Abstract
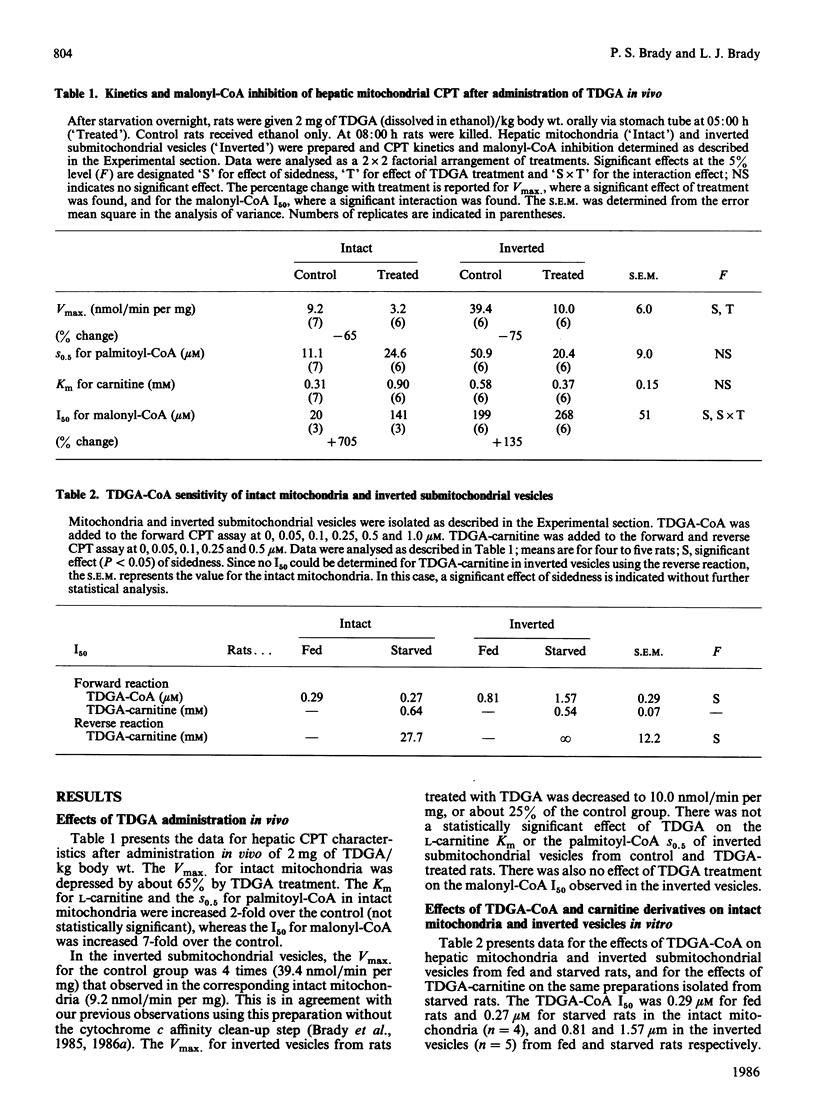
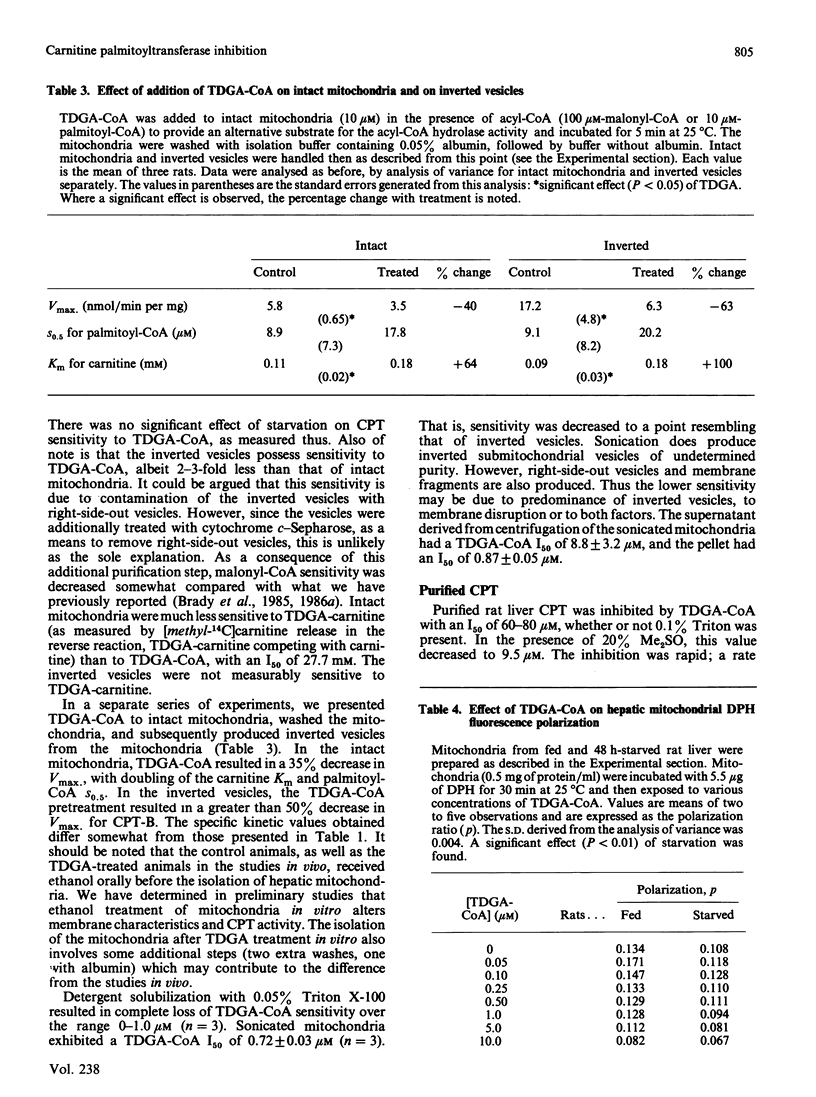
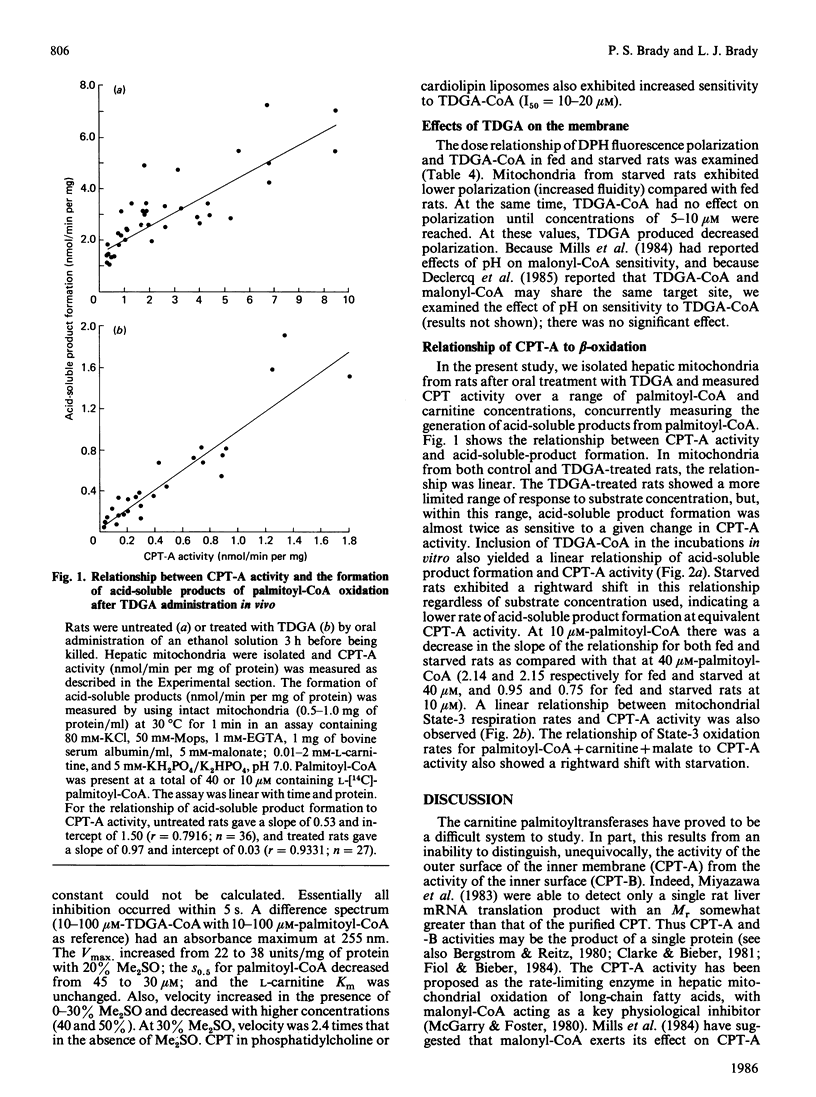
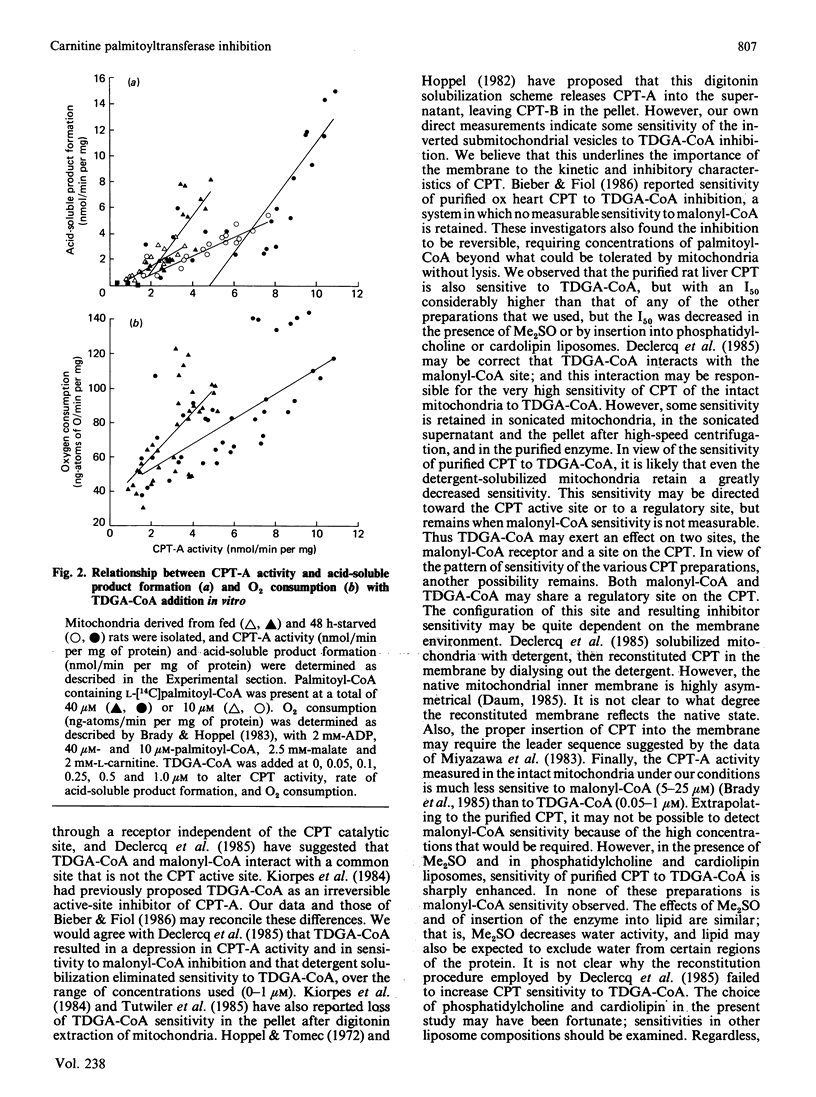
The effects of 2-tetradecylglycidic acid (TDGA), TDGA-CoA and TDGA-carnitine were examined in purified hepatic CPT (carnitine palmitoyltransferase) and in hepatic mitochondria and inverted submitochondrial vesicles derived from Sprague-Dawley rats. Since TDGA has been reported as a specific inhibitor of carnitine palmitoyltransferase-A (CPT-A), the focus was on kinetics and inhibition of CPT-A, and the relationship of this key enzyme to beta-oxidation. After administration of TDGA in vivo to overnight-starved rats, the Vmax. of CPT in intact mitochondria and in inverted vesicles (CPT-B) was depressed by 66%. The S0.5 for palmitoyl-CoA and Km for carnitine were unchanged. The I50 (concn. giving 50% inhibition) for malonyl-CoA was significantly increased from 20 to 141 microM in intact mitochondria, but unchanged (199 versus 268 microM) in inverted vesicles. The addition in vitro of TDGA-CoA (0-1.0 microM) gave I50 values of 0.29 and 0.27 microM (S.E.M. = 0.19) in intact mitochondria from fed and 48 h-starved rats, and 0.81 and 1.57 microM (S.E.M. = 0.29) for inverted vesicles derived from fed and starved rats. Addition in vitro of TDGA-carnitine to mitochondria from starved rats yielded an I50 value of 27.7 mM (S.E.M. = 12.2) for L-[methyl-14C]carnitine release from palmitoyl-L-[methyl-14C]carnitine and 0.64 mM (S.E.M. = 0.07) for palmitoyl-L-[methyl-14C]carnitine formation from L-[methyl-14C]carnitine in intact mitochondria. Inverted vesicles were not measurably sensitive to TDGA-carnitine up to 500 microM for the assay of L-[methyl-14C]carnitine release, but were as sensitive as intact mitochondria when inhibition was determined in the direction of palmitoyl-L-[methyl-14C]carnitine formation (I50 = 0.54 +/- 0.07 microM). When TDGA-CoA was added to intact mitochondria, then incubated for 5 min at room temperature and subsequently washed out, Vmax. of CPT decreased from 5.8 to 3.5 (S.E.M. = 0.6) in intact mitochondria, and from 17.2 to 6.3 (S.E.M. = 4.8) in inverted vesicles. The Km for L-carnitine and the S0.5 for palmitoyl-CoA increased 2-fold with TDGA-CoA pretreatment in both intact mitochondria and inverted vesicles. Detergent solubilization (0.05% Triton X-100) resulted in a complete loss of TDGA-CoA sensitivity (up to 1.0 microM measured). Sonicated mitochondria exhibited an I50 of 0.72 +/- 0.03 microM.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Bieber L. L., Abraham T., Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972 Dec;50(2):509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- Bieber L. L., Fiol C. J. Characterization and properties of carnitine acyltransferases. Biochem Soc Trans. 1986 Aug;14(4):674–676. doi: 10.1042/bst0140674. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner-membrane properties, beta-oxidation and carnitine palmitoyltransferases A and B. Effects of genetic obesity and starvation. Biochem J. 1986 Jan 15;233(2):427–433. doi: 10.1042/bj2330427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Hoppel C. L. Hepatic mitochondrial function in lean and obese Zucker rats. Am J Physiol. 1983 Sep;245(3):E239–E245. doi: 10.1152/ajpendo.1983.245.3.E239. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Silverstein L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner membrane properties and carnitine palmitoyltransferase A and B. Effect of diabetes and starvation. Biochem J. 1985 Dec 1;232(2):445–450. doi: 10.1042/bj2320445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel K., Bressler R. The resolution of (plus or minus)-carnitine and the synthesis of acylcarnitines. Biochim Biophys Acta. 1967 Feb 14;137(1):98–106. doi: 10.1016/0005-2760(67)90012-4. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Lieber C. S., Beattie D. S., Rubin E. Effect of chronic ethanol ingestion on fatty acid oxidation by hepatic mitochondria. J Biol Chem. 1975 Jul 10;250(13):5122–5129. [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific alkylation of a histidine residue in carnitine acetyltransferase by bromoacetyl-L-carnitine. Biochem J. 1970 Feb;116(4):713–720. doi: 10.1042/bj1160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Venincasa M. D., Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and 2-tetradecylglycidyl-CoA with mitochondrial carnitine palmitoyltransferase I. J Biol Chem. 1985 Oct 15;260(23):12516–12522. [PubMed] [Google Scholar]
- Federer W. T., Zelen M. Analysis of multifactor classifications with unequal numbers of observations. Biometrics. 1966 Sep;22(3):525–552. [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Fleischer S., Meissner G., Smigel M., Wood R. Preparation of submitochondrial vesicles using nitrogen decompression. Methods Enzymol. 1974;31:292–299. doi: 10.1016/0076-6879(74)31030-0. [DOI] [PubMed] [Google Scholar]
- Garland P. B. Control of citrate synthesis in mitochondria. Biochem Soc Symp. 1968;27:41–60. [PubMed] [Google Scholar]
- Godinot C., Gautheron D. C. Separation of right-side-out and inside-out submitochondrial particles by affinity chromatography on Sepharose-cytochrome c. Methods Enzymol. 1979;55:112–114. doi: 10.1016/0076-6879(79)55015-0. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. Citrate, pyruvate, and lactate contaminants of commercial serum albumin. J Lipid Res. 1968 Sep;9(5):667–668. [PubMed] [Google Scholar]
- Hoppel C. L. Carnitine and carnitine palmitoyltransferase in fatty acid oxidation and ketosis. Fed Proc. 1982 Oct;41(12):2853–2857. [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- Kiorpes T. C., Hoerr D., Ho W., Weaner L. E., Inman M. G., Tutwiler G. F. Identification of 2-tetradecylglycidyl coenzyme A as the active form of methyl 2-tetradecylglycidate (methyl palmoxirate) and its characterization as an irreversible, active site-directed inhibitor of carnitine palmitoyltransferase A in isolated rat liver mitochondria. J Biol Chem. 1984 Aug 10;259(15):9750–9755. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Noël H., Goswami T., Pande S. V. Solubilization and reconstitution of rat liver mitochondrial carnitine acylcarnitine translocase. Biochemistry. 1985 Aug 13;24(17):4504–4509. doi: 10.1021/bi00338a003. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Bartlett K., Younan S. I., Sherratt H. S. The effects of 2[5(4-chlorophenyl)pentyl]oxirane-2-carbonyl-Co-A on mitochondrial oxidations. Biochem Pharmacol. 1984 Feb 1;33(3):475–481. doi: 10.1016/0006-2952(84)90243-0. [DOI] [PubMed] [Google Scholar]
- Tutwiler G. F., Brentzel H. J., Kiorpes T. C. Inhibition of mitochondrial carnitine palmitoyl transferase A in vivo with methyl 2-tetradecylglycidate (methyl palmoxirate) and its relationship to ketonemia and glycemia. Proc Soc Exp Biol Med. 1985 Feb;178(2):288–296. doi: 10.3181/00379727-178-42012. [DOI] [PubMed] [Google Scholar]
- Tutwiler G. F., Ho W., Mohrbacher R. J. 2-Tetradecylglycidic acid. Methods Enzymol. 1981;72:533–551. doi: 10.1016/s0076-6879(81)72042-1. [DOI] [PubMed] [Google Scholar]
- Tutwiler G. F., Ryzlak M. T. Inhibition of mitochondrial carnitine palmitoyl transferase by 2-tetradecylglycidic acid (McN-3802) (preliminary communication). Life Sci. 1980 Feb 4;26(5):393–397. doi: 10.1016/0024-3205(80)90156-3. [DOI] [PubMed] [Google Scholar]


