Abstract
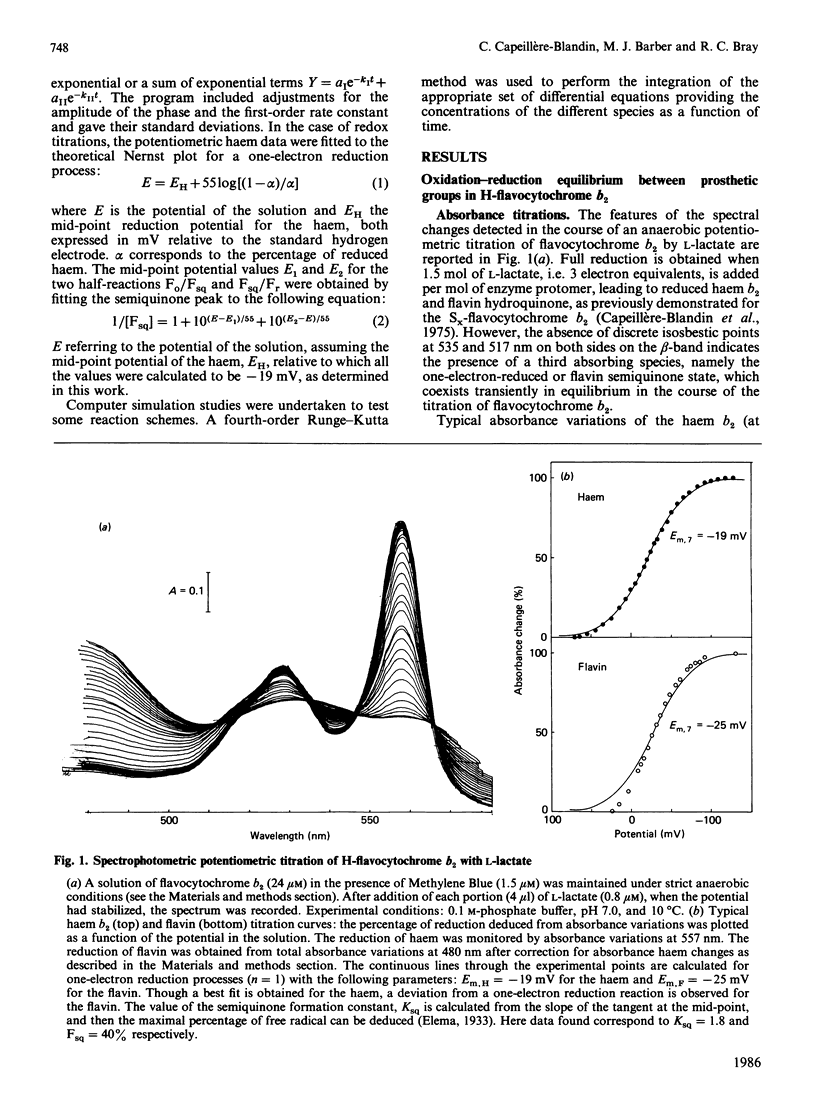
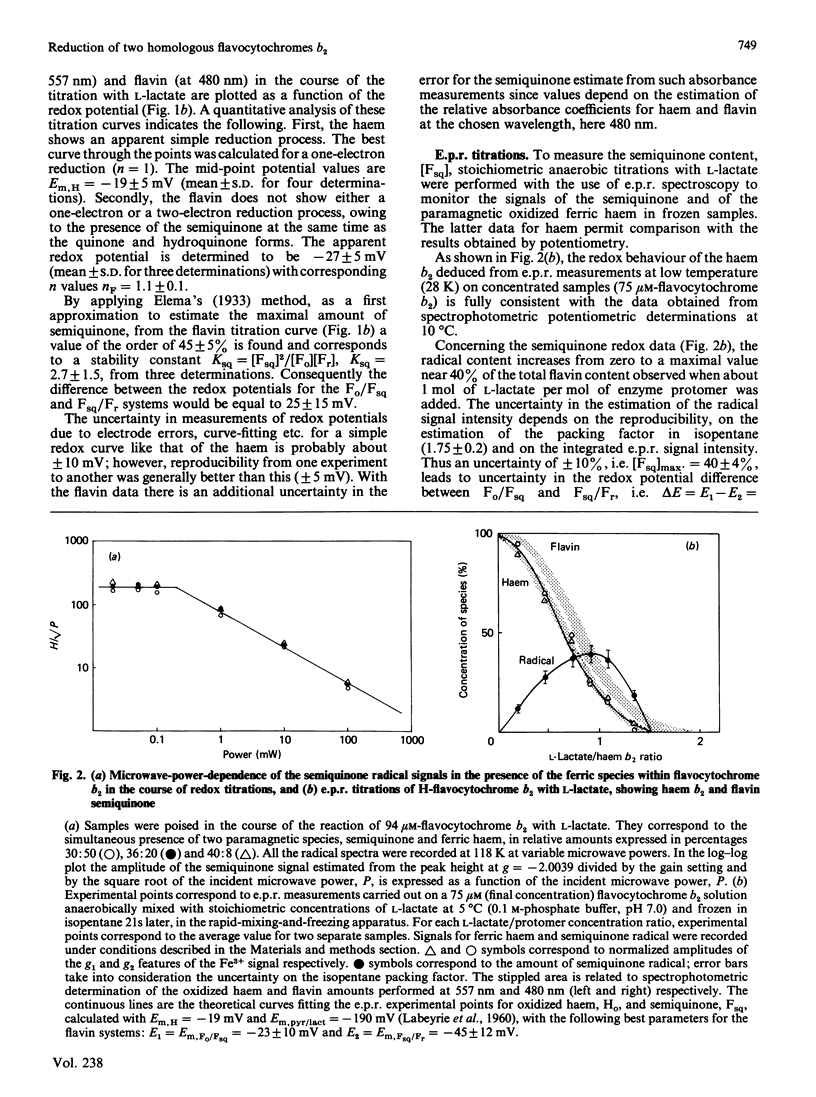
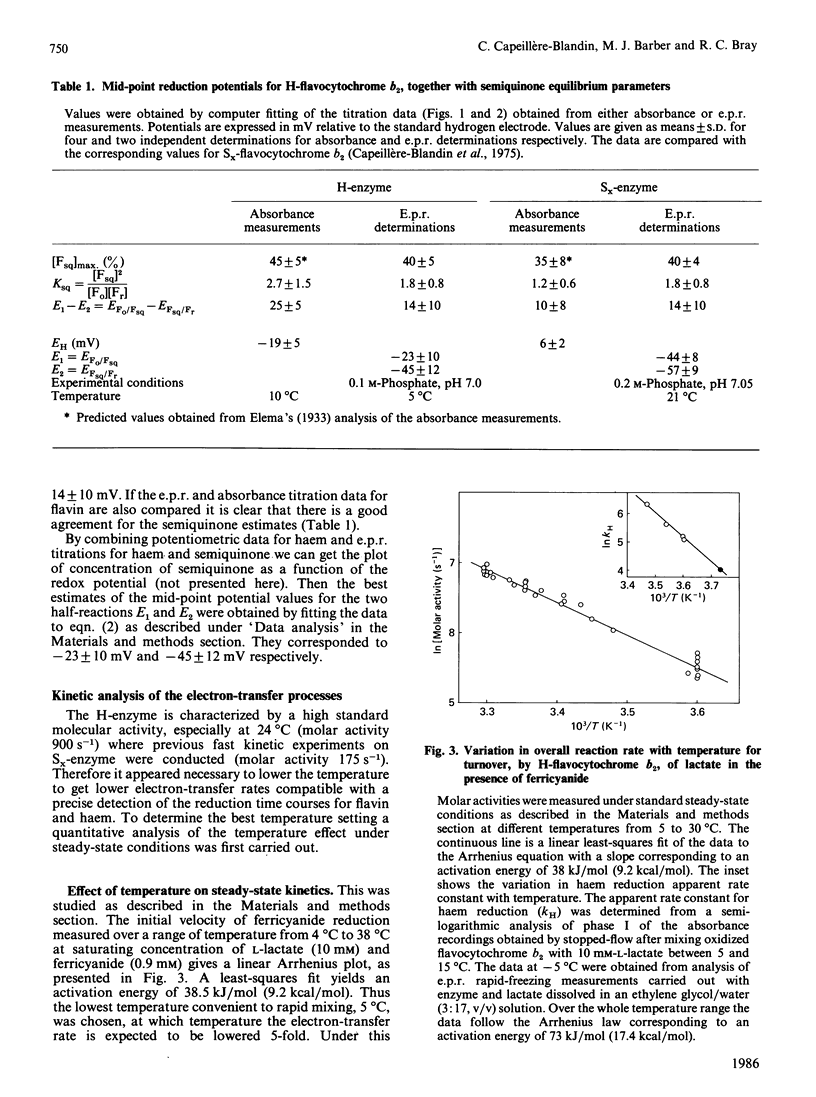
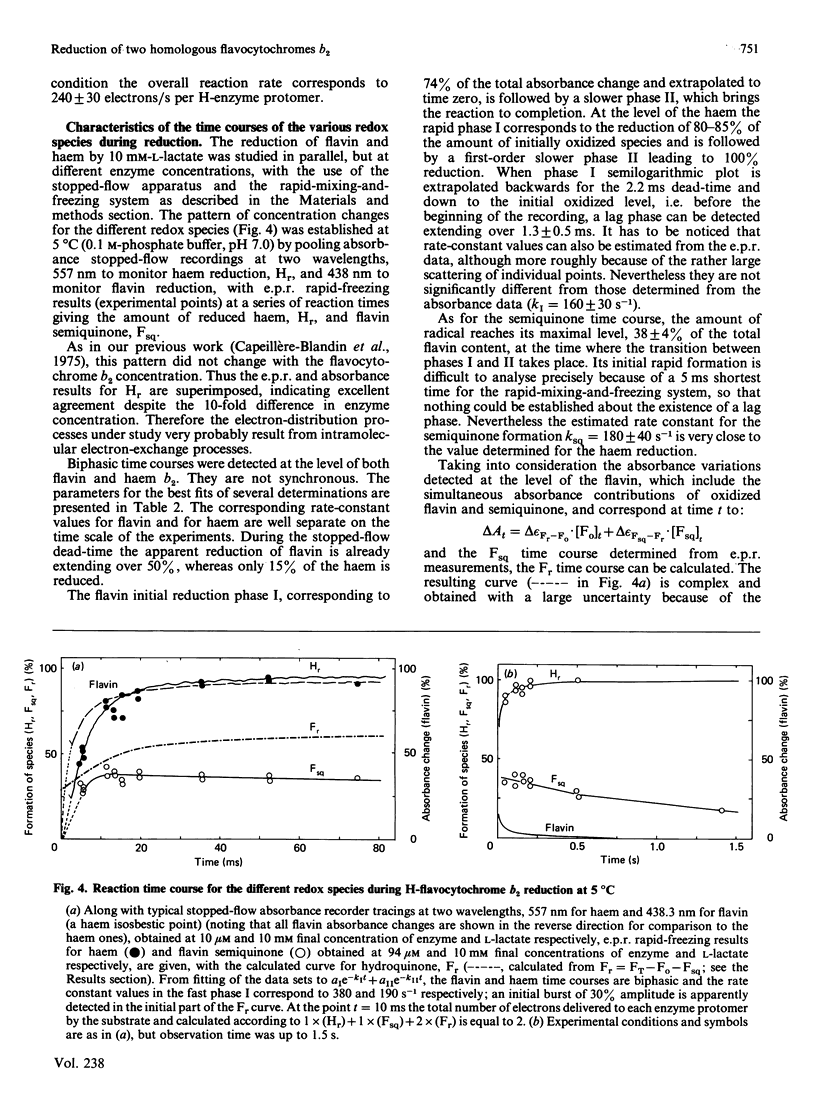
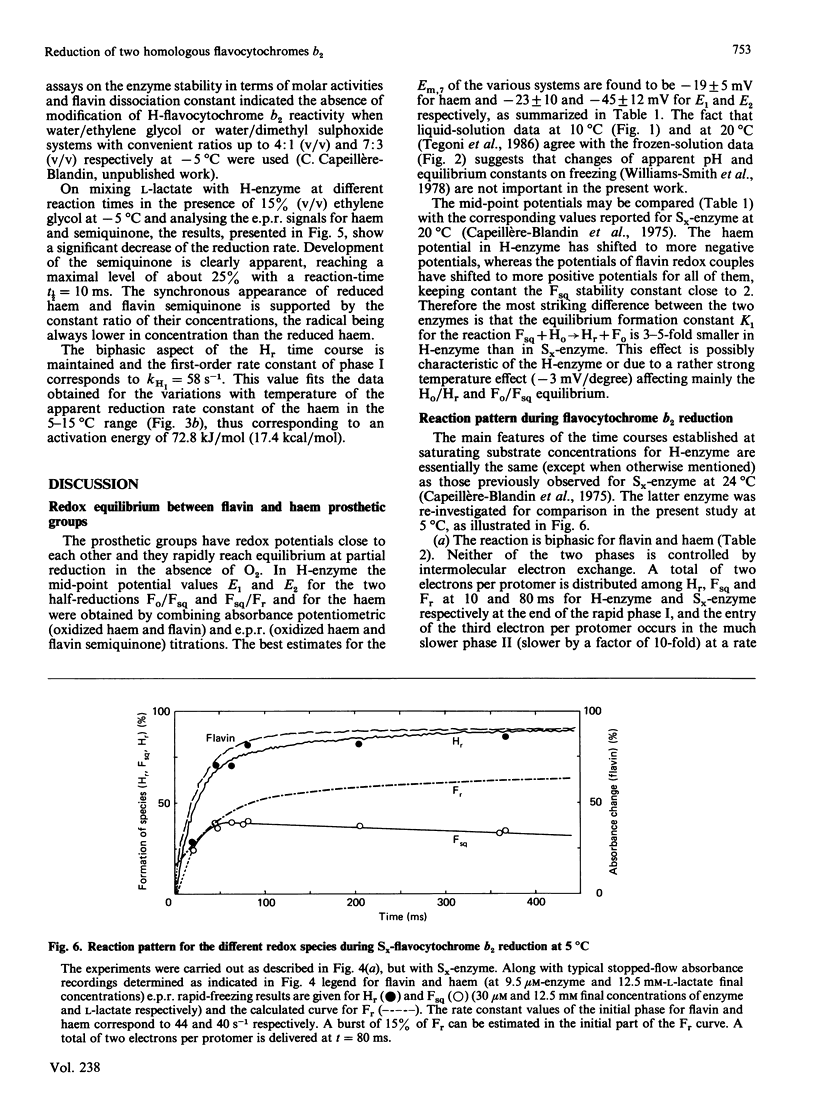
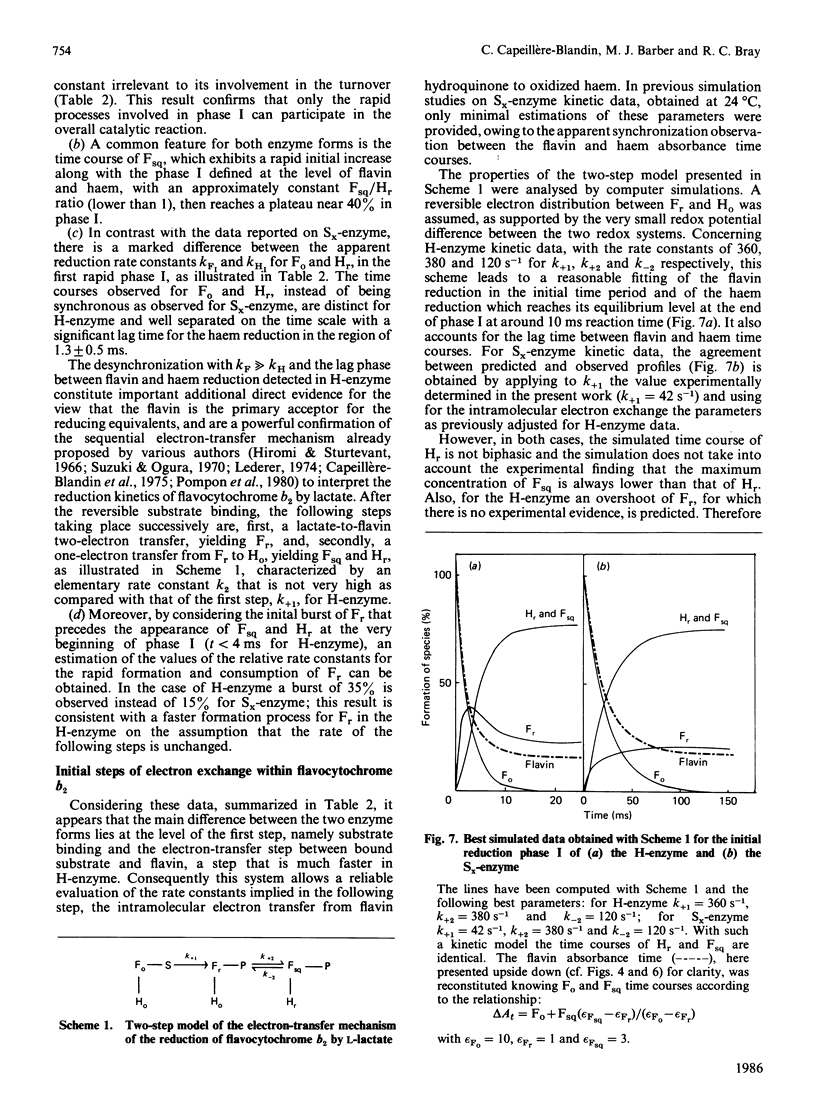
A detailed study of the electron exchanges involved between FMN and haem b2 groups within flavocytochrome b2 of yeast Hansenula anomala (H-enzyme) was performed. The results were compared with those for the homologous enzyme of yeast Saccharomyces cerevisiae (Sx-enzyme) re-investigated at 5 degrees C. The mid-point reduction potentials of FMN and haem were determined by two complementary methods: potentiometric titration with substrate, L-lactate, in the presence of dye mediators with quantification of the reduced species performed by spectrophotometry at suitable wavelengths; anaerobic titration of the enzyme by its substrate by monitoring the e.p.r. signals of the semiquinone and Fe3+ species. Values of Em,7 = -19, -23 and -45 V were determined respectively from the data for the three redox systems Ho/Hr, Fo/Fsq and Fsq/Fr in the H-enzyme instead of +6, -44 and -57 mV respectively in the Sx-enzyme [Capeillère-Blandin, Bray, Iwatsubo & Labeyrie (1975) Eur. J. Biochem. 54, 549-566]. Parallel e.p.r rapid-freezing and absorbance stopped-flow studies allowed determination of the time courses of the various redox species during their reduction by L-lactate. The flavin and the haem reduction time courses were biphasic. In the initial fast phase the reduction of flavin monitored by absorbance measurements is accomplished with a rate constant kF = 360 s-1. The reduction of the haem lags the reduction of flavin with a rate constant kH = 170 s-1. The appearance of flavin free radical is slower than the reduction in flavin absorbance and occurs with a rate constant close to that of the reduction of the haem. At saturating L-lactate concentration the initial rapid phase (up to 15 ms) involved in the overall turnover can be adequately simulated with a two-step reaction scheme. The main difference between the enzymes lies especially at the level of the first step of electron exchange between bound lactate and flavin, which for the H-enzyme is no longer the rate-limiting step in the haem reduction and becomes 8-fold faster than in the Sx-enzyme. Consequently in the H-enzyme for the following step, the intramolecular transfer from flavin hydroquinone to oxidized haem, a reliable evaluation of the rate constants becomes possible. Preliminary values are k+2 = 380 s-1 and k-2 = 120 s-1 at 5 degrees C.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Lactic dehydrogenase and cytochrome b2 of baker's yeast; purification and crystallization. Biochem J. 1959 Mar;71(3):492–499. doi: 10.1042/bj0710492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C. Sudden freezing as a technique for the study of rapid reactions. Biochem J. 1961 Oct;81:189–193. doi: 10.1042/bj0810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY R. C. The chemistry of xanthine oxidase. 8. Electronspin-resonance measurements during the enzymic reaction. Biochem J. 1961 Oct;81:196–199. doi: 10.1042/bj0810196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudras A. Pure L-lactate: cytochrome c oxidoreductase of the yeast Hansenula anomala offers new prospect for the study of intramolecular multi-electron transfer. Biochimie. 1971;53(8):929–933. doi: 10.1016/s0300-9084(71)80156-6. [DOI] [PubMed] [Google Scholar]
- Bray G. A., Gallagher T. F., Jr Suppression of appetite by bile acids. Lancet. 1968 May 18;1(7551):1066–1067. doi: 10.1016/s0140-6736(68)91415-3. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C., Pucheault J., Ferradini C. The reduction of flavocytochrome b2 by carboxylate radicals. A pulse radiolysis study. Biochim Biophys Acta. 1984 Apr 27;786(1-2):67–78. doi: 10.1016/0167-4838(84)90155-9. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C. Transient kinetics of the one-electron transfer reaction between reduced flavocytochrome b2 and oxidized cytochrome c. Evidence for the existence of a protein complex in the reaction. Eur J Biochem. 1982 Nov 15;128(2-3):533–542. doi: 10.1111/j.1432-1033.1982.tb06998.x. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C., Bray R. C., Iwatsubo M., Labeyrie F. Flavocytochrome b2: kinetic studies by absorbance and electron-paramagnetic-resonance spectroscopy of electron distribution among prosthetic groups. Eur J Biochem. 1975 Jun;54(2):549–566. doi: 10.1111/j.1432-1033.1975.tb04168.x. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C. Flavocytochrome b2: simulation studies of the electron-transfer reactions among the prosthetic groups. Eur J Biochem. 1975 Aug 1;56(1):91–101. doi: 10.1111/j.1432-1033.1975.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Devault D. Quantum mechanical tunnelling in biological systems. Q Rev Biophys. 1980 Nov;13(4):387–564. doi: 10.1017/s003358350000175x. [DOI] [PubMed] [Google Scholar]
- Douzou P. The use of subzero temperatures in biochemistry: slow reactions. Methods Biochem Anal. 1974;22:401–512. doi: 10.1002/9780470110423.ch9. [DOI] [PubMed] [Google Scholar]
- Gervais M., Labeyrie F., Risler Y., Vergnes O. A flavin-mononucleotide-binding site in Hansenula anomala nicked flavocytochrome b2, requiring the association of two domains. Eur J Biochem. 1980 Oct;111(1):17–31. doi: 10.1111/j.1432-1033.1980.tb06071.x. [DOI] [PubMed] [Google Scholar]
- Gervais M., Risler Y., Corazzin S. Proteolytic cleavage of Hansenula anomala flavocytochrome b2 into its two functional domains. Isolation of a highly active flavodehydrogenase and a cytochrome b2 core. Eur J Biochem. 1983 Feb 1;130(2):253–259. doi: 10.1111/j.1432-1033.1983.tb07144.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. The molybdenum centre of native xanthine oxidase. Evidence for proton transfer from substrates to the centre and for existence of an anion-binding site. Biochem J. 1978 Dec 1;175(3):869–878. doi: 10.1042/bj1750869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo M., Mével-Ninio M., Labeyrie F. Rapid kinetic studies of partial reactions in the heme free derivative of L-lactate cytochrome c oxidoreductase (flavocytochrome b2); the flavodehydrogenase function. Biochemistry. 1977 Aug 9;16(16):3558–3566. doi: 10.1021/bi00635a009. [DOI] [PubMed] [Google Scholar]
- LABEYRIE F., NASLIN L., CURDEL A., WURMSER R. [A new determination of the redox potential of the lactate-pyruvate system]. Biochim Biophys Acta. 1960 Jul 15;41:509–515. doi: 10.1016/0006-3002(60)90049-4. [DOI] [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Lederer F. On the first steps of lactate oxidation by bakers' yeast L-(plus)-lactate dehydrogenase (cytochrome b2). Eur J Biochem. 1974 Jul 15;46(2):393–399. doi: 10.1111/j.1432-1033.1974.tb03632.x. [DOI] [PubMed] [Google Scholar]
- MORTON R. K., STURTEVANT J. M. KINETIC INVESTIGATIONS OF YEAST L-LACTATE DEHYDROGENASE (CYTOCHROME B2). I. THE DEHYDROGENATION OF L-LACTATE IN THE PRESENCE AND ABSENCE OF FERRICYANIDE AS ELECTRON ACCEPTOR. J Biol Chem. 1964 May;239:1614–1624. [PubMed] [Google Scholar]
- Massey V., Curti B. On the reaction mechanism of Crotalus adamanteus L-amino acid oxidase. J Biol Chem. 1967 Mar 25;242(6):1259–1264. [PubMed] [Google Scholar]
- Pajot P., Groudinsky O. Molecular weight and quaternary structure of yeast L-lactate dehydrogenase (cytochrome b2). 2. Revised heme extinction coefficients and minimal molecular weight. Eur J Biochem. 1970 Jan;12(1):158–164. doi: 10.1111/j.1432-1033.1970.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Pompon D. Flavocytochrome b2 from baker's yeast. Computer-simulation studies of a new scheme for intramolecular electron transfer. Eur J Biochem. 1980 May;106(1):151–159. [PubMed] [Google Scholar]
- Pompon D., Iwatsubo M., Lederer F. Flavocytochrome b2 (Baker's yeast). Deuterium isotope effect studied by rapid-kinetic methods as a probe for the mechanism of electron transfer. Eur J Biochem. 1980 Mar;104(2):479–488. doi: 10.1111/j.1432-1033.1980.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Ogura Y. The kinetic behavior of the FMN and protoheme moieties of yeast L(plus)-lactate dehydrogenase (cytochrome b2). J Biochem. 1970 Feb;67(2):277–289. doi: 10.1093/oxfordjournals.jbchem.a129251. [DOI] [PubMed] [Google Scholar]
- Tegoni M., Janot J. M., Labeyrie F. Regulation of dehydrogenases/one-electron transferases by modification of flavin redox potentials. Effect of product binding on semiquinone stabilization in yeast flavocytochrome b2. Eur J Biochem. 1986 Mar 17;155(3):491–503. doi: 10.1111/j.1432-1033.1986.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Tegoni M., Silvestrini M. C., Labeyrie F., Brunori M. A temperature-jump study of the electron transfer reactions in Hansenula anomala flavocytochrome b2. Eur J Biochem. 1984 Apr 2;140(1):39–45. doi: 10.1111/j.1432-1033.1984.tb08064.x. [DOI] [PubMed] [Google Scholar]
- Williams-Smith D. L., Heathcote P., Sihra C. K., Evans M. C. Quantitative electron-paramagnetic-resonance measurements of the electron-transfer components of the photosystem-I reaction centre. Biochem J. 1978 Feb 15;170(2):365–371. doi: 10.1042/bj1700365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K., Sugiura N., Okamura K., Kotaki A. Mechanism of enzyme action. 3. Crystallization of the semiquinoid form of D-amino-acid oxidase. Biochim Biophys Acta. 1968 Feb 5;151(2):343–352. doi: 10.1016/0005-2744(68)90101-0. [DOI] [PubMed] [Google Scholar]


