Highlights
-
•
BW sequencing can be used to diagnose nodules suspected of early-stage lung cancer.
-
•
BW sequencing shows high diagnostic accuracy in patients with necrotic lesions.
-
•
BW sequencing could provide guidance in determining next diagnostic steps.
-
•
BW sequencing can provide information about the molecular profile of tumors.
-
•
BW sequencing could reduce invasive diagnostic tests and subsequent complications.
Keywords: Bronchial washing, Sequencing, Early-stage lung cancer, Non-invasive diagnosis, Tumor necrosis
Abstract
Background
Early-stage lung cancers detected by low-dose computed tomography (CT) often require confirmation through invasive procedures due to the absence of endobronchial lesions. This study assesses the diagnostic utility of bronchial washing fluid (BW) sequencing, a less invasive alternative, aiming to identify patient characteristics most suited for this approach.
Methods
From June 2017 to March 2018, we conducted a prospective cohort study by enrolling patients with incidental lung lesions suspected of early-stage lung cancer at two independent hospitals, and 114 were diagnosed with lung cancer while 50 were diagnosed with benign lesions. BW sequencing was performed using a targeted gene panel, and the clinical characteristics of patients detected with cancer through sequencing were identified.
Results
Malignant cells were detected in 33 patients (28.9 %) through BW cytology. By applying specificity-focused mutation criteria, BW sequencing classified 42 patients (36.8 %) as having cancer. Among the cancer patients who were BW sequencing positive and BW cytology negative, 15 patients (75.0 %) showed necrosis on CT. The sensitivity of BW sequencing was particularly enhanced in patients with necrotic tumors, reaching 75 %.
Conclusions
BW sequencing presents a viable, non-invasive diagnostic option for early-stage lung cancer, especially valuable in patients with necrotic lesions. By potentially reducing the reliance on more invasive diagnostic procedures, this method could streamline clinical workflows, decrease patient burden, and improve overall diagnostic efficiency.
Introduction
Screening for lung cancer with low-dose computed tomography (CT) has increased the incidental identification of lung lesions. Cancers identified with low-dose CT are typically early stage without endobronchial lesions and most of them requires a pathological diagnosis using invasive diagnostic tests. Complications from invasive diagnostic tests are a major obstacle in diagnosing cancer [1]. Moreover, because lung cancer commonly occurs in elderly patients with comorbidities, some of these patients cannot tolerate invasive diagnostic tests. Although advanced bronchoscopic techniques such as radial endobronchial ultrasound and electromagnetic navigation have been introduced, the reported diagnostic yields for evaluating lung nodules have been inconsistent and molecular testing is often unavailable due to the limited amount of sample [2,3].
Bronchial washing (BW) is minimally invasive, rarely causes complications, and repeatable. In addition, BW fluid can reflect the characteristics of the lung lesion. However, the clinical use of BW fluid has significant limitations, including a low cytological diagnostic yield and minimal availability of tumor cells for molecular testing [4]. The emergence of liquid biopsy, which is based on circulating tumor DNA (ctDNA), allows noninvasive diagnosis of advanced-stage non-small cell lung cancer with driver mutations in patients [5]. Previous studies have examined patients with advanced-stage disease or having endobronchial lesions to determine whether mutations can be detected in BW fluids [6]. However, the application of BW fluid to diagnose early-stage lung cancer without endobronchial lesions is a clinical unmet need.
In our previous study, we demonstrated that BW supernatants are reflective of tumor-associated mutations and presented its potential for use in early-stage lung cancer diagnosis [7]. In this study, we applied a panel sequencing method without a normal control for lung cancer diagnosis using BW fluid from patients with suspected early-stage lung cancer. And, we elucidate the characteristics of patients for whom this diagnostic method can be effectively applied.
Methods
Patients
We prospectively enrolled 188 patients in a cohort study, focusing on those with an incidental lung lesion on chest CT suspected of early-stage lung cancer and no visible endobronchial lesion on bronchoscopy from June 2017 to March 2018 at two independent hospitals (cohort 1: Inha University Hospital, Incheon, South Korea; cohort 2: Wonju Severance Hospital, Wonju, South Korea) (Supplementary Figure 1). Of the patients, 24 patients were excluded because of no further diagnostic test (n = 6), poor DNA quality (n = 14), and loss of follow-up (n = 4). Finally, 164 patients were enrolled in this study (90 patients in cohort 1 and 74 patients in cohort 2). Information on smoking habit, Charlson comorbidity index score, pulmonary function testing, size of lung lesion, laterality, histology, clinical stage by 8th TNM classification, T, N, and M stage, surgical lung biopsy, and invasive diagnostic test and its complications were scrutinized.
Radiological review
Contrast material-enhanced chest CT scans were performed within one month before bronchoscopy in all patients. All chest CT scans were reviewed by two radiologists blinded to the chest CT reports. Radiological characteristics of the lung lesions including location, size, bronchus sign, and tumor necrosis were evaluated. Tumor necrosis on chest CT was defined as a relatively low-density area within the lung lesions on contrast-enhanced CT images [8,9]. Tumor locations were measured based on the inner-most part of the tumor on chest CT [10,11]. Locations of tumor were categorized into three areas: inner, mid, and outer, based on concentric lines drawn from the hilum and midline, dividing the hemithorax into thirds in both axial and coronal images.
Diagnosis
After no endobronchial lesion was identified with bronchoscopic examination, physician decided on further diagnostic work-up for diagnosis. Invasive diagnostic work-up was classified into surgical lung biopsy and invasive diagnostic tests including percutaneous needle biopsy (PCNB), endobronchial ultrasound (EBUS), and other biopsies. Technique of invasive diagnostic test was dictated by physician discretion. Each invasive diagnostic test was performed by subspecialty-trained thoracic radiology or pulmonology attending physicians. Diagnosis was classified into lung cancer and benign disease. Lung cancer was histologically diagnosed. Benign disease primarily was defined by results from histological examination or microbiological test or otherwise serial follow-up of CT imaging over one year with evaluating response to treatment.
Complications
After invasive diagnostic test, chest radiography and clinical assessment were performed to evaluate procedure-related complication. The complications, such as pneumothorax, pneumothorax requiring a chest tube, pulmonary hemorrhage, pulmonary hemorrhage requiring intensive care unit care, and extrapleural hematoma were evaluated.
Acquisition of specimens
After it was determined that no endobronchial lesion was present, non-guided BW was preferentially performed on each patient by placing 15 mL of sterile saline in the subsegmental bronchus where the lung lesion was suspected to be located. Our prior study demonstrated that BW supernatant was appropriate specimen for mutational analysis compared to the precipitate [7]. The BW supernatants and buffy coat that were separated from BW fluid, and 5 mL of whole blood in heparinized tube after centrifugation at 1000 × g for 20 min at 4 °C were stored at −80 °C. The matched whole blood was sampled from cohort 2 only and the sequence data from blood was used at the filtering step as the panel of normal.
DNA extraction
Leukocyte DNA was prepared using a Puregene blood DNA kit (Gentra Inc. Minneapolis, MN, USA), following the manufacturer's protocol. DNA from BW supernatants was extracted using the QIAamp® Circulating Nucleic Acid kit with the QIAvac 24 Plus instrument (QIAGEN, Hilden, Germany) following the manufacturer's instructions and eluted with 30 μL of Buffer AVE. Extracted DNA samples were quantified with Qubit 2.0 (Invitrogen, Carlsbad, CA, USA), and DNA quality was evaluated with the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). DNA quantity and purity were measured with the Nanodrop 8000 Qubit 2.0, and DNA quality was evaluated by 1.0 % agarose gel electrophoresis.
Targeted panel sequencing
Different panels were applied to each of the two cohorts. The cohort 1 covered 0.7 Mbp and contained 6938 probes for 77 genes. The cohort 2 covered 1.705 Mbp and contained 27,480 probes for 113 genes. Target enrichment bait was designed using Agilent SureDesign software. Target regions in 200 ng or 1 μg of genomic DNA were captured using the Agilent SureSelectXT Custom kit with target enrichment bait following the manufacturer's instructions (Agilent, Santa Clara, CA, USA). Briefly, DNA was sheared using the Covaris system (Covaris, Woburn, MA, USA) and purified with Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA). The ends of the fragments were repaired and adaptors were ligated to the fragments. The resulting DNA library was purified with Agencourt AMPure XP beads and amplified by PCR. The quality and quantity of the DNA library was assessed with the Agilent 2100 Bioanalyzer. The DNA library was captured by hybridization to biotinylated RNA library baits. Bound genomic DNA was purified with streptavidin-coated magnetic Dynabeads (Invitrogen, Carlsbad, CA, USA) and then re-amplified. The targeted DNA library was sequenced on Illumina Hiseq2500 with 100 base-pair paired-end reads using the protocols recommended by the manufacturer (Illumina, San Diego, CA, USA).
Preprocessing and variant analysis
Sequence QC was done through FastqQC 0.11.2 [Andrews, S. (2010). FastQC. A quality control tool for high throughput sequence data. http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/], and it was mapped to human reference genome sequence NCBI b37 using bwa 0.7.12 [12]. BAM files were sorted and deduplicated with Picard Tools v2.2.1 and realigned with the Genome Analysis Toolkit 3.5 (GATK) IndelRealigner, and base quality scores were recalibrated by the GATK base quality recalibration tool [13]. Variants were detected with LoFreq v2.1.3.1 with default parameter setting [14]. Then, the variants’ additional information was annotated using ANNOVAR [15]. We applied several steps to filter putative germline and false variants: (i) variants with population allele frequency >1 % in the normal samples in population databases (1000 genome project [16], ExAC [17], gnomAD [18], and KRGDB [19]); (ii) variants with very high variant allele frequency (VAF) (≥98 %); (iii) variants detected in low depth region (<50X); (iv) nonfunctional variants (synonymous alterations, located in non-coding region, etc.); (v) variants found at least 3 times in the panel of normal of cohort 2; and (vi) other frequently detected variants (20 % or more in the entire cohort) that were likely to be alignment artifacts or were in hard-to-sequence regions.
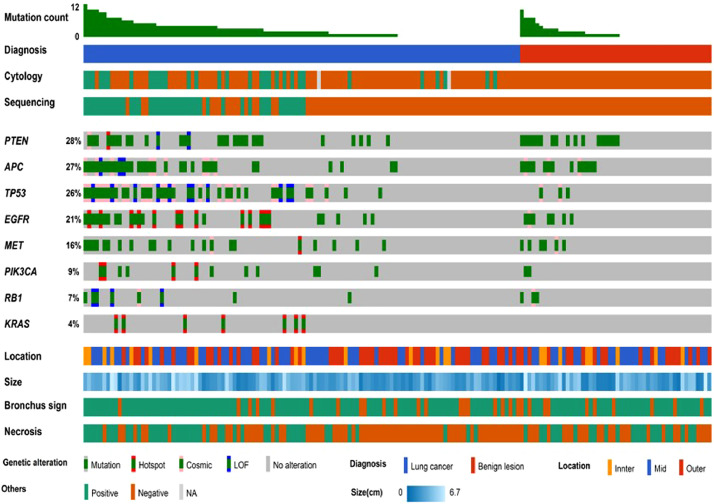
In general, high depth panel sequencing without matched control are difficult to avoid numerous false positives due to technical errors and individual germline polymorphisms [20]. Despite the basic filtering described previously, there were many variants in our cohorts (Supplementary Figure 2A-2B). Among shared genes between the panels for each cohort, we selected 8 genes (APC, EGFR, KRAS, MET, PIK3CA, PTEN, RB1, and TP53) that were frequently mutated known oncogenes/tumor suppressor genes in lung cancer [21]. Although we limited the signal range to 8 genes, there were still many variants in benign samples (Fig. 1). Thus, several additional diagnostic criteria were considered to differentiate cancer (Supplementary Figure 2C-2D). The diagnostic criteria are as follows: Patients are considered to meet criterion ‘a’ if they have at least one variant with VAF of ≥ 1 %. For criterion ‘b’, patients should have at least one COSMIC [22] variant with VAF ≥ 1 %. Criterion ‘c’ is met by patients with at least two COSMIC variants with VAF ≥ 1 %. Criterion ‘d’ is satisfied if patients meet the criteria for c or have at least one definitive somatic mutation (known hotspot mutations [20] or loss of function mutations with VAF ≥ 2 %). Criterion ‘a’ and ‘b’ showed high sensitivity, but high false positives. Criterion ‘c’ achieved 0 % false positive rate, the sensitivity was lower than that of BW cytology [Sensitivity of BW cytology: 23.3 % (14/60) and 31.5 % (17/54) in cohort 1 and 2, respectively]. Since criterion ‘d’ included additional definitive somatic mutations with the criterion ‘c’, the sensitivity was increased without reducing the precision and the sensitivity of sequencing was higher than BW cytology. The difference in performance between the two cohorts was also the least in criterion ‘d’. Thus, we selected the specificity-focused criterion 'd' as our standard.
Fig. 1.
Results of BW sequencing with the panel sequencing by characteristics of lung lesions.
Statistical analysis
All data were expressed as the number (percentage) for categorical variables and as median (interquartile range) for continuous variables. The Pearson chi-square test of Fisher's exact test was used to compare categorical variables, and Mann Whitney U test was used to compare continuous variables. Univariate and multivariate logistic regression were used to evaluate the association between positivity of BW sequencing and variables. Variables that were found to have a p value of 0.1 or less in univariate analysis were included in multivariate logistic regression. All significance testing was done at the two-sided P < 0.05 level. All analyses were performed using the IBM SPSS statistical software package (version 19.0; SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 164 patients were enrolled, including 114 patients with lung cancer (60 patients in cohort 1 and 54 patients in cohort 2) and 50 patients with benign lesions (30 patients in cohort 1 and 20 patients in cohort 2) (Table 1). One of the patients was diagnosed with a benign disease, bronchial anthracofibrosis, but showed a positive result for malignancy in BW sequencing. Seven months later, he turned out to have adenocarcinoma through bronchoscopic biopsy for a newly developed endobronchial lesion. Twenty-seven had squamous cell carcinoma (SQC, 17 patients in cohort 1 and 10 patients in cohort 2), 76 had adenocarcinoma (ADC, 39 patients in cohort 1 and 37 patients in cohort 2), and 11 had small cell carcinoma (4 patients in cohort 1 and 7 patients in cohort 2). Of 114 lung cancer patients, tumor necrosis was observed on chest CT in 44 (38.6 %) patients [23 (38.3 %) patients in cohort 1 and 21 (38.9 %) patients in cohort 2] and malignant cells were identified upon BW cytological examination in 33 (28.9 %) patients [15 (25.0 %) patients in cohort 1 and 18 (33.3 %) patients in cohort 2]. Among the total 164 patients, complications related to invasive diagnostic tests occurred in 33 (20.1 %) [15 (16.7 %) in cohort 1 and 18 (24.3 %) in cohort 2].
Table 1.
Characteristics of patients with lung lesions suspected of having early-stage lung cancer.
| Lung Cancer* | Benign | P | ||
|---|---|---|---|---|
| (n = 114) | (n = 50) | |||
| Age, years | Median (IQR) | 70 (59–77) | 62 (59–76) | 0.357 |
| Sex | Men | 71 (62.3) | 21 (42.0) | 0.016 |
| Women | 43 (37.7) | 29 (58.0) | ||
| Smoking history | Never | 40 (35.1) | 23 (46.0) | 0.186 |
| Ever | 74 (64.9) | 27 (54.0) | ||
| CCI score | 1 | 67 (60.9) | 25 (73.5) | 0.181 |
| ≥ 2 | 43 (39.1) | 9 (26.5) | ||
| COPD | No | 78 (70.9) | 19 (45.2) | 0.003 |
| Yes | 32 (29.1) | 23 (54.8) | ||
| Location | Inner | 15 (13.2) | 8 (16.0) | 0.786 |
| Mid | 52 (45.6) | 24 (48.0) | ||
| Outer | 47 (41.2) | 18 (36.0) | ||
| Diameter, cm | Median (IQR) | 2.8 (2.1–3.9) | 3.0 (1.6–4.0) | 0.797 |
| < 3 | 58 (50.9) | 24 (48.0) | 0.734 | |
| ≥ 3 | 56 (49.1) | 26 (52.0) | ||
| Bronchus sign | Yes | 94 (82.5) | 40 (80.0) | 0.708 |
| No | 20 (17.5) | 10 (20.0) | ||
| Necrosis | Yes | 44 (38.6) | 23 (46.0) | 0.375 |
| No | 70 (61.4) | 27 (54.0) | ||
| Laterality | Right | 65 (57.0) | 35 (70.0) | 0.117 |
| Left | 49 (43.0) | 15 (30.0) | ||
| BW cytology | Positive | 33 (28.9) | 0 (0.0) | <0.001 |
| Negative | 81 (71.1) | 50 (100.0) | ||
| Diagnosis | Clinical | 0 (0.0) | 28 (56.0) | <0.001 |
| Surgical lung biopsy | 16 (14.0) | 0 (0.0) | ||
| PCNB | 85 (74.6) | 21 (42.0) | ||
| EBUS | 8 (7.0) | 0 (0.0) | ||
| TBLB | 3 (2.6) | 1 (2.0) | ||
| Pleural biopsy | 2 (1.8) | 0 (0.0) | ||
| Complication | No | 89 (78.1) | 42 (84.0) | 0.383 |
| Yes | 25 (21.9) | 8 (16.0) | ||
| Pneumothorax | 9 (5.5) | 4 (2.4) | ||
| Pneumothorax requiring chest tube | 2 (1.2) | 1 (0.6) | ||
| Pulmonary hemorrhage | 13 (7.9) | 3 (1.8) | ||
| Pulmonary hemorrhage requiring ICU care | 0 (0.0) | 1 (0.6) | ||
| Pneumothorax + Pulmonary hemorrhage | 3 (1.8) | 0 (0.0) | ||
| Extrapleural hematoma | 0 (0.0) | 1 (0.6) |
IQR = interquartile range; CCI = Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; BW, bronchial washing; PCNB = percutaneous needle biopsy; EBUS = endobronchial ultrasound; TBLB = transbronchial lung biopsy; ICU = intensive care unit.
Of 114 lung cancer patients, 27 had squamous cell carcinoma, 76 had adenocarcinoma, and 11 had small cell carcinoma.
BW sequencing for lung cancer diagnosis
By the previous described panel sequencing, 42 patients [23 patients (25.6 %) in cohort 1 and 19 patients (25.7 %) in cohort 2] were classified as cancer; 122 patients [67 patients (74.4 %) in cohort 1 and 55 patients (74.3 %) in cohort 2] as benign (Fig. 1). The panel showed 100 % of specificity, 100 % of positive predictive value (PPV) for diagnosing cancer in two cohorts whereas it had relatively low sensitivity [38.3 % (23/60) and 35.2 % (19/54) in cohorts 1 and 2, respectively] and low negative predictive value [44.8 % (30/67) and 36.4 % (20/55) in cohorts 1 and 2, respectively]. Among 11 patients with small cell lung cancer, 6 patients showed neuroendocrine features. We observed that mutations in RB1 (50% vs. 0 %, p = 0.064) and TP53 (100% vs. 40 %, p = 0.026) were more common in patients with neuroendocrine features.
Clinical utility of BW sequencing in patients with necrotic tumor
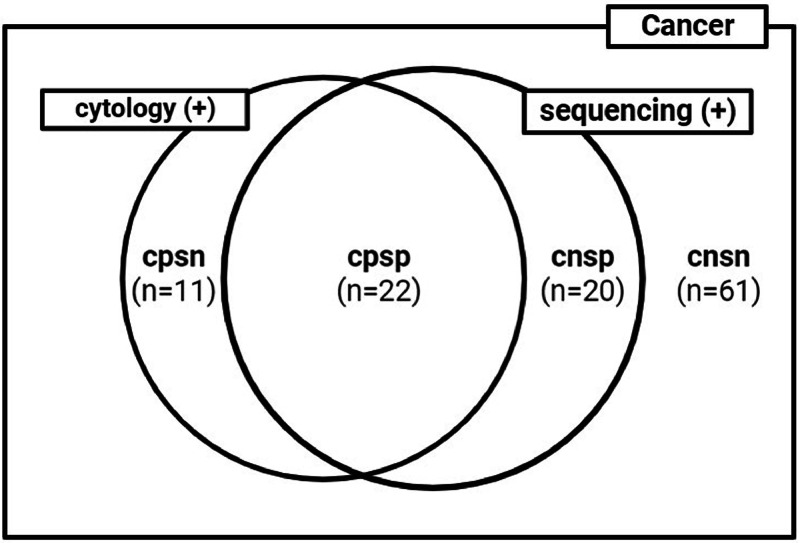
We noticed that 20 of the 42 patients (11 patients in cohort 1 and 9 patients in cohort 2) classified as having cancer by sequencing had negative for cytology (Fig. 2). These patients could be the candidates who have benefit from BW sequencing for diagnosis in clinical practice. Among these 20 patients, complications related to invasive diagnostic tests occurred in 7 (35 %) patients [3 (27.3 %) patients in cohort 1 and 4 (44.4 %) patients in cohort 2].
Fig. 2.
Venn diagram showing the positivity by BW cytology and by BW sequencing among lung cancer patients. cpsp = cytology positive/sequencing positive; cnsp = cytology negative/sequencing positive; cpsn = cytology positive/sequencing negative; cnsn = cytology negative/sequencing negative.
To find a method to identify these patients before bronchoscopy, we aimed to determine the radiologic characteristics of these 20 patients (Table 2). The proportion of BW that was positive for both cytology and sequencing was higher in patients whose primary tumors were inner-located, were larger than 3 cm of diameter, or observed bronchus sign or tumor necrosis on chest CT compared to those did not. Among patients with tumor necrosis observed on chest CT, the proportion of BW positive for sequencing only was significantly higher than that of BW positive for cytology only. In addition, 75 % of BW from patients with tumor necrosis observed on chest CT was positive by sequencing (82.6 % in cohort 1 and 66.7 % in cohort 2). Moreover, in multivariable analysis, tumor necrosis on chest CT was an independent variable associated with positivity BW sequencing (odds ratio: 6.45 and 95 % confidence interval: 2.62–15.85, P < 0.001) (Table 3). We found that the proportion of tumor necrosis observed on chest CT was significantly higher among patients with SQC than those with ADC. We compared the tumor size between BW sequencing positive and negative patients among the 44 patients and found no statistically significant difference (median tumor size 3.7 cm vs. 3.1 cm, p = 0.116).
Table 2.
BW cytology, BW sequencing, and histological classification by the characteristics of lung lesions in patients with early-stage lung cancer.
| Location |
Size |
Bronchus sign |
Necrosis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inner | Mid | Outer | P | ≥3cm | <3cm | P | Yes | No | P | Yes | No | P | |
| Positivity of BW cytology and BW sequencing, number of patients (%) | |||||||||||||
| cpsp (n = 22) | 8 (53.3) | 10 (19.2) | 4 (8.5) | 0.014* | 19 (33.9) | 3 (5.2) | 0.002* | 21 (22.3) | 1 (5.0) | 0.196* | 18 (40.9) | 4 (5.7) | <0.001* |
| cnsp (n = 20) | 2 (13.3) | 12 (23.1) | 6 (12.8) | 0.493* | 13 (23.2) | 7 (12.1) | 0.230* | 19 (20.2) | 1 (5.0) | 0.198* | 15 (34.1) | 5 (7.1) | 0.003* |
| cpsn (n = 11) | 3 (20.0) | 5 (9.6) | 3 (6.4) | 0.391* | 7 (12.5) | 4 (6.9) | 0.530* | 10 (10.6) | 1 (5.0) | 0.689* | 4 (9.1) | 7 (10.0) | >0.999* |
| cnsn (n = 61) | 2 (13.3) | 25 (48.1) | 34 (72.3) | 0.050* | 17 (30.4) | 44 (75.9) | 0.010* | 44 (46.8) | 17 (85.0) | 0.123* | 7 (15.9) | 54 (77.1) | <0.001* |
| Sensitivity of BW cytology and BW sequencing, % | |||||||||||||
| Cytology | 73.3 | 28.8 | 14.9 | <0.001 | 46.4 | 12.1 | <0.001 | 33.0 | 10.0 | 0.056 | 50.0 | 15.7 | <0.001 |
| Seqencing | 66.7 | 42.3 | 21.3 | 0.004 | 57.1 | 17.2 | <0.001 | 42.6 | 10.0 | 0.005 | 75.0 | 12.9 | <0.001 |
| Cytology+Sequencing | 86.7 | 51.9 | 27.7 | <0.001 | 69.6 | 24.1 | <0.001 | 53.2 | 15.0 | 0.002 | 84.1 | 22.9 | <0.001 |
| Histological classification, number of patients (%) | |||||||||||||
| ADC | 11 (73.3) | 36 (69.2) | 29 (61.7) | 0.964# | 32 (57.1) | 44 (75.9) | 0.125# | 59 (62.8) | 17 (85.0) | 0.037# | 24 (31.6) | 52 (68.4) | 0.038# |
| SQC | 4 (26.7) | 12 (23.1) | 11 (23.4) | 16 (28.6) | 11 (19.0) | 26 (27.6) | 1 (5.0) | 15 (55.6) | 12 (44.4) | ||||
| SCLC | 0 (0.0) | 4 (7.7) | 7 (14.9) | 8 (14.3) | 3 (5.2) | 9 (9.6) | 2 (10.0) | 5 (45.5) | 6 (54.5) | ||||
BW = bronchial washing; cpsp = cytology positive / sequencing positive; cnsp = cytology negative / sequencing positive; cpsn = cytology positive / sequencing negative; cnsn = cytology negative / sequencing negative; ADC = adenocarcinoma; SQC = squamous cell carcinoma; SCLC = small cell carcinoma.
The numbers in parentheses represent the percentage of patients relative to the total number of patients in that column.
vs. total.
ADC vs. SQC.
Table 3.
Association of positivity by BW sequencing with characteristics of lung lesions.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | |
| Age, continuous | 1.00 (0.97–1.04) | 0.781 | ||
| Sex | ||||
| Men | 1.21 (0.59–2.46) | 0.604 | ||
| Women | Reference | |||
| Smoking history | ||||
| Ever | 1.02 (0.50–2.10) | 0.961 | ||
| Never | Reference | |||
| Location | ||||
| Inner | 4.23 (1.46–12.27) | 0.008 | 1.62 (0.48–5.42) | 0.435 |
| Mid | 2.24 (0.97–5.17) | 0.059 | 1.56 (0.60–4.11) | 0.365 |
| Outer | Reference | Reference | ||
| Size | ||||
| ≥3cm | 4.61 (2.08–10.22) | <0.001 | 1.99 (0.80–4.98) | 0.140 |
| <3cm | Reference | Reference | ||
| Bronchus sign | ||||
| Yes | 5.96 (1.35–26.21) | 0.018 | 2.10 (0.40–10.86) | 0.378 |
| No | Reference | Reference | ||
| Tumor necrosis on chest CT | ||||
| Yes | 9.49 (4.11–21.91) | <0.001 | 6.45 (2.62–15.85) | <0.001 |
| No | Reference | Reference | ||
BW = bronchial washing; OR = odds ratio; CI = confidence interval.
Of the 44 patients with tumor necrosis observed on chest CT, 20 underwent surgical resection and histologic tumor necrosis was found in the surgical specimens of 18 patients (90 %). Particularly, among the 20 patients underwent surgical resection, patients with BW positive for sequencing only were seven, and histologic tumor necrosis was found in all of their tumors.
Representative cases for applications of BW sequencing in patients with necrotic tumor
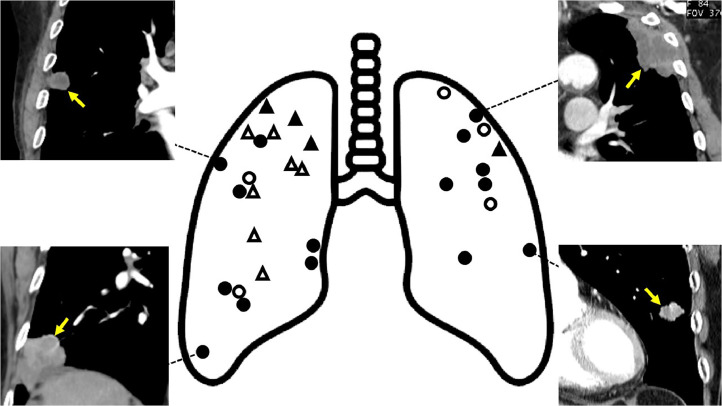
Tumor necrosis was observed on chest CT in 66.7 % of patients whose primary tumors were located in the outer area and who were BW-positive for sequencing only (Fig. 3). Fig. 4 shows representative cases of patients with tumor necrosis on chest CT, a positive for sequencing only, and a complication of an invasive diagnostic tests. If BW sequencing had been conducted prior to the invasive diagnostic test, potential side effects could have been avoided.
Fig. 3.
Location and necrosis of lung lesions in lung cancer patients whose BW were positive for sequencing only and those whose BW were positive for cytology only. Circles represent lesions with positive for BW sequencing only and triangles represent those with positive for BW cytology only. Filled shapes represent lung lesions with necrosis. Chest CT scan images show representative peripheral lung lesions (arrow) with necrosis and positive for BW sequencing only.
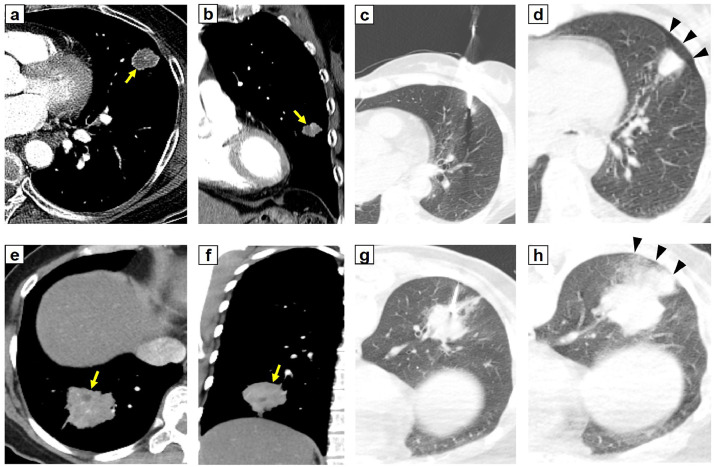
Fig. 4.
Two representative cases of patients with necrosis in a lung lesion, a positive sequencing only, and a complication of an invasive diagnostic test. (A-D) In a 78 years old woman, pneumothorax (arrowhead) occurred after percutaneous needle biopsy (PCNB) of a lung lesion (arrow) in outer location on lingula segment of the left upper lobe with necrosis. The patient was diagnosed with stage I lung adenocarcinoma and epidermal growth factor receptor (EGFR) exon 19 deletion was detected by EGFR PCR method. Result of BW sequencing also showed an EGFR exon 19 deletion mutation, E746_A750del. The patient underwent radiosurgery rather than surgical resection considering her old age and low performance status. (A) axial view (B) coronal view (C) PCNB (D) pneumothorax occurred after PCNB
(E-H) In a 51 years old woman, pulmonary hemorrhage (arrowhead) with hemoptysis developed after PCNB of a lung lesion (arrow) in mid location on right lower lobe with necrosis. The patient was diagnosed with stage II lung adenocarcinoma and EGFR exon 20 insertion was detected by EGFR PCR method. Result of BW sequencing also showed an EGFR exon 20 insertion mutation, D770_N771insSVD. The patient underwent lobectomy. After 1 year, recurrence of lung cancer was observed and the patient received amivantamab, a target agent for EGFR exon 20 insertion mutation, and the partial response of lung lesions was observed thereafter. (E) axial view (F) coronal view (G) PCNB (H) pulmonary hemorrhage developed after PCNB.
Additionally, 3 cancer patients with wild-type EGFR confirmed by PCR in the primary tumor showed activating EGFR mutations in the BW sequencing results. All of these patients were female, never smokers, and had ADC and necrotic tumors. One of these patients experienced a recurrence one year after surgery. Despite undergoing chemotherapy, the disease progressed. However, subsequent treatment with an EGFR tyrosine kinase inhibitor resulted in a partial response by RECIST criteria.
Discussion
In this study, we assessed the diagnostic utility of a panel sequencing without normal control in BW fluid for patients with lung nodules suspected of early-stage lung cancer on CT scans. Our findings suggest that its application could potentially mitigate the need for more invasive tests and their associated complications. However, the panel's low sensitivity remains a limitation, which could be significantly improved, as we found in cases showing radiologic tumor necrosis on CT scans. Thus, our research suggests that the panel sequencing could be particularly valuable for distinguishing early-stage lung cancer in patients with radiologic tumor necrosis on CT, providing a crucial tool for accurate and less invasive diagnostics.
A previous study has suggested that bronchoalveolar lavage (BAL) fluid offers superior genomic profiling capabilities compared to plasma, particularly effective in detecting tumor-derived mutations in NSCLC patients [23]. This indicates that specimens directly obtained from the bronchus through bronchoscopy may more specifically reflect tumor characteristics than plasma. In this study, we further validate the utility of BW fluid, showing that our panel sequencing, when applied to BW samples, delivers high diagnostic specificity and positive predictive value, thereby sparing patients from unnecessary and potentially harmful follow-up procedures. While the sensitivity of BW sequencing was observed to be slightly lower than that of study utilizing BAL fluid, this difference likely results from the smaller sampling volumes and less invasive nature of the BW method. Nonetheless, BW fluid presents distinct advantages: it is less invasive, requires a smaller amount of physiological saline, and can be performed regardless of the lesion's location, making it accessible to a broader range of patients, including those for whom BAL may be contraindicated or overly risky. Therefore, BW fluid remains a valuable diagnostic tool in clinical settings where patient safety and comfort are of the utmost concern. In addition, this study overcomes the limitation of lower sensitivity by demonstrating that BW fluid exhibits enhanced sensitivity in patients with radiological evidence of tumor necrosis.
The 20 patients who were only positive for BW sequencing are those who would benefit clinically from the diagnostic method using the panel. A significant proportion of these patients exhibited tumors with radiological necrosis observed on CT scans, suggesting that radiological tumor necrosis could serve as an indicator for identifying candidates for the panel diagnostic approach. This trend was similarly observed in tumors located in the outer areas. Tumors in the outer regions are particularly difficult to access via bronchoscopy in most cases, often necessitating invasive diagnostic procedures such as PCNB or surgical biopsy. The diagnostic method proposed in this study could prevent complications associated with invasive diagnostic tests in these patients.
A recent study showed that driver mutations, such as EGFR or KRAS mutations, can be detected in bronchial brushing samples from non-cancerous sites [24]. Therefore, if malignancy is diagnosed solely based on the presence of a specific genetic mutation, non-cancerous lesions could be mistaken for cancer. In this study, with the diagnostic panel and strict criteria we applied, the specificity of BW sequencing was 100 %. This means that BW sequencing has never misdiagnosed a non-cancer case as cancer. Additionally, if BW sequencing is positive, the lesion can be considered malignant regardless of the results of BW cytology.
Furthermore, the use of the panel sequencing in BW fluid enables molecular testing for biomarkers, which is crucial in the current clinical landscape where a variety of targeted therapies are available. Notably, our results highlighted instances where patients, initially tested negative for EGFR mutations in tissue samples, were found to be positive in BW sequencing. This discrepancy can be attributed to tumor heterogeneity, suggesting that different parts of a tumor or different tumors within the same individual may exhibit unique genetic profiles. Consequently, BW sequencing can detect these variations, providing a more comprehensive genetic landscape of the lung cancer. Therefore, the diagnostic method we have developed does not only confirm the presence of cancer but also facilitates the detection of actionable biomarkers. This capability is especially significant, as identifying specific mutations can guide the selection of appropriate targeted therapies, enhancing personalized treatment strategies and potentially improving patient outcomes.
A previous study suggested a significant correlation between pathologic tumor necrosis and increased detection of ctDNA in plasma [25]. In alignment with this finding, our study observed that patients with radiologic tumor necrosis exhibited higher sensitivity in BW sequencing. This enhancement in sensitivity can be attributed to more effective ctDNA shedding from necrotic tumors, indicating that necrosis facilitates the release of ctDNA into the BW fluid [26]. Additionally, a previous study indicated a higher prevalence of necrosis in squamous cell carcinoma, and our research similarly demonstrates this result, reinforcing the association between this type of cancer and tumor necrosis. However, unlike the previous study which focused primarily on blood samples, our study extends these findings by demonstrating a similar correlation between necrosis and increased mutation detectability in BW fluid, a specimen more specific to the site. These parallels not only validate our findings but also highlight the importance of considering tumor necrosis as a predictive marker for ctDNA shedding in BW fluid. Our results also demonstrated a strong correlation between pathologic tumor necrosis observed in surgical specimens and radiologic tumor necrosis identified on CT scans. This correlation not only reinforces the reliability of our findings but also suggests that by preliminarily identifying patients most likely to benefit from BW sequencing based on CT findings, the need for invasive procedures can be significantly reduced.
A case presented in the Results section highlights the advantage of BW sequencing in detecting lung cancer at an early stage. This patient initially had bronchial narrowing due to anthracofibrosis, and no definite lesion suggestive of lung cancer was observed on CT, which made biopsy impossible. Nevertheless, sequencing of the BW fluid proved to be sensitive enough to detect genetic mutations in the lesion. Specifically, it demonstrates that detection with BW sequencing is possible more sensitively and earlier than with imaging findings.
This study demonstrates that BW sequencing can be applied for the diagnosis of patients with necrotic lung lesions in chest CT images suspected to be early-stage lung cancer. In these patients, BW sequencing could provide guidance in determining next steps such as CT follow-up, biopsy, or surgical resection. BW sequencing can also provide information about the molecular profile of tumors. Therefore, BW sequencing would be possible to reduce the frequency of unnecessary invasive diagnostic tests and subsequent complications.
Funding
This work was supported by grant (NRF-2021R1A5A2031612) from the National Research Foundation of Korea (NRF). This research was supported by the Korean Association for Lung Cancer (KALC-20210625).
Patient consent for publication
Informed consent was obtained from patients.
Ethics approval
The study protocol was approved by the Institutional Review Boards of Inha University Hospital (2016–11–013) and Wonju Severance Hospital (CR323090).
CRediT authorship contribution statement
Jun Hyeok Lim: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Hyun-Tae Shin: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Sunmin Park: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Woo Kyung Ryu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Lucia Kim: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Kyung-Hee Lee: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Sung Min Ko: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Seung Jae Lee: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Jung Soo Kim: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Jeong-Seon Ryu: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102134.
Appendix. Supplementary materials
Supplementary Figure 1. Patient flow diagram
Supplementary Figure 2. Landscape of mutations in total gene set, and filtering criteria to differentiate cancer. (A) Landscape of mutations in the total gene set of cohort 1. (B) Landscape of mutations in the total gene set of cohort 2. (C) Precision (alias positive predictive value) and sensitivity (alias recall) depending on criteria. (D) Receiver operating characteristic curve. COSMIC, Catalogue of Somatic Mutations in Cancer, definitive somatic mutation: known hotspot mutations or loss of function mutations with VAF ≥ 2 %.
References
- 1.Wiener R.S., Schwartz L.M., Woloshin S., Welch H.G. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann. Intern. Med. 2011;155:137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folch E.E., Bowling M.R., Pritchett M.A., Murgu S.D., Nead M.A., Flandes J., et al. NAVIGATE 24-month results: electromagnetic navigation bronchoscopy for pulmonary lesions at 37 centers in Europe and the United States. J. Thoracic Oncol. 2022;17:519–531. doi: 10.1016/j.jtho.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Robin M., Mhanna L., Chaltiel L., Plat G., Héluain V., Basset C., et al. Feasibility of comprehensive genotyping specimens from radial endobronchial ultrasonography and electromagnetic navigation bronchoscopy. ERJ. Open. Res. 2021;7 doi: 10.1183/23120541.00942-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon D.A., Solliday N.H., Gracey D.R. Cytology in fiberoptic bronchoscopy. Comparison of bronchial brushing, washing and post-bronchoscopy sputum. Chest. 1974;65:616–619. doi: 10.1378/chest.65.6.616. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J.D., Li L., Wang Y., Thoburn C., Afsari B., Danilova L., et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science (1979) 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roncarati R., Lupini L., Miotto E., Saccenti E., Mascetti S., Morandi L., et al. Molecular testing on bronchial washings for the diagnosis and predictive assessment of lung cancer. Mol. Oncol. 2020 doi: 10.1002/1878-0261.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu J.S., Lim J.H., Lee M.K., Lee S.J., Kim H.J., Kim M.J., et al. Feasibility of Bronchial Washing Fluid-Based Approach to Early-Stage Lung Cancer Diagnosis. Oncologist. 2019;24:e603–e6e6. doi: 10.1634/theoncologist.2019-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swensen S.J., Viggiano R.W., Midthun D.E., Müller N.L., Sherrick A., Yamashita K., et al. Lung nodule enhancement at CT: multicenter study. 2000;214:73–80. [DOI] [PubMed]
- 9.Hollings N., Shaw PJERJ. Diagnostic imaging of lung cancer. 2002;19:722–42. [DOI] [PubMed]
- 10.Shin S.H., Jeong D.Y., Lee K.S., Cho J.H., Choi Y.S., Lee K., et al. Which definition of a central tumour is more predictive of occult mediastinal metastasis in nonsmall cell lung cancer patients with radiological N0 disease? Eur. Respiratory J. 2019;53 doi: 10.1183/13993003.01508-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casal R.F., Vial M.R., Miller R., Mudambi L., Grosu H.B., Eapen G.A., et al. What exactly is a centrally located lung tumor? Results of an online survey. Ann. Am. Thorac. Soc. 2017;14:118–123. doi: 10.1513/AnnalsATS.201607-568BC. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilm A., Aw P.P., Bertrand D., Yeo G.H., Ong S.H., Wong C.H., et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic. Acids. Res. 2012;40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic. Acids. Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genomes Project C., Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karczewski K.J., Weisburd B., Thomas B., Solomonson M., Ruderfer D.M., Kavanagh D., et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic. Acids. Res. 2017;45:D840–D8D5. doi: 10.1093/nar/gkw971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung K.S., Hong K.W., Jo H.Y., Choi J., Ban H.J., Cho S.B., et al. KRGDB: the large-scale variant database of 1722 Koreans based on whole genome sequencing. Database (Oxford) 2020;2020 doi: 10.1093/database/baaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin H.-T., Choi Y.-L., Yun J.W., Kim N.K., Kim S.-Y., Jeon H.J., et al. Prevalence and detection of low-allele-fraction variants in clinical cancer samples. Nat. Commun. 2017;8:1377. doi: 10.1038/s41467-017-01470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B., Veeriah S., et al. Tracking the evolution of non–small-cell lung cancer. New England Journal of Medicine. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 22.Tate J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D9D7. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair V.S., Hui A.B.-Y., Chabon J.J., Esfahani M.S., Stehr H., Nabet B.Y., et al. Genomic profiling of bronchoalveolar lavage fluid in lung cancer. Cancer Res. 2022;82:2838–2847. doi: 10.1158/0008-5472.CAN-22-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill W., Lim E.L., Weeden C.E., Lee C., Augustine M., Chen K., et al. Lung adenocarcinoma promotion by air pollutants. Nature. 2023;616:159–167. doi: 10.1038/s41586-023-05874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbosh C., Birkbak N.J., Wilson G.A., Jamal-Hanjani M., Constantin T., Salari R., et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz L.A., Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J. Clinical Oncol. 2014;32:579. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Patient flow diagram
Supplementary Figure 2. Landscape of mutations in total gene set, and filtering criteria to differentiate cancer. (A) Landscape of mutations in the total gene set of cohort 1. (B) Landscape of mutations in the total gene set of cohort 2. (C) Precision (alias positive predictive value) and sensitivity (alias recall) depending on criteria. (D) Receiver operating characteristic curve. COSMIC, Catalogue of Somatic Mutations in Cancer, definitive somatic mutation: known hotspot mutations or loss of function mutations with VAF ≥ 2 %.