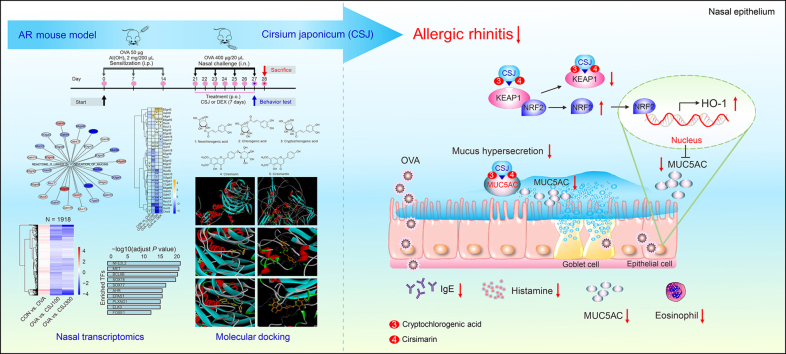
Graphical abstract
Highlights
-
•
Cirsium japonicum (CSJ) alleviates ovalbumin (OVA)-induced allergic rhinitis (AR).
-
•
CSJ reduces serum anti-OVA IgE, histamine, and IL-13 levels and nasal inflammation.
-
•
O-Linked glycosylation of mucins, MUC5AC, and NRF2 are key targets of CSJ in AR.
-
•
Cryptochlorogenic acid and cirsimarin in CSJ bind directly to NRF2–KEAP1 and MUC5AC.
-
•
CSJ shows potential for AR therapeutic agent via the NRF2 pathway and MUC5AC expression regulation.
Allergic rhinitis (AR) is an immunoglobulin (Ig)E-mediated allergic disease frequently recurring following seasonal changes and significantly impacting quality of life [1]. Worsening AR symptoms can lead to more severe conditions. Cirsium japonicum (CSJ, milk thistle) is an edible and medicinal plant with anti-oxidant, anti-inflammatory, and hepatoprotective properties; however, its effects and mechanisms of action in AR remain unclear. Here, we investigated the CSJ targets in AR and the underlying mechanisms in an ovalbumin (OVA)-induced AR mouse model.
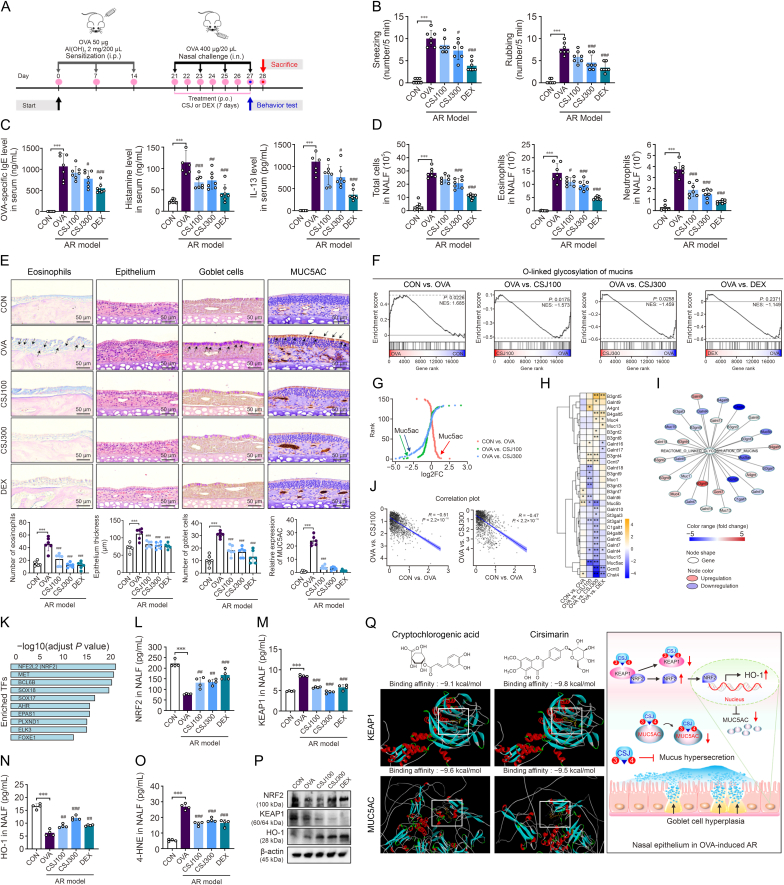
To this end, we modeled AR mice with an OVA challenge according to the experimental timeline shown in Fig. 1A. Animal experiments were performed according to the ARRIVE guidelines and were approved by the Institutional Animal Care and Use Committee of the Chonnam National University (Approval number: CNU IACUC-YB-2022-83). Allergic nasal symptoms such as sneezing and nose rubbing were measured within 5 min after the last OVA exposure to evaluate the anti-allergenic effect of CSJ. CSJ administration ameliorated these representative clinical symptoms and reduced the serum OVA-specific IgE, histamine, and interleukin (IL)-13 levels (Figs. 1B and C). Infiltration of inflammatory cells, such as eosinophils and neutrophils, in the nasal lavage fluid (NALF) was also inhibited by CSJ treatment (Figs. 1D and S1). The OVA-induced AR mouse model showed increased eosinophil infiltration and accumulation in the nasal mucosal epithelium, leading to increased infiltration of various inflammatory cells and thickening of the nasal mucosal epithelial layer. CSJ treatment effectively inhibited eosinophilia and thickening of the nasal mucosal epithelium (Fig. 1E).
Fig. 1.
Therapeutic effects of Cirsium japonicum (CSJ) on the nasal mucosal inflammatory response in the ovalbumin (OVA)-induced allergic rhinitis (AR) mouse model, as determined via systematic transcriptomic and molecular docking analyses. (A) Schematic of the experimental protocol for establishing the OVA-induced AR mouse model and CSJ treatment. (B) Sneezing and rubbing frequencies during 5 min after the last OVA intranasal challenge. ∗∗∗P < 0.001 compared with the CON group; #P < 0.05 and ###P < 0.001 compared with the OVA group. (C) Serum OVA-specific immunoglobulin (Ig)E, histamine, and interleukin (IL)-13 levels evaluated using enzyme-linked immunosorbent assay (ELISA). ∗∗∗P < 0.001 compared with the CON group; #P < 0.05, ##P < 0.01, and ###P < 0.001 compared with the OVA group. (D) Numbers of infiltrated total immune cells, eosinophils, and neutrophils in nasal lavage fluid (NALF) were calculated. Data are expressed as the mean ± standard deviation (SD), n = 7. ∗∗∗P < 0.001 compared with the CON group; #P < 0.05 and ###P < 0.001 compared with the OVA group. (E) Giemsa staining showing eosinophil infiltration; eosinophils stained in bright red are indicated with black arrows. Hematoxylin and eosin staining showing changes in the nasal epithelium thickness. Periodic acid-Schiff staining showing goblet cell hyperplasia; goblet cells stained in purple are indicated with black arrows. Immunohistochemical staining showing the expression of mucin 5 subtype AC (MUC5AC), a major mucus protein secreted by the airway epithelium; MUC5AC stained in brown is indicated with black arrows. ∗∗∗P < 0.001 compared with the CON group; ###P < 0.001 compared with the OVA group. (F) Gene set enrichment analysis plot for the “O-linked glycosylation of mucins” pathway under three conditions. (G) Ranking of genes by expression change (Log2FC) under the three conditions. (H) Heatmap for “O-linked glycosylation of mucins” pathway genes. ∗P < 0.05, ∗∗P < 0.01. (I) Altered gene expression network for “O-linked glycosylation of mucins” pathway genes following CSJ treatment. (J) Correlation plot of expression changes (Log2FC) significantly correlated with MUC5AC expression. (K) Bar plot of enriched transcription factors (TFs) significantly correlated with MUC5AC expression. (L–O) Levels of nuclear factor erythroid 2-related factor 2 (NRF2) (L), Kelch-like ECH-associated protein 1 (KEAP1) (M), heme oxygenase (HO-1) (N), and 4-hydroxynonenal (4-HNE) (O) in NALF measured using ELISA. Data are presented as the mean ± standard deviation (SD), n = 3–4. ∗∗∗P < 0.001 compared with the CON group; ##P < 0.01 and ###P < 0.001 compared with the OVA group. (P) NRF2, KEAP1, and HO-1 protein levels in homogenized nasal tissues evaluated using Western blotting. Protein levels were normalized to the total β-actin level, and relative protein band intensities were calculated using ImageJ software relative to those in the CON group. (Q) The compounds directly bind to KEAP1 with a binding energy of −9.1 kcal/mol (cryptochlorogenic acid) and −9.8 kcal/mol (cirsimarin). The binding site of each major CSJ compound docked to the Kelch domain of KEAP1 is indicated by white squares. The compounds directly bind to MUC5AC with a binding energy of −9.6 kcal/mol (cryptochlorogenic acid) and −9.5 kcal/mol (cirsimarin). Schematic illustration of the dual function of CSJ and two key compounds (cryptochlorogenic acid, CSJ compound no. 3, and cirsimarin, CSJ compound no. 4) through direct binding with the NRF2/KEAP1-dependent signaling pathway and MUC5AC (right-hand side). The binding site of each major CSJ compound docked to the CysD of MUC5AC is indicated by white squares. Experimental groups: CON: control; OVA: OVA-induced AR model; CSJ100: OVA-induced AR model treated with CSJ (100 mg/kg/mouse); CSJ300: OVA-induced AR model treated with CSJ (300 mg/kg/mouse); DEX: OVA-induced AR model treated with dexamethasone (1 mg/kg/mouse).
Mucins are the major constituents of mucus, with mucin 5 subtype AC (MUC5AC; secreted/gel-forming mucin) accounting for the highest amount of mucus secretion in the airway epithelium [2]. MUC5AC expression is higher in goblet cells than that in other epithelial cells [2]. Herein, CSJ inhibited mucus hypersecretion by reducing goblet cell proliferation and MUC5AC expression (Fig. 1E), suggesting its therapeutic potential in downregulating allergic responses at all AR stages.
We then performed transcriptome analysis to investigate CSJ-driven gene expression signatures and identify the major mechanisms of action and regulators of CSJ in the AR model (Figs. S2–S5 and Tables S1 and S2). Among pathways enriched by CSJ, the “O-linked glycosylation of mucins” was significantly downregulated with two doses of CSJ (Fig. 1F and Table S3). MUC5AC was highly ranked by expression change in three conditions (Fig. 1G and Table S4) and identified as a core gene related to reducing nasal inflammation (Figs. 1H and I). Histopathological results also confirmed that CSJ suppressed MUC5AC expression (Fig. 1E). To identify the core transcription factors (TFs) that regulate MUC5AC, we performed gene set enrichment analysis using a list of 1918 MUC5AC-associated genes (Figs. 1J and S6, and Table S5) via co-expression analysis. Nuclear factor erythroid 2-related factor 2 (NRF2) was the most significantly enriched TF in MUC5AC-associated genes (Fig. 1K and Tables S6 and S7). We confirmed expression profiling of the NRF2-associated pathways and NRF2 downstream genes included in MSigDB (Fig. S7). Additionally, previous studies have reported that NRF2 is a core TF that regulates the expression of MUC5AC and genes related to anti-oxidant activity [3]. These results suggest that MUC5AC may be a key protein involved in the anti-inflammatory effect of CSJ and that NRF2 is an important regulator associated with reduction of nasal inflammation by CSJ.
In AR, increased amounts of reactive oxygen species are generated from damaged cells and inflammatory reactions, which limit the anti-oxidant system and continue to promote inflammatory responses because of increased oxidative stress [4]. NRF2, which defends against oxidative stress, dissociates from the cytoplasmic NRF2-Kelch-like ECH-associated protein 1 (KEAP1) complex during AR development and migrates to the nucleus to regulate the expression of anti-oxidant and anti-inflammatory genes [4]. Heme oxygenase (HO)-1, an NRF2-dependent anti-oxidant enzyme, is involved in reducing oxidative damage. TF analysis using MUC5AC-related genes showed that NRF2 was the major TF regulating the expression of genes correlated with MUC5AC, a key gene in regulating nasal inflammation; this was experimentally validated using NALF (Figs. 1L–O) and nasal tissues (Figs. 1P and S8) of the OVA-induced AR model. Compared with the OVA group, the CSJ treatment group showed increased NRF2 levels, significantly decreased KEAP1 levels, and induced HO-1 expression. Moreover, 4-hydroxynonenal (an oxidative stress marker) was strongly expressed in the OVA group, unlike that in the control (CON) group. This oxidative damage was significantly inhibited by CSJ treatment. Thus, CSJ may exert anti-oxidant effects by upregulating the NRF2/HO-1 signaling pathway, which is uncoupled from KEAP1 because of oxidative and inflammatory responses.
We also identified five active ingredients of CSJ via high-performance liquid chromatography analysis, namely neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, cirsimarin, and cirsimaritin (Fig. S9, and Tables S8–S10), which are important flavonoids and polyphenolic compounds with anti-oxidant or free radical-scavenging abilities. The effects of interactions between these main compounds and the NRF2–KEAP1 complex on the anti-oxidant and anti-allergic effects of CSJ were predicted using molecular docking. Cryptochlorogenic acid and cirsimarin were found to bind strongly to KEAP1 (binding energies < −9.0 kcal/mol), demonstrating that CSJ ameliorates AR by regulating NRF2 activity (Figs. 1Q and S10). Furthermore, we used molecular docking to explore the interactions between the major compounds of CSJ and MUC5AC, critical for mucus hypersecretion, as a signature immune response in AR. Similar to KEAP1, cryptochlorogenic acid and cirsimarin were found to dock stably to MUC5AC (binding energy < −8.919 kcal/mol) (Figs. 1Q and S11). Mucin production can be inhibited when these two compounds bind to the cysteine-rich domain of MUC5AC [5], an important region for mucin production that acts as a competitive antagonist. Cryptochlorogenic acid and cirsimarin showed remarkable binding affinities, suggesting the potential dual role of CSJ in modulating AR-related signaling pathways and mucus (Fig. 1Q, right panel).
In conclusion, we established that the alleviation of AR-related oxidative damage and inflammation in the nasal mucosa by CSJ is mediated by increased activation of the NRF2/KEAP1/HO-1 pathway and reduced MUC5AC production. To our knowledge, this is the first study to investigate the hub targets and major bioactive pathways of CSJ via systematic transcriptome analysis and highlight the potential of CSJ for AR treatment. Considering its dual function in regulating the NRF2 pathway and MUC5AC expression, CSJ presents as a promising AR candidate drug.
Data statement
The raw sequence and processed data in this study were deposited in NCBI Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE246623.
CRediT authorship contribution statement
Bo-Jeong Pyun: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Su-Jin Baek: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Kyuhyung Jo: Validation. Ik Soo Lee: Writing – original draft, Validation, Formal analysis. Musun Park: Writing – review & editing, Writing – original draft, Formal analysis. Hye Jin Kim: Validation. Joo Young Lee: Validation. Susanna Choi: Validation. Yun Hee Kim: Supervision, Data curation, Conceptualization. Taesoo Kim: Supervision, Project administration, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Korea Institute of Oriental Medicine, Republic of Korea (Grant No.: KSN2023331). We thank the laboratory members of the Department of Veterinary Pharmacology, College of Veterinary Medicine, Chonnam National University, for their support in animal experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2024.101000.
Contributor Information
Yun Hee Kim, Email: ddyunee@kiom.re.kr.
Taesoo Kim, Email: xotn91@kiom.re.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shao Y., Zhou Y., Hu M., et al. The anti-allergic rhinitis effect of traditional Chinese medicine of Shenqi by regulating mast cell degranulation and Th1/Th2 cytokine balance. Molecules. 2017;22:504. doi: 10.3390/molecules22030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samsuzzaman M., Uddin M.S., Shah M.A., et al. Natural inhibitors on airway mucin: Molecular insight into the therapeutic potential targeting MUC5AC expression and production. Life Sci. 2019;231:116485. doi: 10.1016/j.lfs.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Wang J., Li J., He Y., et al. The SIRT3 activator ganoderic acid D regulates airway mucin MUC5AC expression via the NRF2/GPX4 pathway. Pulm. Pharmacol. Ther. 2023;83:102262. doi: 10.1016/j.pupt.2023.102262. [DOI] [PubMed] [Google Scholar]
- 4.Van Nguyen T., Piao C.H., Fan Y.J., et al. Anti-allergic rhinitis activity of α-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling. Sci. Rep. 2020;10:12528. doi: 10.1038/s41598-020-69234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khmelnitsky L., Milo A., Dym O., et al. Diversity of CysD domains in gel-forming mucins. FEBS J. 2023;290:5196–5203. doi: 10.1111/febs.16918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.