Abstract
This study investigated the effects of different storage times (0–24 months) and temperatures (−20 °C, 4 °C, 25 °C, and 35 °C) on the volatile compounds and color of vanilla beans from Taiwan using ISO 5565-1:1999 as the commercial quality indicators. The vanillin level was highest at −20 °C for 6 months (p < 0.05) and no color-perceived differences (ΔE < 1). At 35 °C, lipid metabolism, enzymatic oxidation, and Maillard reaction products, such as 2-acetylpyrrole and 5-hydroxymethylfurfural, were detected after 18 months of storage, leading to significant color-perceived differences (ΔE > 5) and changes in the odor profile, which did not meet ISO standards. To sum up, as the storage temperature and duration increased, oxidative metabolites increased. Furthermore, storage at −20 °C or 4 °C is stable, but considering vanillin quality, the storage temperature of −20 °C can extend the storage period by 12 months.
Keywords: vanilla planifolia, Storage, GC–MS, HS-SPME, Volatile compounds
Highlights
-
•
Vanilla beans stored at −20 °C for 6 months showed the highest vanillin levels.
-
•
Lowest phenolic content was stored at −20 °C.
-
•
Storage at 35 °C enhanced lipid metabolism and Millard reaction.
-
•
Nonanal and 3,5-octadien-2-one levels can indicate oxidation.
-
•
Samples stored at −20 °C and 4 °C meet ISO 335565–1:1999 color standards.
1. Introduction
Vanilla beans (Vanilla planifolia Andrews) are naturally occurring aroma compounds derived from the processed fruit pods of Vanilla spp. (Ramachandra Rao & Ravishankar, 2000). Renowned for their highly desirable vanilla aroma, vanilla beans are extensively utilized as flavoring agents in beverages, food products, medicines, and cosmetics, making them immensely popular among consumers (Verma et al., 2009). The aromatic profile of vanilla beans is a complex blend of volatile components (Zhang & Mueller, 2012). The primary component of vanilla pods is vanillin, while phenolic compounds are significant volatile elements that influence the aroma of V. planifolia (Dignum et al., 2004). Phenolic compounds in vanilla beans are derived from the metabolism of the phenylalanine pathway (Peña-Barrientos et al., 2022), and they possess strong phenolic, woody, and smoky aromas, which are considered characteristic aromas of V. planifolia (Brunschwig et al., 2012). The composition of these aroma constituents is influenced by various factors, including environmental conditions, pod maturity, botanical variety, processing techniques, and storage conditions (Baqueiro-Pena & Guerrero-Beltran, 2017). Previous studies conducted by Yeh et al. (2021) and Yeh et al. (2022) have extensively researched and discussed the processing conditions, origin, and extraction methods of vanilla beans in Taiwan. However, there is currently no literature available that addresses the impact of subsequent storage conditions on the aroma quality of vanilla beans.
Storage is a crucial stage following the processing of vanilla beans, as it aims to maintain the aroma quality obtained through cultivation and processing. The aroma quality during storage is influenced by factors such as temperature, humidity, gas composition, microorganisms, and packaging type (Havkin-Frenkel & Frenkel, 2006; Perez-Cacho & Rouseff, 2008). Through research on natural spices such as star anise, cardamom, and cinnamon, Biao et al. (2019) discovered that storage temperature has a significant impact on the aroma quality. Temperature can induce enzymatic reactions or chemical changes, leading to the formation or loss of specific volatile compounds (Dixon & Hewett, 2000; Karolkowski et al., 2021; Perez-Cacho & Rouseff, 2008). However, Havkin-Frenkel and Frenkel (2006) reported that vanilla beans, after processing, are considered nonliving material and do not undergo enzymatic reactions during storage. Nevertheless, studies by Dignum et al. (2002) and Márquez and Waliszewski (2008) indicated that some enzymes in vanilla beans may remain active even after heat treatment. Previous research has focused mainly on the processing of vanilla beans (Gu et al., 2017); thus, further investigation and clarification of the chemical and physiological changes that occur during storage have practical relevance (Pazmiño-Arteaga et al., 2022).
In this study, Taiwanese vanilla beans were subjected to long-term storage (0–24 months) at temperatures of −20 °C, 4 °C, 25 °C, and 35 °C. The volatile compounds in the vanilla beans were analyzed using HS-SPME combined with GC and GC–MS techniques, and the changes in color were measured using a colorimeter. The objective of this study is to provide an important reference for understanding the quality variation of vanilla beans during the time and the temperature of storage.
2. Materials and methods
2.1. Samples and chemicals
The vanilla variety utilized in this study is V. planifolia, and both the cultivation and processing of vanilla pods were conducted by the Taoyuan District Agricultural Research and Extension Station, a division of the Agricultural Committee (Taiwan). The cultivation of vanilla beans for this study took place in Taoyuan City, Taiwan, and the processing procedure is outlined in Table 1. After completing the curing process, the vanilla pods were hermetically sealed in PET/AL/LLDPE aluminum bags and subjected to long-term storage at temperatures of −20 °C, 4 °C, 25 °C, and 35 °C. The vanilla pods used in the experiments were processed and stored on January 25, 2021, and their aroma analysis during long-term storage at different temperatures was conducted every 6 months, with storage durations of 0 months, 6 months, 12 months, 18 months, and 24 months.
Table 1.
Curing conditions of vanilla beans.
| procedures | method |
|---|---|
| killing | Soaking the freshly harvested pods in hot water at 60 °C for 3 min. |
| sweating | The vanilla beans are placed in a high-humidity environment at 40–50 °C for 2 days, followed by 10 days of storage in a high-humidity environment at 35–40 °C. |
| drying | The vanilla beans which have completed the sweating process, are placed in a controlled environment at 25 °C. They are dried using a dehumidifier until their moisture content reaches 35–38%. |
| condition | After drying, the vanilla beans are transferred to wooden crates for a maturation period of 3 months. |
Standard samples of volatile compounds in the C5–C25 alkane range were obtained from Sigma–Aldrich (St. Louis, MO, USA) for identification.
2.2. Color analysis
The Hunter L, a, b coordinates (L, a, b values) of the color of the vanilla beans were measured using the HunterLab color difference meter (model MSEZ-4500 L) from HunterLab in Virginia, USA. The total color difference (ΔE) was calculated. The L value represents brightness, where higher values (closer to 100) indicate brighter colors, while lower values (closer to 0) indicate darker colors. The a value represents the red–green axis, with positive values indicating a reddish color and negative values indicating a greenish color. The b value represents the yellow–blue axis, with positive values indicating a yellowish color and negative values indicating a bluish color. Additionally, (Δ E)=. Before conducting the color analysis, color calibration was performed using the dedicated black and white reference standards provided by the HunterLab color difference meter, and the average color value was obtained from three repeated measurements.
2.3. Sample preparation and HS–SPME extraction
This extraction test refers to the method of Yeh et al. (2021) with slight modifications. Approximately 30 g of vanilla beans under different storage conditions was cut in half to obtain the seeds, and 1 g of the seeds was randomly placed in a 4 mL transparent cylindrical glass sample bottle and then covered with a Teflon silicone thermal insulation gasket, and sealed. The sample bottle containing vanilla seeds was placed in a 50 °C water bath, and the top air was extracted with 65 μm PDMS/DVB (Supelco, Bellefonte, PA, USA) absorbent fibers for 40 min. Then, the adsorption fiber was placed in a GC inlet at a temperature of 250 °C to desorb for 5 mm for subsequent identification and analysis of volatile compounds.
2.4. Extraction of volatile compounds
2.4.1. Gas chromatography with flame ionization detection (GC-FID)
The instrument setting conditions refer to the method of Yeh et al. (2021). The instrument used was a 7890 A GC (Agilent, Palo Alto, CA, USA) with a DB-1 nonpolar capillary column (60 m × 0.25 mm, film thickness of 0.25 μm, Agilent, Agilent Technologies) and flame ionization detector (flame ionization detector, FID), and the detector temperature was 300 °C. The temperature of the inlet of the instrument is 250 °C, and the injection mode adopts the splitless mode. The heating program of GC was as follows: the initial temperature was 40 °C and maintained for 1 min, then the temperature was raised to 150 °C at 5 °C/min and maintained for 1 min, and then the temperature was raised to 200 °C at 10 °C/min and maintained for 21 min. The carrier gas was nitrogen with a flow rate of 1 mL/min.
2.4.2. Gas chromatography–mass spectrometry (GC–MS)
An Agilent 7890 B GC coupled with an Agilent 5977 A quadrupole mass spectrometer was used to analyze and identify the volatile compounds in vanilla pods. The conditions set by the GC are the same as those of the GC-FID, only the carrier gas is replaced by helium, and the flow rate is 1 mL/min. The mass spectrum data measured by the instrument will be compared and judged with the Wiley 7 N database. The gas chromatograph retention index (GC-index) of volatile components is a mixture of C5-C25 normal alkanes (Sigma–Aldrich Chem. Co., St. Louis, MO, USA) under the same operating conditions Next, the GC residence time was taken as a reference and calculated according to the method of Kovát (1985). The results are presented as peak areas. The total amount of volatile compounds was estimated by summing all the areas in the chromatogram (Sánchez-García et al., 2024).
2.5. Statistical analysis
All experiments were analyzed in triplicate, and the analysis results are expressed as the mean ± standard deviation. Followed the method from Chiang and Chiang (2024), the software XLSTAT 2019 (Addinsoft, New York, NY, USA) was used for principal component (PCA) and heatmap analysis. PCA is a multivariate statistical analysis technique used for dimensionality reduction and visualization of analytical data. By analyzing the contribution rates of principal component factors, PCA can highlight differences in the distribution of volatile components between samples, thereby facilitating the evaluation of sample regularities and dissimilarities (Guo et al., 2022). The analysis results were analyzed using IBM SPSS Statistics 23 (IBM, Armonk, NY, USA) for one-way analysis of variance (ANOVA), and the post hoc test was performed to compare the significant differences with Tukey's range test (p < 0.05).
3. Results and discussion
3.1. Changes in volatile compounds during long-term storage at different temperatures
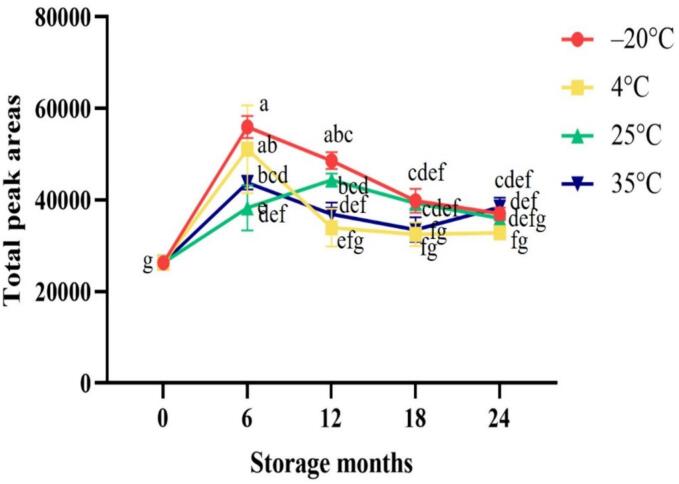
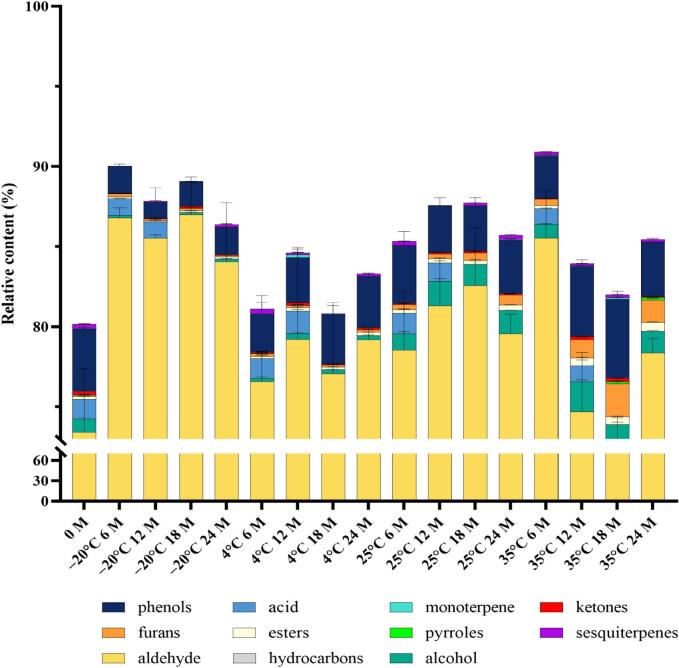
In this study, Taiwanese vanilla beans were subjected to storage at temperatures of −20 °C, 4 °C, 25 °C, and 35 °C to observe the effects of storage time and temperature on their quality. The changes in total aroma content during the storage of Taiwanese vanilla beans at different temperatures are shown in Fig. 1. The changes in identified volatile compounds and their concentrations during the storage period were examined and are presented in Table 2 and Table 3. All the samples showed significant decreases after 18 and 12 months of storage even the samples stored at −20 °C and 4 °C. The analysis of vanilla beans under different storage conditions was conducted using HS-SPME combined with GC–MS, leading to the identification of a total of 50 volatile compounds. These compounds encompassed various chemical groups, among them were 11 aldehydes, 7 alcohols, 6 esters, 1 acid, 5 ketones, 4 furans, 1 hydrocarbon, 3 phenols, 1 pyrrole, 1 monoterpene, and 10 sesquiterpenes. Notably, aldehydes exhibited the highest quantity and content among the identified chemical groups, accounting for approximately 70–85% (Fig. 2). Consequently, aldehydes emerged as the predominant chemical constituents of vanilla beans from Taiwan.
Fig. 1.
Changes in the total peak areas of vanilla beans at different storage temperatures. Values are means ± SD of triplicates. Values having different superscripts are significantly (p < 0.05) different.
Table 2.
Changes in volatile components of vanilla beans from Taiwan stored at −20 °C and 4 °C.
| Compounds | RI b | RI c | peak areasa, b, e, f |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| -20°C d |
4°C |
||||||||||
| 0 M d | 6 M | 12 M | 18 M | 24 M | 6 M | 12 M | 18 M | 24 M | |||
| alcohols | |||||||||||
| 1-hexanolg | 852 | 859 | - f | - | 24.99 ± 1.18 | 23.15 ± 1.61 | - | - | - | 32.44 ± 3.64 | - |
| benzyl alcoholg | 1008 | 996 | 41.63 ± 9.65 | 40.91 ± 0.23 | 119.98 ± 4.81 | 100.83 ± 6.87 | 105.57 ± 14.68 | 48.36 ± 21.51 | 182.37 ± 18.28 | 154.12 ± 17.69 | 155.89 ± 9.24 |
| 2-ethylhexanoli | 1012 | 1007 | 37.49 ± 2.05 | - | 45.96 ± 6.52 | ||||||
| 1-octanolg | 1056 | 1044 | - | 32.81 ± 1.71 | 27.77 ± 2.79 | 26.88 ± 3.34 | 27.27 ± 3.40 | - | 33.85 ± 4.66 | - | - |
| phenethyl alcoholg | 1080 | 1077 | 100.58 ± 10.64 | 124.26 ± 3.94 | 114.84 ± 6.39 | 93.40 ± 2.64 | 101.31 ± 7.52 | 141.31 ± 21.06 | 148.40 ± 4.28 | 131.80 ± 7.29 | 142.44 ± 3.94 |
| anisyl alcoholg | 1253 | 1243 | 81.56 ± 4.65 | 131.99 ± 13.74 | 103.36 ± 3.22 | 91.78 ± 9.92 | 81.11 ± 1.59 | 142.40 ± 27.03 | 108.29 ± 14.60 | 70.74 ± 47.65 | 117.16 ± 1.51 |
| cinnamyl alcoholg | 1278 | 1271 | - | - | - | 10.99 ± 7.27 | 10.42 ± 4.19 | - | - | 13.62 ± 6.37 | 14.44 ± 1.3 |
| aldehydes | |||||||||||
| benzaldehydeg | 932 | 931 | 39.06 ± 10.63 | 39.66 ± 1.04 | 37.04 ± 2.36 | 33.06 ± 1.50 | 36.90 ± 0.98 | 45.75 ± 22.59 | 46.68 ± 14.15 | 199.48 ± 35.84 | 36.06 ± 5.45 |
| phenylacetaldehydeg | 1012 | 1004 | - | - | - | 39.39 ± 4.15 | 42.74 ± 5.43 | - | - | - | - |
| nonanalg | 1084 | 1074 | 51.07 ± 7.26 | 52.76 ± 15.95 | 36.21 ± 6.57 | 59.12 ± 3.51 | 28.52 ± 0.73 | 91.40 ± 39.04 | 84.72 ± 8.96 | 144.15 ± 49.55 | 23.43 ± 1.63 |
| safranalg | 1183 | 1172 | 19.31 ± 4.15 | 21.37 ± 1.51 | 20.46 ± 1.24 | 18.79 ± 2.51 | 20.52 ± 2.16 | 24.43 ± 5.17 | 24.53 ± 2.58 | 21.73 ± 1.33 | 24.73 ± 3.22 |
| decanali | 1186 | 1180 | 9.15 ± 1.67 | - | - | - | - | - | - | - | - |
| β-cyclocitralh | 1194 | 1205 | 16.36 ± 2.65 | - | 19.51 ± 1.72 | 16.26 ± 0.53 | 15.47 ± 4.72 | - | 22.07 ± 2.54 | 13.88 ± 0.86 | 13.82 ± 2.54 |
| anisaldehydeg | 1221 | 1210 | 32.6 ± 3.43 | 25.41 ± 1.82 | 23.83 ± 1.44 | 20.95 ± 0.30 | 24.28 ± 2.71 | 32.64 ± 0.89 | 26.69 ± 1.87 | 23.18 ± 0.70 | 26.68 ± 4.21 |
| cinnamaldehydeg | 1239 | 1231 | 11.11 ± 2.78 | - | - | - | - | - | - | - | - |
| p-hydroxybenzaldehydeg | 1315 | 1318 | 175.71 ± 8.28 | 581.67 ± 130.40 | 283.97 ± 19.49 | 359.11 ± 34.78 | 283.24 ± 23.62 | 436.01 ± 138.94 | 273.38 ± 35.37 | 323.72 ± 27.71 | 367.45 ± 29.82 |
| vanilling | 1368 | 1361 | 18927.06 ± 1503.70 | 47807.07 ± 2175.99 | 41137.19 ± 1583.66 | 34112.35 ± 2770.10 | 30677.20 ± 2434.51 | 34711.22 ± 5454.09 | 26591.77 ± 5240.30 | 24368.45 ± 3156.22 | 25490.96 ± 1139.68 |
| methylvanillini | 1427 | 1426 | 14.46 ± 2.33 | 43.72 ± 4.51 | - | - | - | 57.09 ± 2.54 | - | - | - |
| acids | |||||||||||
| 2-ethylhexanoic acidg | 1096 | 1094 | 319.05 ± 44.5 | 590.70 ± 21.93 | 494.74 ± 32.03 | - | - | 597.55 ± 39.97 | 473.79 ± 21.52 | - | - |
| esters | |||||||||||
| benzyl acetateg | 1135 | 1125 | 7.72 ± 0.63 | 9.05 ± 0.74 | 6.44 ± 1.83 | - | 11.47 ± 1.23 | 10.63 ± 1.81 | 11.22 ± 2.76 | - | 8.85 ± 2.62 |
| methyl salicylateg | 1176 | 1166 | 40.08 ± 7.84 | 44.11 ± 1.83 | 44.1 ± 2.63 | 40.89 ± 4.58 | 39.75 ± 1.31 | 54.80 ± 14.70 | 60.05 ± 5.76 | 50.64 ± 4.16 | 50.90 ± 4.07 |
| furans | |||||||||||
| furfuralh | 799 | 796 | 23.8 ± 12.18 | 39.92 ± 4.07 | 40.43 ± 2.10 | 29.96 ± 0.35 | 33.58 ± 1.64 | 48.57 ± 15.63 | 51.91 ± 10.86 | 46.04 ± 9.66 | 52.08 ± 4.41 |
| 2-pentylfurang | 980 | 974 | 36.09 ± 8.65 | 76.75 ± 9.81 | 34.18 ± 2.99 | 34.76 ± 2.40 | - | 44.40 ± 20.02 | - | - | - |
| hydrocarbons | |||||||||||
| 3,4-dimethoxytoluenei | 1199 | 1199 | 7 ± 1.11 | 7.34 ± 1.65 | - | 6.66 ± 0.50 | - | 9.96 ± 1.83 | 5.77 ± 1.09 | - | - |
| ketones | |||||||||||
| acetophenoneg | 1039 | 1027 | 11.02 ± 3.55 | 10.63 ± 0.40 | 9.70 ± 1.74 | 9.36 ± 1.07 | 6.98 ± 1.08 | - | 13.81 ± 3.59 | - | 12.62 ± 2.55 |
| 3,5-octadien-2-oneg | 1044 | 1046 | 13.36 ± 3.10 | - | 14.38 ± 2.34 | 13.31 ± 1.67 | - | - | - | - | - |
| isophoronei | 1086 | 1085 | 19.68 ± 3.50 | - | 16.57 ± 1.20 | 15.62 ± 1.28 | 17.22 ± 0.91 | 21.81 ± 6.10 | 20.31 ± 3.30 | 19.77 ± 1.16 | 20.70 ± 2.23 |
| β-iononei | 1477 | 1476 | 8.33 ± 0.51 | - | - | - | - | - | 7.68 ± 2.02 | - | 12.46 ± 2.30 |
| phenols | |||||||||||
| p-cresolh | 1043 | 1041 | 65.68 ± 24.56 | 79.45 ± 10.70 | 51.71 ± 11.99 | 65.95 ± 11.84 | 48.58 ± 4.44 | 108.44 ± 11.44 | 117.08 ± 5.29 | 160.53 ± 14.04 | 195.81 ± 9.97 |
| guaiacolh | 1056 | 1053 | 831.40 ± 33.28 | 721.03 ± 25.42 | 541.12 ± 17.62 | 438.60 ± 50.71 | 466.81 ± 49.83 | 938.86 ± 226.63 | 724.52 ± 99.02 | 704.49 ± 88.5 | 698.75 ± 24.72 |
| p-creosolg | 1170 | 1160 | 125.08 ± 19.25 | 133.50 ± 9.39 | 116.86 ± 8.22 | 102.65 ± 13.54 | 111.92 ± 9.91 | 146.30 ± 27.40 | 149.95 ± 10.39 | 146.51 ± 11.96 | 158.24 ± 4.31 |
| monoterpene | |||||||||||
| limoneneg | 1026 | 1027 | 11.96 ± 4.46 | - | - | 6.73 ± 0.93 | - | - | 18.34 ± 16.38 | - | - |
| sesquiterpenes | |||||||||||
| α-copaeneh | 1373 | 1379 | 38.94 ± 6.99 | 7.47 ± 1.19 | 20.25 ± 3.26 | - | 48.99 ± 5.78 | 33.54 ± 13.52 | 44.28 ± 3.61 | < 0.01 | 46.37 ± 3.43 |
| α-santaleneg | 1433 | 1426 | 10.97 ± 1.23 | - | 13.60 ± 1.75 | - | - | - | - | - | - |
| γ-muurolenei | 1477 | 1478 | 8.33 ± 0.53 | 17.54 ± 2.62 | 19.35 ± 2.40 | 8.18 ± 2.13 | 9.09 ± 1.11 | 20.03 ± 3.48 | 14.29 ± 0.31 | < 0.01 | 16.17 ± 3.95 |
| β-cadinenei | 1521 | 1518 | 7.35 ± 0.43 | - | - | - | - | - | - | - | - |
| δ-cadineneg | 1526 | 1522 | - | - | - | - | 7.72 ± 0.81 | - | - | - | 7.72 ± 0.81 |
Value are mean ± SD of triplicates.
literature retention indices obtain from g, h, and i.
retention indices, using n-paraffin (C5-C25) as references.
storage temperature.
storage time (month).
undetectable.
Zhang et al., 2012.
Yeh et al., 2021.
Table 3.
Changes in volatile components of vanilla beans from Taiwan stored at 25 °C and 35 °C.
| Compounds | RI b | RI c | peak areasa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25°C df |
35°C |
||||||||||
| 0 M e | act6 M | 12 M | 18 M | 24 M | 6 M | 12 M | 18 M | 24 M | |||
| alcohols | |||||||||||
| 1-hexanolg | 852 | 847 | - f | - | 31.07 ± 5.75 | 28.70 ± 6.16 | - | - | 41.01 ± 4.51 | - | - |
| benzyl alcoholg | 1008 | 997 | 41.63 ± 9.65 | 60.73 ± 15.60 | 194.56 ± 7.09 | 195.35 ± 20.94 | 215.84 ± 5.63 | 42.23 ± 8.69 | 269.61 ± 13.39 | 247.04 ± 11.18 | 209.28 ± 0.64 |
| 2-ethylhexanoli | 1012 | 998 | - | - | 39.75 ± 5.73 | - | - | - | 49.84 ± 4.78 | - | - |
| phenethyl alcoholg | 1080 | 1078 | 100.58 ± 10.64 | 177.70 ± 11.69 | 164.31 ± 10.04 | 153.75 ± 14.73 | 163.09 ± 0.30 | 170.48 ± 2.94 | 198.10 ± 4.94 | 171.97 ± 3.25 | 152.43 ± 4.17 |
| anisyl alcoholg | 1253 | 1245 | 81.56 ± 4.65 | 142.95 ± 21.06 | 159.13 ± 18.58 | 145.55 ± 5.81 | 143.13 ± 3.71 | 161.48 ± 9.47 | 141.47 ± 8.98 | 113.50 ± 17.00 | 145.11 ± 9.95 |
| cinnamyl alcoholg | 1278 | 1278 | - | - | 23.73 ± 4.62 | - | - | - | - | 7.92 ± 0.18 | 9.51 ± 0.32 |
| aldehydes | |||||||||||
| benzaldehydeg | 932 | 932 | 39.06 ± 10.63 | 10.19 ± 2.43 | 37.88 ± 6.58 | 41.93 ± 9.71 | 51.82 ± 3.49 | 55.51 ± 10.35 | 92.18 ± 8.36 | - | - |
| phenylacetaldehydeg | 1012 | 1004 | - | - | - | 41.48 ± 7.72 | - | - | - | - | - |
| nonanalg | 1084 | 1076 | 51.07 ± 7.26 | 65.62 ± 2.35 | 35.93 ± 5.45 | 32.05 ± 6.43 | 30.83 ± 2.13 | 37.61 ± 2.82 | 33.38 ± 1.27 | 44.61 ± 10.31 | 21.01 ± 3.26 |
| safranalg | 1183 | 1174 | 19.31 ± 4.15 | 32.03 ± 4.51 | 30.67 ± 3.33 | 29.77 ± 4.27 | 29.33 ± 1.51 | 31.08 ± 2.62 | 46.87 ± 1.04 | 54.52 ± 0.76 | 51.65 ± 0.90 |
| decanali | 1186 | 1180 | 9.15 ± 1.67 | - | - | - | - | - | - | - | - |
| β-cyclocitralh | 1194 | 1196 | 16.36 ± 2.65 | 14.18 ± 3.27 | 16.66 ± 1.47 | - | - | 7.52 ± 0.16 | - | - | 29.43 ± 1.51 |
| anisaldehydeg | 1221 | 1212 | 32.60 ± 3.43 | 33.58 ± 1.50 | 38.29 ± 5.64 | 40.10 ± 3.88 | 44.73 ± 1.33 | 42.78 ± 0.99 | 65.98 ± 1.98 | 68.90 ± 1.45 | 75.19 ± 0.36 |
| cinnamaldehydeg | 1239 | 1231 | 11.11 ± 2.78 | - | - | - | - | - | - | - | - |
| p-hydroxybenzaldehydeg | 1315 | 1320 | 175.71 ± 8.28 | 251.05 ± 37.81 | 333.07 ± 18.01 | 334.18 ± 24.74 | 333.27 ± 16.67 | 333.26 ± 13.93 | 253.28 ± 14.78 | 277.79 ± 12.23 | 341.83 ± 3.18 |
| vanilling | 1368 | 1362 | 18927.06 ± 1503.70 | 29796.49 ± 5255.91 | 34565.09 ± 2790.76 | 31938.82 ± 2358.46 | 28204.37 ± 313.05 | 35283.99 ± 2771.47 | 27402.61 ± 1970.15 | 23645.26 ± 2722.59 | 29572.04 ± 1379.52 |
| methylvanillini | 1427 | 1428 | 14.46 ± 2.33 | 15.45 ± 2.66 | - | - | - | 23.53 ± 2.72 | - | - | - |
| acids | |||||||||||
| 2-ethylhexanoic acidg | 1096 | 1094 | 319.05 ± 44.5 | 489.82 ± 48.49 | 471.98 ± 49.01 | - | - | 435.39 ± 16.62 | 299.07 ± 120.79 | - | - |
| esters | |||||||||||
| methyl decanoatei | 1306 | 1300 | - | - | - | - | - | - | - | 11.46 ± 0.48 | 13.62 ± 1.12 |
| benzyl acetateg | 1135 | 1127 | 7.72 ± 0.63 | 9.86 ± 1.45 | 14.43 ± 2.23 | 13.36 ± 2.95 | 22.49 ± 6.44 | 12.73 ± 1.15 | 18.16 ± 2.84 | 18.85 ± 2.31 | 23.31 ± 5.59 |
| methyl salicylateg | 1176 | 1168 | 40.08 ± 7.84 | 72.34 ± 10.53 | 66.94 ± 8.12 | 63.50 ± 9.10 | 66.04 ± 2.24 | 74.05 ± 7.58 | 117.22 ± 6.69 | 82.36 ± 0.51 | 67.63 ± 1.83 |
| methyl nonanoatei | 1205 | 1197 | - | - | 15.68 ± 1.69 | 17.93 ± 2.12 | 23.66 ± 0.09 | 4.04 ± 0.11 | 29.80 ± 1.38 | 29.54 ± 4.36 | 68.06 ± 15.65 |
| 2-phenylethyl acetatei | 1221 | 1219 | - | - | 13.90 ± 2.37 | - | 12.45 ± 1.36 | - | 14.77 ± 1.25 | - | 15.44 ± 0.05 |
| methyl laurateg | 1508 | 1504 | - | - | - | - | - | - | - | 16.65 ± 4.20 | 20.20 ± 9.22 |
| furans | |||||||||||
| furfuralh | 799 | 798 | 23.8 ± 12.18 | 112.91 ± 17.82 | 121.92 ± 16.81 | 166.56 ± 26.59 | 223.77 ± 11.16 | 182.83 ± 25.38 | 410.63 ± 30.53 | 553.29 ± 12.51 | 412.91 ± 7.03 |
| 5-methylfurfuralg | 930 | 928 | - | - | - | 9.56 ± 3.44 | - | - | - | 120.73 ± 4.28 | 99.55 ± 2.90 |
| 5-hydroxymethylfurfurali | 1188 | 1182 | - | - | - | - | - | - | - | 22.01 ± 0.43 | 21.79 ± 0.51 |
| hydrocarbons | |||||||||||
| 3,4-dimethoxytoluenei | 1199 | 1200 | 7.00 ± 1.11 | - | - | - | - | - | - | - | - |
| ketones | |||||||||||
| acetophenoneg | 1039 | 1029 | 11.02 ± 3.55 | - | 13.04 ± 1.97 | 13.39 ± 2.79 | 14.84 ± 1.05 | - | 17.92 ± 0.72 | 21.64 ± 0.84 | - |
| 3,5-octadien-2-oneg | 1044 | 1036 | 13.36 ± 3.10 | - | - | - | - | - | - | - | - |
| isophoronei | 1086 | 1086 | 19.68 ± 3.50 | 22.74 ± 3.80 | 18.61 ± 2.17 | 18.03 ± 3.16 | 17.91 ± 0.50 | 19.51 ± 2.41 | 22.15 ± 1.82 | 27.14 ± 0.70 | 21.73 ± 2.04 |
| α-iononei | 1408 | 1406 | - | - | - | - | - | - | 30.52 ± 3.2 | 26.15 ± 1.31 | - |
| β-iononei | 1477 | 1467 | 8.33 ± 0.51 | - | - | 11.29 ± 1.76 | - | 10.37 ± 1.44 | - | - | 12.23 ± 1.38 |
| phenols | |||||||||||
| p-cresolh | 1043 | 1043 | 65.68 ± 24.56 | 278.67 ± 37.49 | 270.07 ± 24.73 | 313.54 ± 53.15 | 347.86 ± 9.12 | 322.72 ± 13.98 | 433.58 ± 11.3 | 392.47 ± 6.11 | 369.59 ± 8.09 |
| guaiacolh | 1056 | 1055 | 831.40 ± 33.28 | 904.19 ± 118.08 | 693.84 ± 66.82 | 619.58 ± 88.89 | 667.13 ± 21.95 | 678.06 ± 65.72 | 912.11 ± 41.34 | 956.36 ± 39.19 | 747.86 ± 20.82 |
| p-creosolg | 1170 | 1162 | 125.08 ± 19.25 | 181.65 ± 16.72 | 185.67 ± 13.87 | 179.96 ± 22.15 | 185.35 ± 5.04 | 196.82 ± 11.61 | 267.37 ± 2.6 | 305.13 ± 6.53 | 193.49 ± 1.68 |
| Pyrroles | |||||||||||
| 2-acetylpyrrolei | 1021 | 1018 | - | - | - | - | - | - | - | 38.84 ± 3.13 | 61.75 ± 0.36 |
| monoterpenes | |||||||||||
| limoneneg | 1026 | 1016 | 11.96 ± 4.46 | - | - | 10.36 ± 1.44 | 20.46 ± 0.96 | 16.70 ± 0.42 | 15.95 ± 3.40 | 33.80 ± 3.94 | - |
| sesquiterpenes | |||||||||||
| α-copaeneh | 1373 | 1381 | 38.94 ± 6.99 | - | 38.94 ± 16.49 | 63.47 ± 12.05 | 56.15 ± 1.27 | - | 58.76 ± 3.35 | 51.38 ± 7.08 | - |
| α-santaleneg | 1433 | 1421 | 10.97 ± 1.23 | - | - | - | - | 16.66 ± 0.84 | - | - | - |
| α-amorpheneh | 1474 | 1478 | - | - | - | 10.16 ± 3.20 | 7.71 ± 0.79 | - | 10.48 ± 0.80 | - | - |
| γ-muurolenei | 1477 | 1498 | 8.33 ± 0.53 | 9.26 ± 1.08 | 23.91 ± 3.54 | 17.90 ± 2.08 | 14.51 ± 6.11 | 16.85 ± 1.77 | - | - | 12.29 ± 0.16 |
| β-cadinenei | 1521 | 1518 | 7.35 ± 0.43 | - | - | - | - | 10.57 ± 1.27 | - | - | - |
| δ-cadineneg | 1526 | 1522 | - | - | - | 9.19 ± 4.21 | - | - | - | - | 9.81 ± 1.72 |
| α-calacoreneh | 1530 | 1532 | - | - | - | - | - | 14.47 ± 1.67 | - | - | - |
| cadaleneg | 1653 | 1667 | - | - | - | - | - | - | - | - | 4.54 ± 0.19 |
Value are mean ± SD of triplicates.
literature retention indices obtain from g, h, and i.
retention indices, using n-paraffin (C5-C25) as references.
storage temperature.
storage time (month).
undetectable
National Library of Medicine, Pubchem.
Fig. 2.
The relative content of chemical groups in vanilla beans stored at different temperatures.
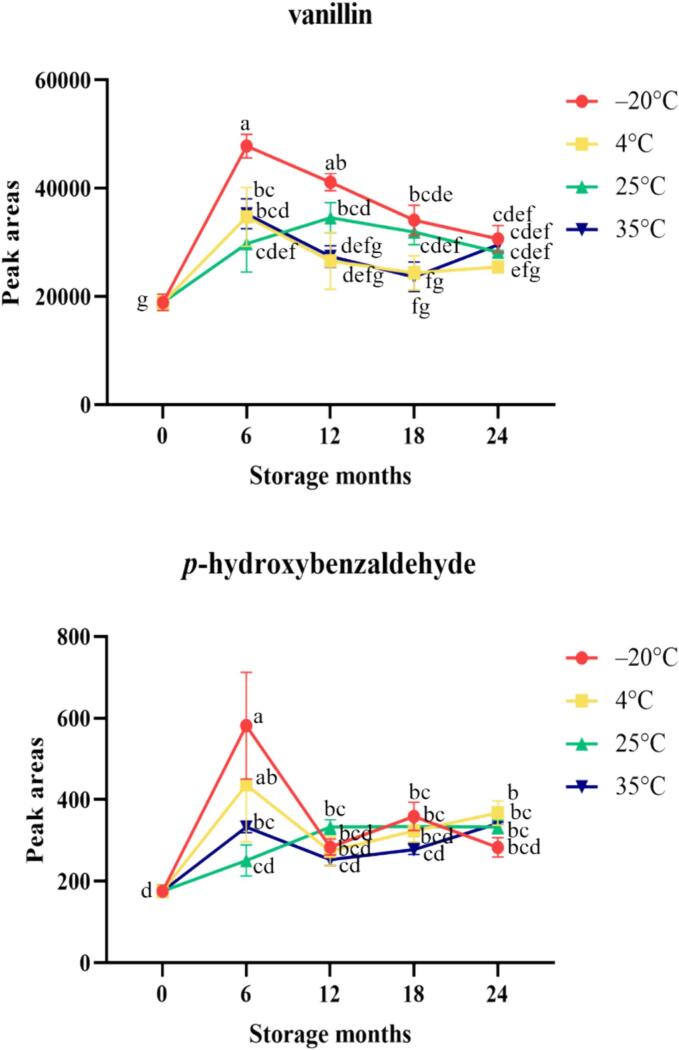
This research identified benzaldehyde, phenylacetaldehyde, nonanal, safranal, decanal, β-cyclocitral, anisaldehyde, cinnamaldehyde, p-hydroxybenzaldehyde, vanillin and methyl vanillin as aldehyde compounds, among which vanillin has a strong vanilla, sweet and creamy aroma. It is the main volatile compound of vanilla beans from Taiwan and is derived from phenylalanine in the shikimic acid pathway (Brunschwig et al., 2016; Sreedhar et al., 2007), p-hydroxybenzaldehyde, which has a vanilla and biscuit aroma, is an important component in the synthetic pathway of vanillin (Pérez-Silva et al., 2006; Sreedhar et al., 2007; Brunschwig et al., 2016).
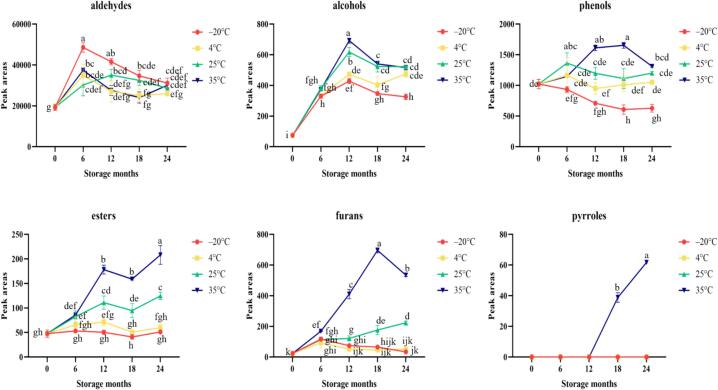
Salazar-Rojas et al. (2012) noted that vanillin, vanillic acid, p-hydroxybenzoic acid, and p-hydroxybenzoic acid, which have relatively high concentrations and play an important role in aroma, so they are used commercially as quality indicators; however, in this experiment, vanillic acid (melting point 213–217 °C, decomposed when boiling) and p-hydroxybenzoic acid (melting point 208–210 °C, boiling point unknown) were not identified in vanilla beans from Taiwan, probably because of their relatively low volatility (Zhang & Mueller, 2012), and it is difficult to analyze them through the headspace technique. Based on Fig. 3 and Fig. 4, it can be observed that the aldehydes and their indicative components in vanilla beans stored at −20 °C, 4 °C, 25 °C, and 35 °C showed an initial upward trend. Gao et al. (2021) investigated the changes in volatile compounds in rice bran under different storage conditions. The results indicated that regardless of the storage conditions, the aldehyde content increased with prolonged storage time. However, this experiment found that the aldehyde content in vanilla bean samples stored at −20 °C and 4 °C showed a decreasing trend after 6 months of storage.
Fig. 3.
Chemical groups of vanilla beans with regular changes during storage at different temperatures. Values are means ± SD of triplicates. Values having different superscripts are significantly (p < 0.05) different.
Fig. 4.
Changes of in the indicative components of vanilla beans stored in different atmosphere-modified packaging. Values are means ± SD of triplicates. Values having different superscripts are significantly (p < 0.05) different.
Phenols are the secondary chemical groups of vanilla beans from Taiwan, and the content of guaiacol is second only to vanillin. The experimental results are the same as those of Zhang and Mueller (2012). Fig. 3 shows that the phenolic content of vanilla bean samples stored at 25 °C and 35 °C initially increased during storage, followed by a significant decrease at the 12th and 18th months. In contrast, samples stored at 4 °C showed no significant change in phenolic content during storage, while samples stored at −20 °C exhibited a decreasing trend throughout the storage period. These findings are consistent with previous research, indicating that higher storage temperatures promote the formation of phenolic compounds (de la Rosa et al., 2019).
Alcohols are usually the main source of sweet and fruity aromas (Javed et al., 2019). Frenkel et al. (2018) indicated that alcohols in vanilla beans are usually derived from lipid oxidation. Fig. 3 shows the alcohol content reaches the highest peak at the 12th month of storage, and the rising trend is most obvious in samples stored at 35 °C. Rogel-Castillo et al. (2015) noted that increasing temperature will increase many reactions (such as chemical, enzymatic and metabolic reactions). This may be the main reason for the higher content of alcohols, esters, furans, pyrroles, ketones and alcohols in samples stored at 35 °C. In addition, this experiment found that among alcohols, the related compounds (phenylethyl alcohol and benzyl alcohol) produced by lipid metabolism and enzyme activity in all samples tended to increase after storage (Rogel-Castillo et al., 2015), so it is speculated that lipid metabolism or oxidation reactions may occur during storage of vanilla beans.
In this experiment, only 2-ethylhexanoic acid was identified among the acids. Zhang and Mueller (2012) previously detected 2-ethylhexanoic acid in Ugandan vanilla. According to previous literature, 2-ethylhexanoic acid has an unpleasant and putrid odor (Javed et al., 2019). Vanilla beans were stored at different temperatures until the 6th month, and the acid compounds were significantly increased and gradually decreased after 6 months of storage, and no volatile compounds were detected in the samples stored for 18 and 24 months (Table 2 and Table 3).
Esters primarily originate from lipid oxidation and amino acid metabolism (Yang et al., 2019), often imparting fruity, fatty, oily, and sweet aromas (Peña-Barrientos et al., 2022). The combination of esters with the phenolic Compound p-cresol contributes to the pronounced spicy, fruity, and rum-like aromas of vanilla beans (Brunschwig et al., 2016). From Table 3 and Fig. 3, it can be found that the content and quantity of ester compounds identified in the samples at 25 °C and 35 °C in the late storage period tended to increase, such as methyl laurate and stale, woody and unpleasant odors and methyl decanoate (Dai et al., 2020), which was detected only in samples stored at 35 °C. There were no significant differences in the levels and quantities of ester compounds identified in samples stored at −20 °C and 4 °C. Yang et al. (2019) suggested that long-term low-temperature storage can inhibit the activity of aroma-metabolizing enzymes, including those related to ester compounds, resulting in a reduction in fruity aroma.
Furan compounds often have a sweet or caramel aroma (Perez-Cacho et al., 2008). The furfural, 2-pentylfuran, 5-methylfurfural and 5-hydroxymethylfurfural identified in this study are furan compounds, and 5-methylfurfural and 5-hydroxymethylfurfural were detected only in samples stored at 25 °C and 35 °C. Additionally, it should be noted that 2-acetylpyrrole and 5-hydroxymethylfurfural were exclusively present in samples stored at 35 °C for 18 and 24 months. These two volatile compounds are important indicators of the Maillard reaction (Delgado-Andrade et al., 2010; Raitio et al., 2011). The reaction rate of the Maillard reaction increases with higher temperatures (Göncüoğlu Taş & Gökmen, 2017) and under appropriate moisture conditions, the reaction can still occur at low temperatures (Wong et al., 2015). The findings of this study align with the results, as shown in Fig. 3, where the levels of furan compounds and pyrroles in samples stored at 35 °C were significantly higher compared to other samples.
The content changes of trace components such as ketones, hydrocarbons, monoterpenes and sesquiterpenes are relatively unstable, and the differences in these changes may be caused by different oxidation and hydrolysis pathways (Liu et al., 2017).
3.2. Principal component analysis of long-term storage of vanilla beans at different temperatures
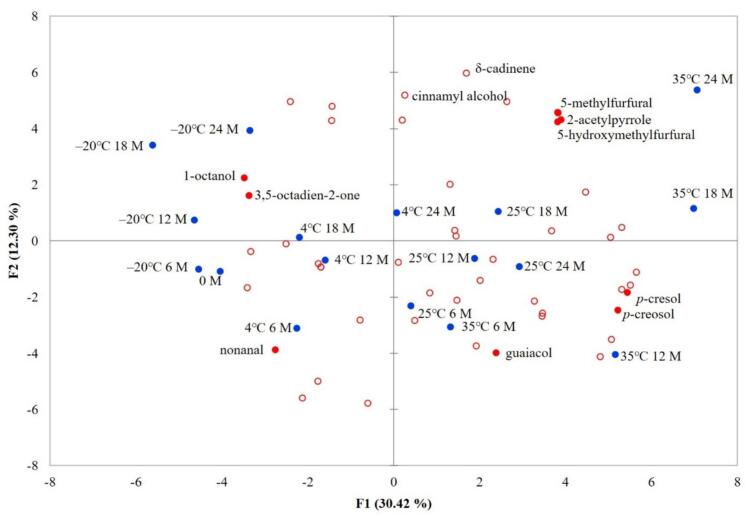
To gain deeper insights into the impact of different storage temperatures on the aroma of vanilla beans, this study employed principal component analysis (PCA) to assess variations in volatile components among samples stored at various temperatures. Fig. 5 presents the PCA results of volatile compounds in vanilla beans during storage at different temperatures. It is evident from the figure that the cumulative variation explained by PC1 and PC2 amounts to 42.72%. Notably, the samples stored at 35 °C for 12, 18, and 24 months exhibit distinct separation from the other samples, indicating that storage temperature significantly influences the formation of volatile components in vanilla beans. Furthermore, the longer the storage duration, the more pronounced the impact of temperature on the samples. Specifically, 5-methylfurfural, 2-acetylpyrrole, and 5-hydroxymethylfurfural exhibit the closest association with the samples stored at 35 °C for 24 months. Moreover, 2-acetylpyrrole and 5-hydroxymethylfurfural were exclusively detected in samples stored at 35 °C for 18 and 24 months. The formation of these components may be linked to the Maillard reaction (Delgado-Andrade et al., 2010; Raitio et al., 2011). Additionally, the PCA plots reveal a strong correlation between the three phenolic compounds, namely, guaiacol, p-creosol, and p-cresol, and samples stored at 35 °C for 6 and 12 months. Brunschwig et al. (2016) highlighted that phenolic compounds in vanilla beans typically contribute to robust phenolic, woody, and smoky aromas, which can detract from the desired vanilla bean aroma. Brunschwig et al. (2016) noted that the phenolic compounds in vanilla beans usually have strong phenolic, woody and smoky aromas, which will neutralize the vanilla aroma of vanilla beans.
Fig. 5.
Principal component analysis of vanilla beans from Taiwan at different storage times and temperatures. (▲: sample (M: months); ●: marked volatile components; ○: unmarked volatile components.)
Based on the results of principal component analysis (PCA), the samples stored at low temperatures (−20 °C and 4 °C) exhibit a closer association with 1-octanol, 3,5-octadien-2-one, and nonanal. These three volatile components are known to be related to oxidation processes (Grosso et al., 2020). Typically, such compounds are characterized by fatty and green aromas, which have the potential to introduce off-flavors in the product (Meetaworn et al., 2022; Hartvigsen et al., 2000). Meethaworn et al. (2022) noted that 1-octanol is related to the formation of off-flavors.
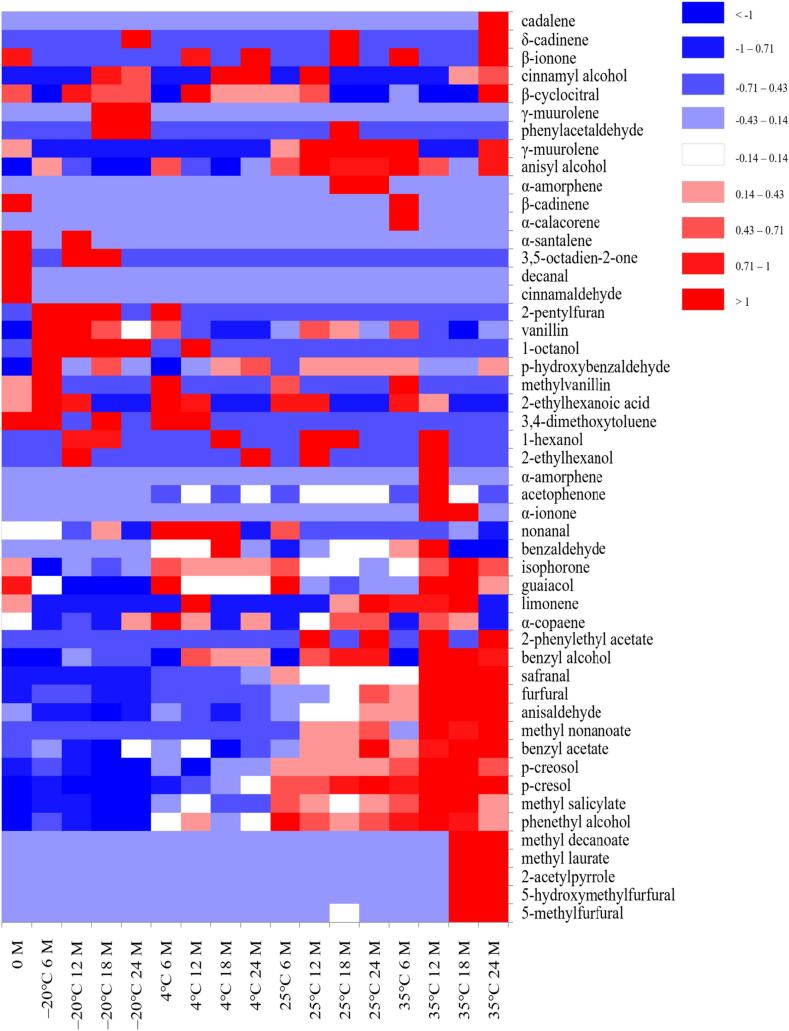
3.3. Heatmap analysis of long-term storage of vanilla beans at different temperatures
A heatmap was generated to show the changes in volatile compound content of Taiwanese vanilla beans during long-term storage at different temperatures (Fig. 6). Color intensities are normalized from a maximum value of 1 (bright red) to a minimum value of −1 (dark blue), representing the relative abundance of compounds from high to low. It can be observed from the heatmap that samples of vanilla beans stored at −20 °C for 6 months exhibited higher levels of the two quality index components, vanillin and p-hydroxybenzaldehyde, compared to other samples. Conversely, samples stored at 35 °C showed lower levels of these components. The quality indicator components of samples stored at 4 °C and 25 °C were found to be less stable during storage. Moreover, the heatmap revealed that samples stored at −20 °C for 6 months had higher contents of 2-pentylfuran, 1-octanol, 2-ethylhexanoic acid, 1-hexanol, and 2-ethylhexanol. These compounds are known to possess aromas such as green, grassy, fatty, woody, and rotten (Hartvigsen et al., 2000; Xiang et al., 2023), which may have a negative impact on the aroma quality of vanilla beans. Although quality indicator components suggest that samples stored at −20 °C for 6 months exhibit better quality, it is still challenging to completely prevent the formation of some off-odors even at low temperatures. Fig. 6 demonstrates that samples stored at 25 °C and 35 °C have higher levels of p-creosol, p-cresol, methyl salicylate, and phenethyl alcohol. Among these, p-creosol and p-cresol, known for their strong phenolic aroma, can neutralize the vanilla aroma of the vanilla beans, resulting in a diminished vanilla fragrance (Brunschwig et al., 2016). Therefore, an increase in p-creosol and p-cresol content may impact consumer acceptance of the vanilla aroma. Previous studies have indicated a positive correlation between vanilla aroma and consumer acceptance (Jaeger et al., 2023). When samples were stored at 35 °C for 18 and 24 months, a higher production of volatile compounds such as methyl decanoate, methyl laurate, 2-acetylpyrrole, 5-hydroxymethylfurfural, and 5-methylfurfural was observed. These compounds are mostly products of the Maillard reaction (Perez-Cacho & Rouseff, 2008), which can lead to food deterioration (Xiang et al., 2021). Thus, the aroma quality of vanilla beans stored at 35 °C exhibits instability during storage.
Fig. 6.
Heat map of vanilla beans at different storage temperatures.
3.4. Results of color analysis
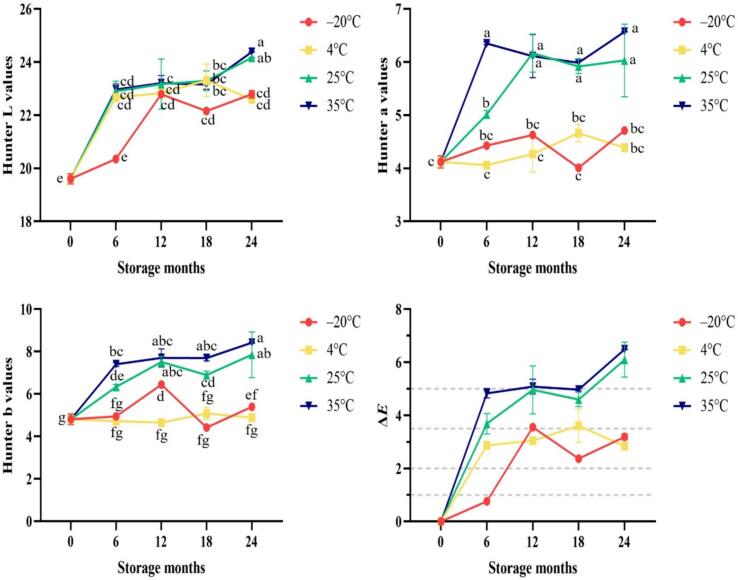
The trend of color changes in Taiwanese vanilla beans during storage at different temperatures was analyzed using a colorimeter and is shown in Fig. 7 Regardless of the storage temperature, there was a significant increase in the L value of the vanilla beans, indicating a lightening of their appearance after storage. Compared to vanilla beans stored at −20 °C and 4 °C, the upward trend in the a and b values was more pronounced in samples stored at 25 °C and 35 °C. ΔE in Fig. 7 represents the overall color parameter changes, and it can be used to assess whether observers can perceive differences in color (Mokrzycki & Tatol, 2011). When vanilla beans were stored at −20 °C for 6 months, the ΔE values ranged from 0 to 1, indicating not significant differences (observers had difficulty perceiving color changes). For the samples stored at −20 °C and 4 °C for 12–24 months, the ΔE values ranged from 2 to 3.5, indicating noticeable color differences (even inexperienced individuals could perceive the differences). For the samples stored at 25 °C and 35 °C for 6–18 months, the ΔE values ranged from 3.5 to 5, indicating very noticeable color differences, and when stored for 24 months, ΔE exceeded 5, indicating that the color of these samples was different from that of the initial month. According to ISO 5565-1:1999, vanilla beans with a dark chocolate brown color are classified as the highest grade. However, in this study, only the vanilla beans stored at −20 °C for 6 months showed no color difference compared to the initial month, while the color of the remaining samples lightened (increased L value) during storage. The samples stored at 25 °C and 35 °C exhibited a transition from dark brown to reddish-brown due to the increase in the +a and + b values. Pola et al. (2021) mentioned that both enzymatic and nonenzymatic reactions can contribute to color changes, and higher temperatures can accelerate the rate of color change. In conclusion, the color of vanilla beans is influenced by storage time and temperature, with higher storage temperatures resulting in more noticeable color differences, while lower temperatures effectively slow color changes.
Fig. 7.
Comparison of the color changes of vanilla beans stored at different temperatures. Values are means ± SD of triplicates. Values having different superscripts are significantly (p < 0.05) different.
4. Conclusions
The results of volatile compound identification and statistical analysis indicate that both storage temperature and time have an impact on the volatile components of vanilla beans. As time increases, the trends and differences in these components become more apparent due to lipid metabolism, enzymatic oxidation, and Maillard reaction. The color analysis results show that samples stored at lower temperatures (−20 °C and 4 °C) exhibit minimal color changes. Based on the above findings, it is evident that higher temperatures (25 °C and 35 °C) significantly affect the quality of vanilla beans, while lower temperatures (−20 °C and 4 °C) have a lesser impact. Therefore, this study recommends storing vanilla beans at −20 °C, to maintain their quality.
CRediT authorship contribution statement
Chih-Hsin Yeh: Writing – review & editing, Validation, Methodology, Formal analysis, Conceptualization. Chia-Yi Chou: Investigation, Data curation. Kai-Min Yang: Writing – review & editing, Investigation. Chin-Sheng Wu: Writing – review & editing, Investigation. Lee-Ping Chu: Writing – review & editing. Yu-Ling Hsu: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Hsin-Chun Chen: Writing – review & editing, Writing – original draft, Validation, Investigation, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
Financial support from the Ministry of Agriculture (Taiwan) 112AS-4.2.2-FD-Z1 (1) and the China Medical University (Taiwan) (CMU112-S-45) are gratefully acknowledged.
Contributor Information
Yu-Ling Hsu, Email: u110044201@cmu.edu.tw.
Hsin-Chun Chen, Email: d91628004@ntu.edu.tw.
Data availability
Data will be made available on request.
References
- Baqueiro-Pena I., Guerrero-Beltran J.A. Vanilla (Vanilla planifolia Andr.), its residues and other industrial by-products for recovering high value flavor molecules: A review. Journal of Applied Research on Medicinal and Aromatic Plants. 2017;6:1–9. [Google Scholar]
- Biao Y., Zhao C., Yan M., Huang D., McClements D.J., Huang Z., Cao C. Influence of gene regulation on rice quality: Impact of storage temperature and humidity on flavor profile. Food Chemistry. 2019;283(15):141–147. doi: 10.1016/j.foodchem.2019.01.042. [DOI] [PubMed] [Google Scholar]
- Brunschwig C., Rochard S., Pierrat A., Rouger A., Senger-Emonnot P., George G., Raharivelomanana P. Volatile composition and sensory properties of Vanilla ×tahitensis bring new insights for vanilla quality control. Journal of the Science of Food and Agriculture. 2016;96(3):848–858. doi: 10.1002/jsfa.7157. [DOI] [PubMed] [Google Scholar]
- Chiang Y.C., Chiang P.Y. Accentuation of the browning characteristics and functional properties of aged tomatoes (Solanum lycopersicum cv.) Food Chemistry. 2024;X doi: 10.1016/j.fochx.2024.101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q., Jin H., Gao J., Ning J., Yang X., Xia T. Investigating volatile compounds' contributions to the stale odour of green tea. International Journal of Food Science & Technology. 2020;55(4):1606–1616. [Google Scholar]
- Delgado-Andrade C., Seiquer I., Haro A., Castellano R., Navarro M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chemistry. 2010;122(1):145–153. [Google Scholar]
- Dignum M.J., Kerler J., Verpoorte R. Vanilla curing under laboratory conditions. Food Chemistry. 2002;79(2):165–171. [Google Scholar]
- Dignum M.J., van der Heijden R., Kerler J., Winkel C., Verpoorte R. Identification of glucosides in green beans of Vanilla planifolia Andrews and kinetics of vanilla β-glucosidase. Food Chemistry. 2004;85(2):199–205. [Google Scholar]
- Dixon J., Hewett E.W. Factors affecting apple aroma/flavour volatile concentration: A review. New Zealand Journal of Crop and Horticultural Science. 2000;28(3):155–173. [Google Scholar]
- Frenkel C., Ranadive A.S., Vázquez J.T., Havkin-Frenkel D. Curing of vanilla. Handbook of Vanilla Science and Technology. 2018:191–221. [Google Scholar]
- Gao C., Li Y., Pan Q., Fan M., Wang L., Qian H. Analysis of the key aroma volatile compounds in rice bran during storage and processing via HS-SPME GC/MS. Journal of Cereal Science. 2021;99 [Google Scholar]
- Göncüoğlu Taş N., Gökmen V. Maillard reaction and caramelization during hazelnut roasting: A multiresponse kinetic study. Food Chemistry. 2017;221:1911–1922. doi: 10.1016/j.foodchem.2016.11.159. [DOI] [PubMed] [Google Scholar]
- Grosso A.L., Riveros C., Asensio C.M., Grosso N.R., Nepote V. Improving walnuts’ preservation by using walnut phenolic extracts as natural antioxidants through a walnut protein-based edible coating. Journal of Food Science. 2020;85(10):3043–3051. doi: 10.1111/1750-3841.15395. [DOI] [PubMed] [Google Scholar]
- Gu F., Chen Y., Hong Y., Fang Y., Tan L. Comparative metabolomics in vanilla pod and vanilla bean revealing the biosynthesis of vanillin during the curing process of vanilla. AMB Express. 2017;7:116. doi: 10.1186/s13568-017-0413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Zhao X., Ma Y., Wang Y., Wang D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130939. [DOI] [PubMed] [Google Scholar]
- Hartvigsen K., Lund P., Hansen L.F., Hølmer G. Dynamic headspace gas chromatography/mass spectrometry characterization of volatiles produced in fish oil enriched mayonnaise during storage. Journal of Agricultural and Food Chemistry. 2000;48(10):4858–4867. doi: 10.1021/jf991385b. [DOI] [PubMed] [Google Scholar]
- Havkin-Frenkel D., Frenkel C. Postharvest handling and storage of cured vanilla beans. Stewart Postharvest Review. 2006;4(6):1–9. [Google Scholar]
- Jaeger S.R., Cardello A.V., Jin D., Ryan G.S., Giacalone D. Consumer perception of plant-based yoghurt: Sensory drivers of liking and emotional, holistic and conceptual associations. Food Research International. 2023;167 doi: 10.1016/j.foodres.2023.112666. [DOI] [PubMed] [Google Scholar]
- Javed H.U., Wang D., Wu G.F., Kaleem Q.M., Duan C.Q., Shi Y. Post-storage changes of volatile compounds in air-and sun-dried raisins with different packaging materials using HS-SPME with GC/MS. Food Research International. 2019;119:23–33. doi: 10.1016/j.foodres.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Karolkowski A., Guichard E., Briand L., Salles C. Volatile compounds in pulses: A review. Foods. 2021;10(12):3140. doi: 10.3390/foods10123140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Li Y., Chen F., Yong F. Lipid oxidation of brown rice stored at different temperatures. International Journal of Food Science & Technology. 2017;52(1):188–195. [Google Scholar]
- Márquez O., Waliszewski K.N. The effect of thermal treatment on β-glucosidase inactivation in vanilla bean (Vanilla planifolia Andrews) International Journal of Food Science & Technology. 2008;43(11):1993–1999. [Google Scholar]
- Meethaworn K., Imsabai W., Zhang B., Chen K., Siriphanich J. Off-flavor and loss of aroma in young coconut fruit during cold storage are associated with the expression of genes derived from the LOX pathway and badh2. The Horticulture Journal. 2022;91(2):209–220. [Google Scholar]
- Mokrzycki W.S., Tatol M. Color difference ∆E-A survey. Mach. Graph. Vis. 2011;20(4):383–411. [Google Scholar]
- National Library of Medicine PubChem. 2024. https://pubchem.ncbi.nlm.nih.gov/ Available online: accessed on 21, May, 2024. [DOI] [PubMed]
- Pazmiño-Arteaga J., Gallardo C., González-Rodríguez T., Winkler R. Loss of sensory cup quality: Physiological and chemical changes during green coffee storage. Plant Foods for Human Nutrition. 2022;77:1–11. doi: 10.1007/s11130-022-00953-8. [DOI] [PubMed] [Google Scholar]
- Peña-Barrientos A., de Jesús Perea-Flores M., Martínez-Gutiérrez H., Patrón-Soberano A., González-Jiménez F.E., Vega-Cuellar M.Á., Davila-Ortiz G. Physicochemical, microbiological, and structural relationship of vanilla beans (Vanilla planifolia, Andrews) during traditional curing process and use of its waste. Journal of Applied Research on Medicinal and Aromatic Plants. 2022;100445 [Google Scholar]
- Perez-Cacho P.R., Rouseff R. Processing and storage effects on orange juice aroma: A review. Journal of Agricultural and Food Chemistry. 2008;56(21):9785–9796. doi: 10.1021/jf801244j. [DOI] [PubMed] [Google Scholar]
- Pérez-Silva A., Odoux E., Brat P., Ribeyre F., Rodriguez-Jimenes G., Robles-Olvera V., Günata Z. GC–MS and GC–olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chemistry. 2006;99(4):728–735. [Google Scholar]
- Pola W., Sugaya S., Srilaong V., Wongs-Aree C., Photchanachai S. Color appearance and physico-chemical changes in dried chili as affected by modified atmosphere packaging and temperature during storage. Journal of Food Processing and Preservation. 2021;45(11) [Google Scholar]
- Raitio R., Orlien V., Skibsted L.H. Storage stability of cauliflower soup powder: The effect of lipid oxidation and protein degradation reactions. Food Chemistry. 2011;128(2):371–379. doi: 10.1016/j.foodchem.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Ramachandra Rao S., Ravishankar G.A. Vanilla flavour: Production by conventional and biotechnological routes. Journal of the Science of Food and Agriculture. 2000;80(3):289–304. [Google Scholar]
- Rogel-Castillo C., Zuskov D., Chan B.L., Lee J., Huang G., Mitchell A.E. Effect of temperature and moisture on the development of concealed damage in raw almonds (Prunus dulcis) Journal of Agricultural and Food Chemistry. 2015;63(37):8234–8240. doi: 10.1021/acs.jafc.5b03121. [DOI] [PubMed] [Google Scholar]
- de la Rosa L.A., Moreno-Escamilla J.O., Rodrigo-García J., Alvarez-Parrilla E. Phenolic compounds. In Postharvest physiology and biochemistry of fruits and vegetables. 2019:253–271. [Google Scholar]
- Salazar-Rojas V.M., Herrera-Cabrera B.E., Delgado-Alvarado A., Soto-Hernández M., Castillo-González F., Cobos-Peralta M. Chemotypical variation in Vanilla planifolia Jack. (Orchidaceae) from the Puebla-Veracruz Totonacapan region. Genetic Resources and Crop Evolution. 2012;59(5):875–887. [Google Scholar]
- Sánchez-García J., Muñoz-Pina S., García-Hernández J., Heredia A., Andrés A. Volatile profile of quinoa and lentil flour under fungal fermentation and drying. Food Chemistry. 2024;430 doi: 10.1016/j.foodchem.2023.137082. [DOI] [PubMed] [Google Scholar]
- Sreedhar R.V., Roohie K., Venkatachalam L., Narayan M.S., Bhagyalakshmi N. Specific pretreatments reduce curing period of vanilla (Vanilla planifolia) beans. Journal of Agricultural and Food Chemistry. 2007;55(8):2947–2955. doi: 10.1021/jf063523k. [DOI] [PubMed] [Google Scholar]
- Verma P.C., Chakrabarty D., Jena S.N., Mishra D.K., Singh P.K., Sawant S.V., Tuli R. The extent of genetic diversity among Vanilla species: Comparative results for RAPD and ISSR. Industrial Crops and Products. 2009;29(2–3):581–589. [Google Scholar]
- Wong C.W., Wijayanti H.B., Bhandari B.R. Water Stress in Biological; Chemical, Pharmaceutical and Food Systems: 2015. Maillard reaction in limited moisture and low water activity environment; pp. 41–63. [Google Scholar]
- Xiang J., Liu F., Wang B., Chen L., Liu W., Tan S. A literature review on maillard reaction based on milk proteins and carbohydrates in food and pharmaceutical products: Advantages, disadvantages, and avoidance strategies. Foods. 2021;10(9):1998. doi: 10.3390/foods10091998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L., Zhu W., Jiang B., Chen J., Zhou L., Zhong F. Volatile compounds analysis and biodegradation strategy of beany flavor in pea protein. Food Chemistry. 2023;402 doi: 10.1016/j.foodchem.2022.134275. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu J., Wang X., Wang R., Ren F., Zhang Q., Ding S. Characterization of volatile component changes in jujube fruits during cold storage by using headspace-gas chromatography-ion mobility spectrometry. Molecules. 2019;24(21):3904. doi: 10.3390/molecules24213904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C.H., Chen K.Y., Chou C.Y., Liao H.Y., Chen H.C. New insights on volatile components of Vanilla planifolia cultivated in Taiwan. Molecules. 2021;26(12):3608. doi: 10.3390/molecules26123608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C.H., Chou C.Y., Wu C.S., Chu L.P., Huang W.J., Chen H.C. Effects of different extraction methods on vanilla aroma. Molecules. 2022;27(14):4593. doi: 10.3390/molecules27144593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Mueller C. Comparative analysis of volatiles in traditionally cured Bourbon and Ugandan vanilla bean (Vanilla planifolia) extracts. Journal of Agricultural and Food Chemistry. 2012;60(42):10433–10444. doi: 10.1021/jf302615s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.