Abstract
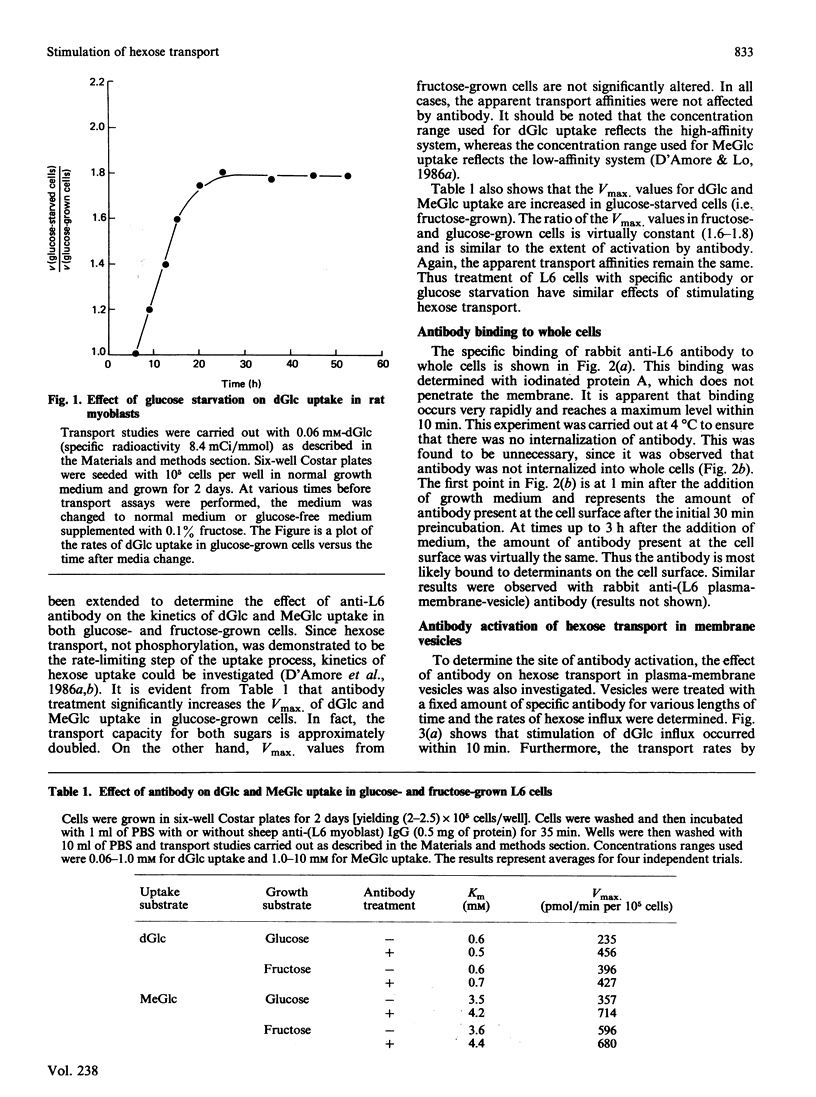
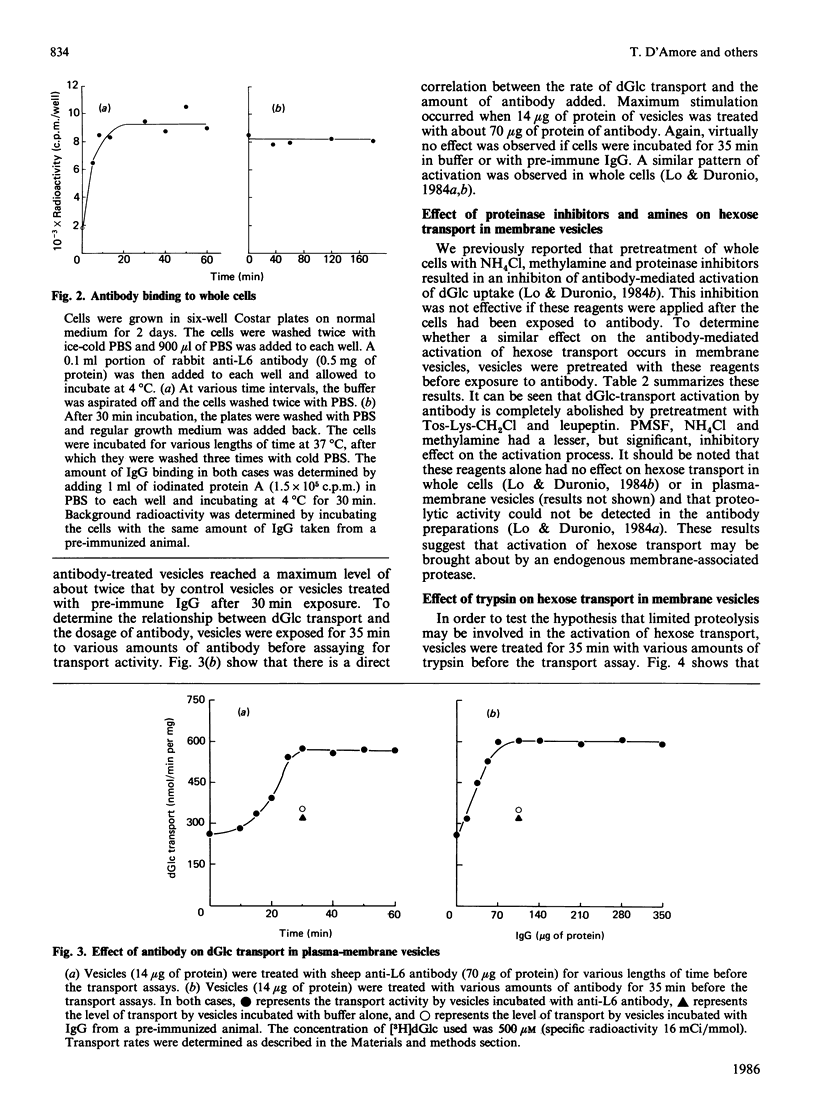
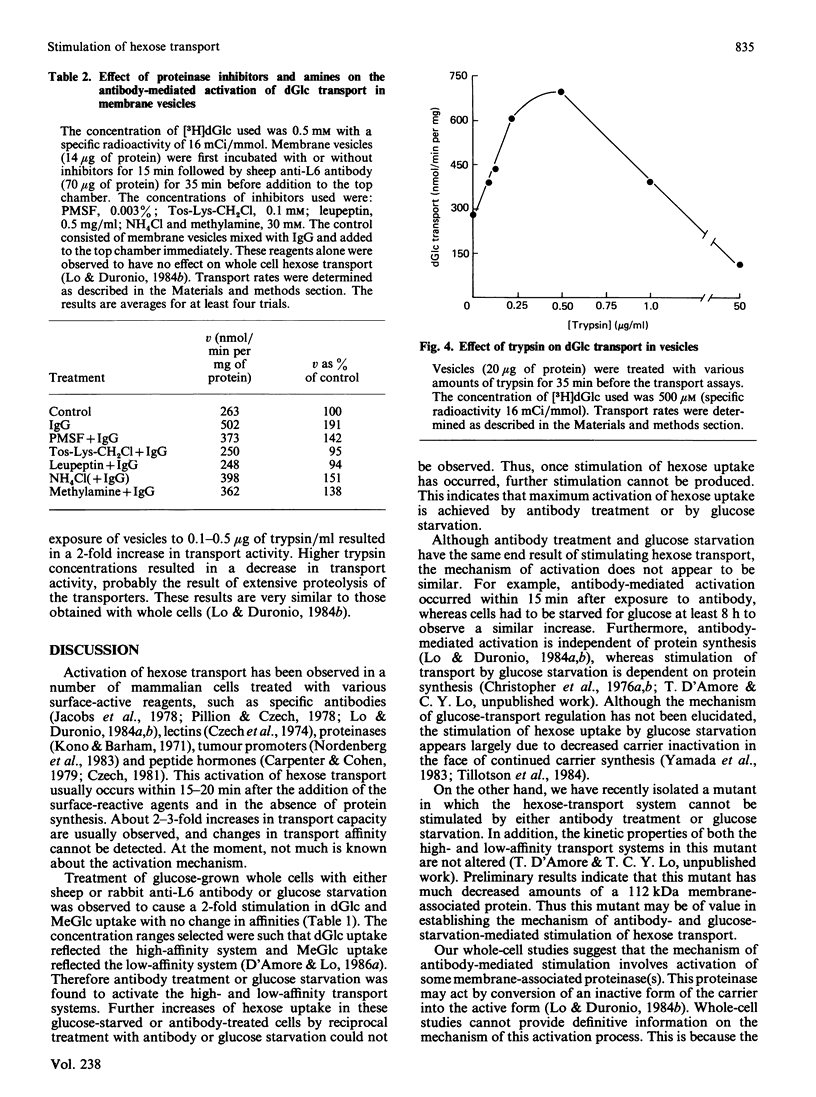
Treatment of glucose-grown L6 rat myoblasts with rabbit or sheep anti-(L6-rat myoblast) antibody for 35 min or glucose starvation for at least 8 h results in a 2-fold increase in the Vmax. of 2-deoxy-D-glucose (dGlc) and 3-O-methyl-D-glucose uptake. In both cases, apparent transport affinities were not affected. Furthermore, once stimulation has occurred, further increases in hexose uptake could not be produced. Assays of antibody binding to whole cells suggested that the antibody is not internalized but remains bound on the cell surface. To elucidate the site and mechanism of antibody action, plasma-membrane vesicles from L6 cells were prepared. Anti-L6 antibody was found to cause a time- and dosage-dependent stimulation of dGlc transport in these vesicles. Maximum activation was achieved after 30 min exposure. This antibody-mediated activation could be inhibited by treatment of vesicles with various proteinase inhibitors. Treatment of vesicles with trypsin was also found to activate dGlc transport to levels observed with antibody. These results are virtually identical with those obtained with whole cells and suggest that antibody-mediated activation of hexose transport results from interaction of antibody with a specific membrane component(s).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Cheung M. O., Lo T. C. Hexose transport in plasma membrane vesicles of rat myoblast L6. Can J Biochem Cell Biol. 1984 Nov;62(11):1217–1227. doi: 10.1139/o84-156. [DOI] [PubMed] [Google Scholar]
- Christopher C. W., Kohlbacher M. S., Amos H. Transport of sugars in chick-embryo fibroblasts. Evidence for a low-affinity system and a high-affinity system for glucose transport. Biochem J. 1976 Aug 15;158(2):439–450. doi: 10.1042/bj1580439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Ullrey D., Colby W., Kalckar M. Paradoxical effects of cycloheximide and cytochalasin B on hamster cell hexose uptake. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2429–2433. doi: 10.1073/pnas.73.7.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Insulin action. Am J Med. 1981 Jan;70(1):142–150. doi: 10.1016/0002-9343(81)90421-6. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Activation of hexose transport by concanavalin A in isolated brown fat cells. Effects of cell surface modification with neuraminidase and trypsin on lectin and insulin action. J Biol Chem. 1974 Dec 10;249(23):7499–7505. [PubMed] [Google Scholar]
- D'Amore T., Duronio V., Cheung M. O., Lo T. C. Isolation and characterization of hexose transport mutants in L6 rat myoblasts. J Cell Physiol. 1986 Jan;126(1):29–36. doi: 10.1002/jcp.1041260105. [DOI] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Hexose transport in L6 rat myoblasts. I. Rate-limiting step, kinetic properties, and evidence for two systems. J Cell Physiol. 1986 Apr;127(1):95–105. doi: 10.1002/jcp.1041270113. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Nicholson M. L., Dulak N. C. Effects of peptide anabolic hormones on growth of myoblasts in culture. Endocrinology. 1977 Jul;101(1):32–41. doi: 10.1210/endo-101-1-32. [DOI] [PubMed] [Google Scholar]
- Gay R. J., Hilf R. Density-dependent and adaptive regulation of glucose transport in primary cell cultures of the R3230AC rat mammary adenocarcinoma. J Cell Physiol. 1980 Feb;102(2):155–174. doi: 10.1002/jcp.1041020207. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Klip A., Logan W. J., Li G. Hexose transport in L6 muscle cells. Kinetic properties and the number of [3H]cytochalasin B binding sites. Biochim Biophys Acta. 1982 May 7;687(2):265–280. doi: 10.1016/0005-2736(82)90555-7. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. Insulin-like effects of trypsin on fat cells. Localization of the metabolic steps and the cellular site affected by the enzyme. J Biol Chem. 1971 Oct 25;246(20):6204–6209. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lo T. C., Duronio V. Activation of hexose transport by antibody. Can J Biochem Cell Biol. 1984 May;62(5):245–254. doi: 10.1139/o84-034. [DOI] [PubMed] [Google Scholar]
- Lo T. C., Duronio V. Mechanism of antibody stimulation of hexose transport in rat myoblasts. Can J Biochem Cell Biol. 1984 May;62(5):255–265. doi: 10.1139/o84-035. [DOI] [PubMed] [Google Scholar]
- Nordenberg J., Stenzel K. H., Novogrodsky A. 12-0-tetradecanoylphorbol-13-acetate and concanavalin A enhance glucose uptake in thymocytes by different mechanisms. J Cell Physiol. 1983 Nov;117(2):183–188. doi: 10.1002/jcp.1041170208. [DOI] [PubMed] [Google Scholar]
- Pillion D. J., Czech M. P. Antibodies against intrinsic adipocyte plasma membrane proteins activate D-glucose transport independent of interaction with insulin binding sites. J Biol Chem. 1978 Jun 10;253(11):3761–3764. [PubMed] [Google Scholar]
- Rapaport E., Christopher C. W., Svihovec S. K., Ullrey D., Kalckar H. M. Selective high metabolic lability of uridine, guanosine and cytosine triphosphates in response to glucose deprivation and refeeding of untransformed and polyoma virus-transformed hamster fibroblasts. J Cell Physiol. 1979 Nov;101(2):229–235. doi: 10.1002/jcp.1041010205. [DOI] [PubMed] [Google Scholar]
- Tillotson L. G., Yamada K., Isselbacher K. J. Regulation of hexose transporters of chicken embryo fibroblasts during glucose starvation. Fed Proc. 1984 May 15;43(8):2262–2264. [PubMed] [Google Scholar]
- Ullrey D., Gammon M. T., Kalckar H. M. Uptake patterns and transport enhancements in cultures of hamster cells deprived of carbohydrates. Arch Biochem Biophys. 1975 Apr;167(2):410–416. doi: 10.1016/0003-9861(75)90481-6. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Tillotson L. G., Isselbacher K. J. Regulation of hexose carriers in chicken embryo fibroblasts. Effect of glucose starvation and role of protein synthesis. J Biol Chem. 1983 Aug 25;258(16):9786–9792. [PubMed] [Google Scholar]


