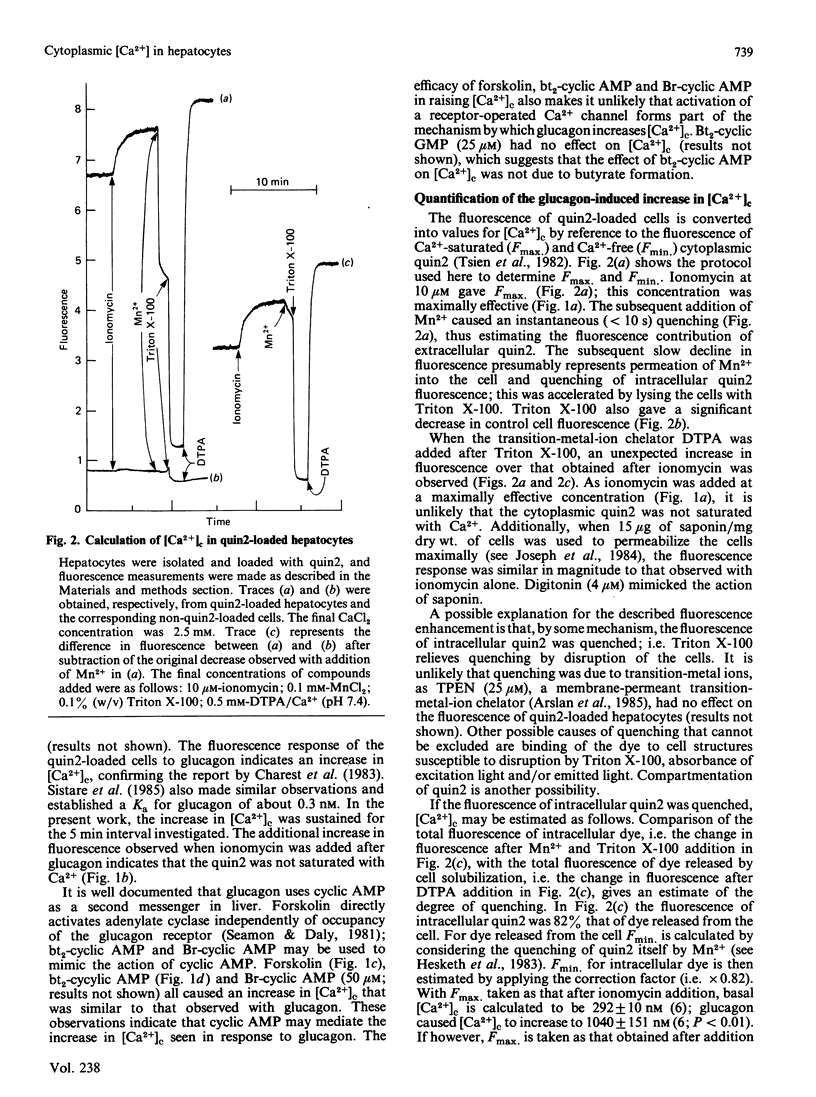
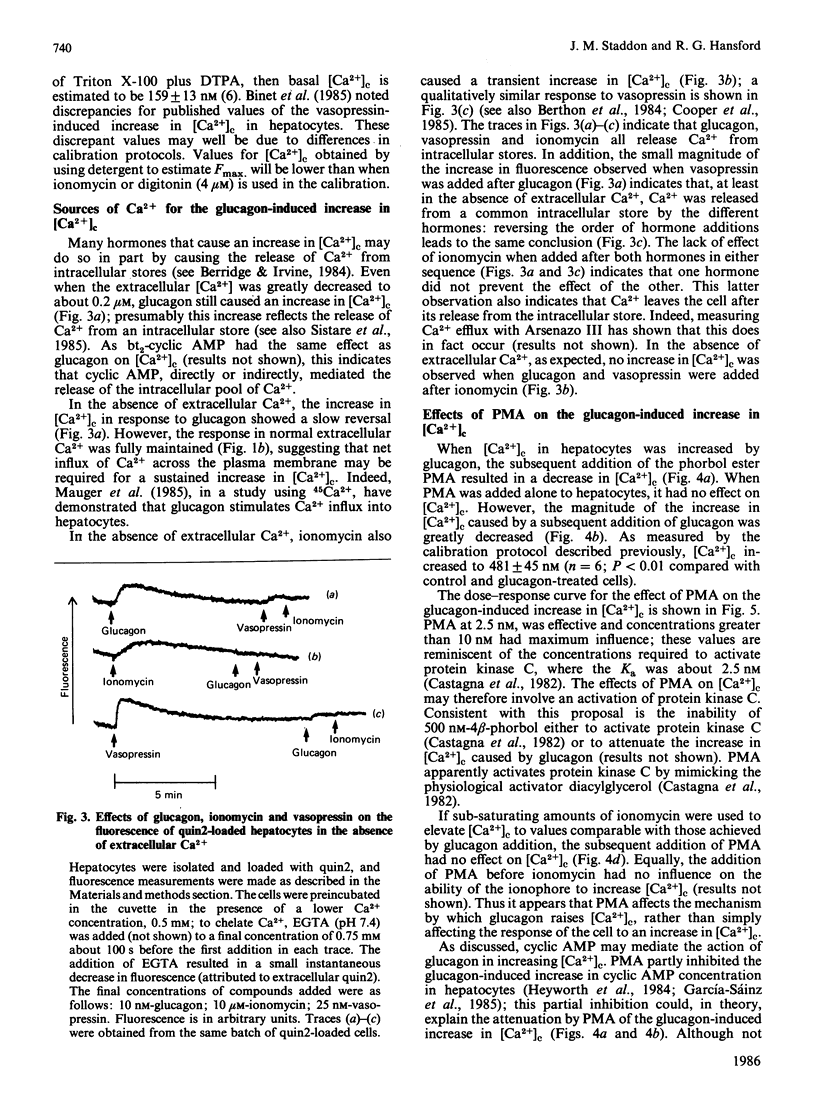
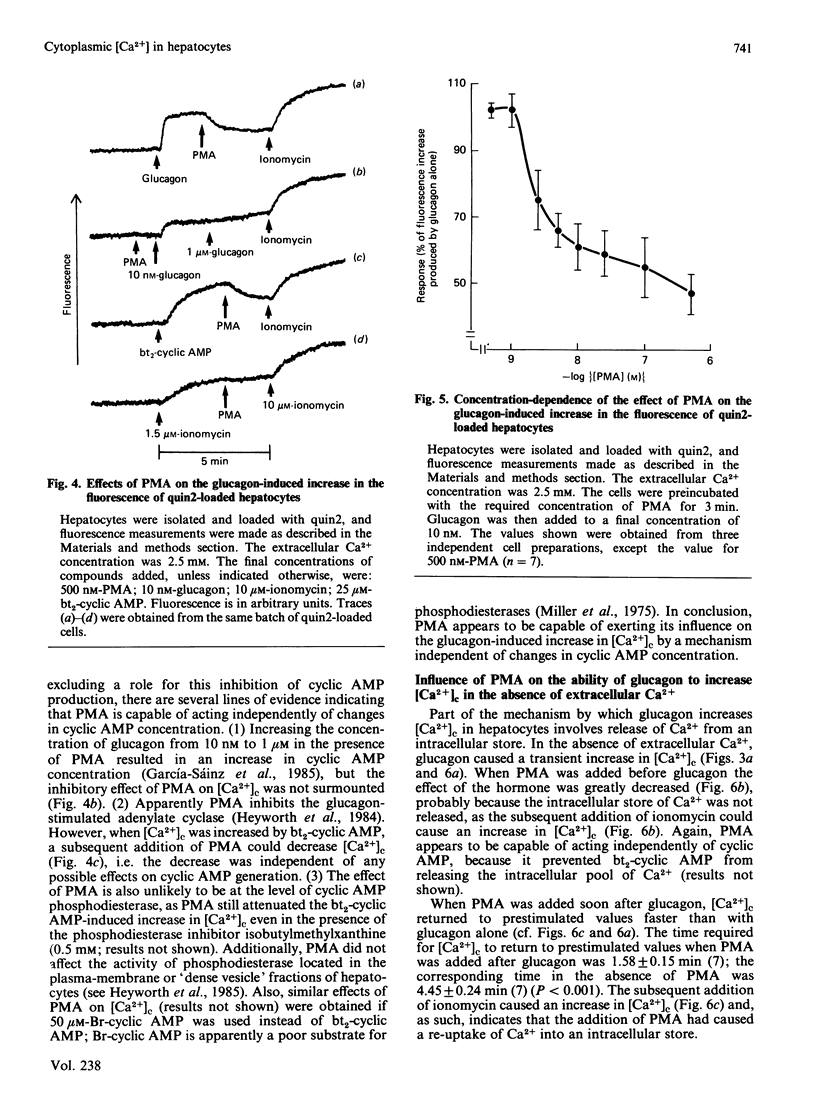
Hepatocytes were isolated from rats and then loaded with the fluorescent Ca2+ indicator quin2. Glucagon caused a sustained increase (at least 5 min) in the fluorescence of the quin2-loaded cells; the increase was much greater than that observed with control, non-quin2-loaded, cells. These observations indicate that glucagon caused an increase in cytoplasmic free Ca2+ concentration [( Ca2+]c). The effects of glucagon were mimicked if forskolin (to activate adenylate cyclase), dibutyryl cyclic AMP or bromo cyclic AMP were added directly to the cells. Thus an increase in cyclic AMP concentration may mediate the effect of glucagon on [Ca2+]c. If 4 beta-phorbol 12-myristate 13-acetate (PMA; an activator of protein kinase C) was added to the cells before glucagon, the magnitude of the increase in [Ca2+]c was greatly diminished. If PMA was added after glucagon it caused a lowering of [Ca2+]c. These effects of PMA on the glucagon-induced increase in [Ca2+]c could not be mimicked if [Ca2+]c was increased by the Ca2+-ionophore ionomycin. Thus an event involved in the mechanism by which glucagon increases [Ca2+]c appears to be required for the action of PMA. If [Ca2+]c was increased by forskolin, dibutyryl cyclic AMP or bromo cyclic AMP, the effect of PMA on [Ca2+]c was similar to that observed when glucagon was used to elevate [Ca2+]c. When [Ca2+]c was raised by dibutyryl cyclic AMP the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine did not prevent the subsequent addition of PMA from causing [Ca2+]c to decrease. These observations suggest that PMA can inhibit the cyclic AMP-induced increase in [Ca2+]c independently of any changes in cyclic AMP concentration. Glucagon appears to increase [Ca2+]c by releasing intracellular stores of Ca2+ and stimulating net influx of Ca2+ into the cell; PMA greatly diminishes both of these effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Binet A., Berthon B., Claret M. Hormone-induced increase in free cytosolic calcium and glycogen phosphorylase activation in rat hepatocytes incubated in normal and low-calcium media. Biochem J. 1985 Jun 15;228(3):565–574. doi: 10.1042/bj2280565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Coll K. E., Williamson J. R. Differential effects of phorbol ester on phenylephrine and vasopressin-induced Ca2+ mobilization in isolated hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3281–3288. [PubMed] [Google Scholar]
- García-Sáinz J. A., Mendlovic F., Martínez-Olmedo M. A. Effects of phorbol esters on alpha 1-adrenergic-mediated and glucagon-mediated actions in isolated rat hepatocytes. Biochem J. 1985 May 15;228(1):277–280. doi: 10.1042/bj2280277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J Biol Chem. 1984 Mar 10;259(5):3283–3292. [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Kinsella A. R., Houslay M. D. The phorbol ester, TPA inhibits glucagon-stimulated adenylate cyclase activity. FEBS Lett. 1984 May 7;170(1):38–42. doi: 10.1016/0014-5793(84)81364-2. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Wilson S. P., Gawler D. J., Houslay M. D. The phorbol ester TPA prevents the expression of both glucagon desensitisation and the glucagon-mediated block of insulin stimulation of the peripheral plasma membrane cyclic AMP phosphodiesterase in rat hepatocytes. FEBS Lett. 1985 Aug 5;187(2):196–200. doi: 10.1016/0014-5793(85)81241-2. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kleineke J., Söling H. D. Mitochondrial and extramitochondrial Ca2+ pools in the perfused rat liver. Mitochondria are not the origin of calcium mobilized by vasopressin. J Biol Chem. 1985 Jan 25;260(2):1040–1045. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- McCormack J. G. Studies on the activation of rat liver pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase by adrenaline and glucagon. Role of increases in intramitochondrial Ca2+ concentration. Biochem J. 1985 Nov 1;231(3):597–608. doi: 10.1042/bj2310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Beck A. H., Simon L. N., Meyer R. B., Jr Induction of hepatic tyrosine aminotransferase in vivo by derivatives of cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1975 Jan 25;250(2):426–431. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]


