Abstract
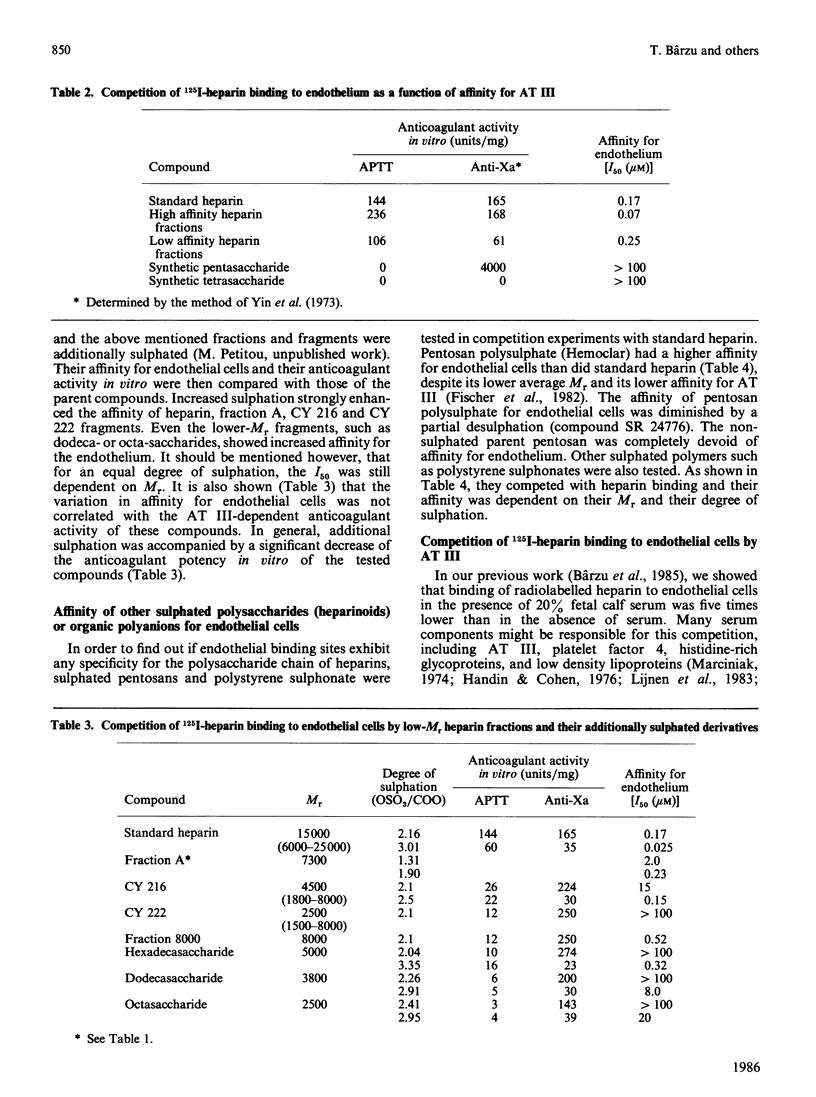
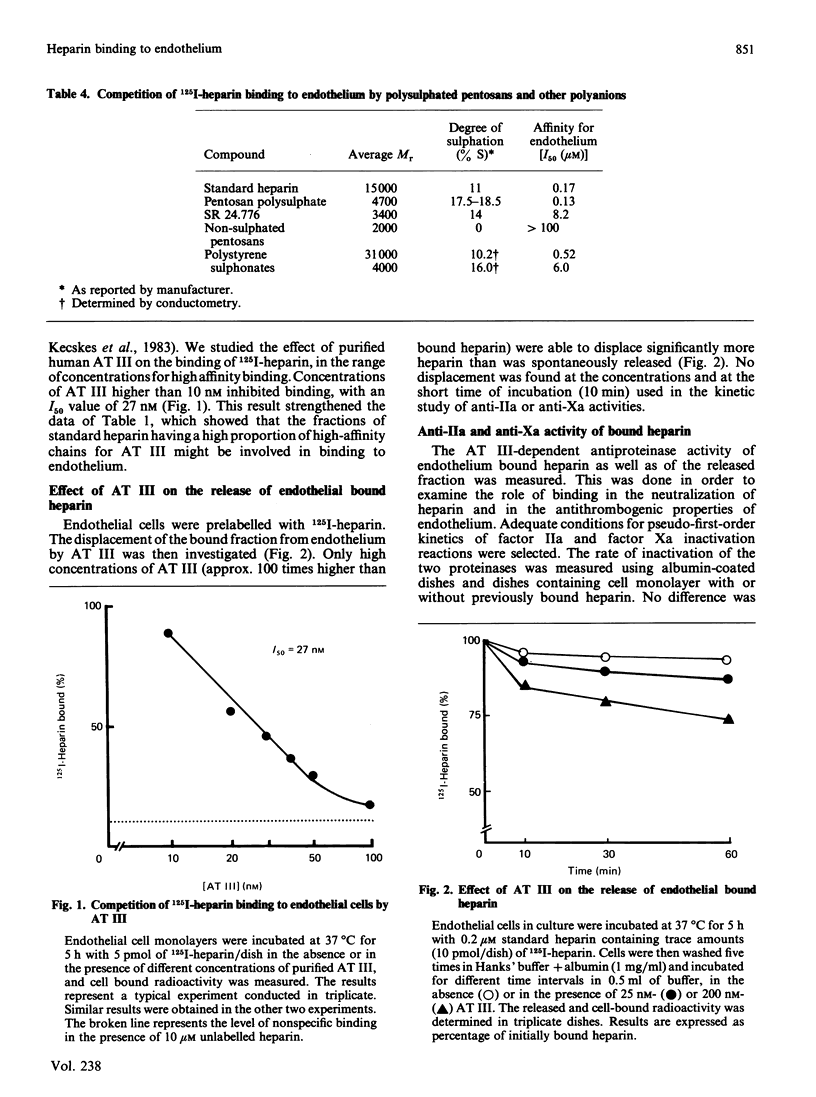
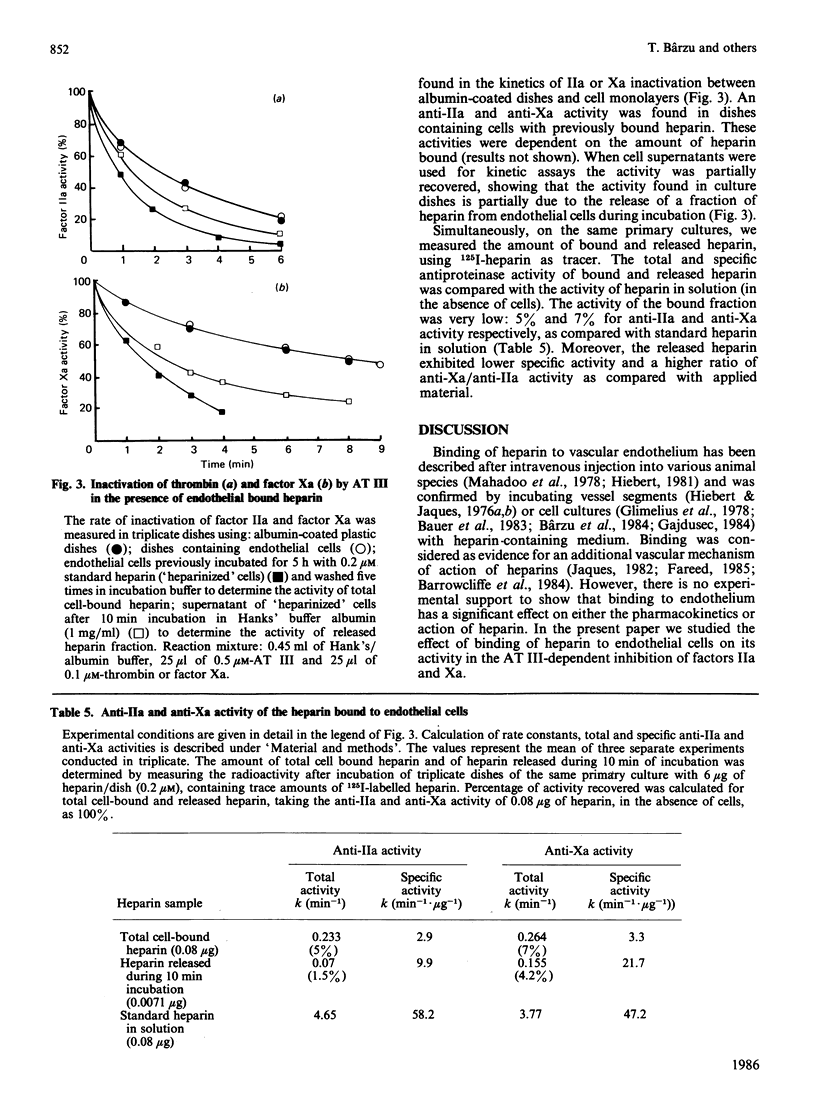
The specificity of endothelial binding sites for heparin was investigated with heparin fractions and fragments differing in their Mr, charge density and affinity for antithrombin III, as well as with heparinoids and other anionic polyelectrolytes (polystyrene sulphonates). The affinity for endothelial cells was estimated by determining I50 values in competition experiments with 125I-heparin. We found that affinity for endothelial cells increases as a function of Mr and charge density (degree of sulphation). Binding sites are not specific receptors for heparin. Other anionic polyelectrolytes, such as pentosan polysulphates and polystyrene sulphonates, competed with heparin for binding to endothelial cells. Fractions of standard heparin with high affinity for antithrombin III also had greater affinity for endothelium. However, these two properties of heparin (affinity for antithrombin III and affinity for endothelial cells) could be dissociated. Oversulphated heparins and oversulphated low-Mr heparin fragments had lower anticoagulant activity and higher affinity for endothelial cells than did their parent compounds. Synthetic pentasaccharides, bearing the minimal sequence for binding to antithrombin III, did not bind to endothelial cells. Binding to endothelial cells involved partial neutralization of heparin. Bound heparin exhibited only 5% and 7% of antifactor IIa and antifactor Xa specific activity, respectively. In the presence of 200 nM-antithrombin III, and in the absence of free heparin, a limited fraction (approx. 30%) of bound heparin was displaced from endothelial cells during a 1 h incubation period. These data suggested that a fraction of surface-bound heparin could represent a pool of anticoagulant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrowcliffe T. W., Merton R. E., Havercroft S. J., Thunberg L., Lindahl U., Thomas D. P. Low-affinity heparin potentiates the action of high-affinity heparin oligosaccharides. Thromb Res. 1984 Apr 15;34(2):125–133. doi: 10.1016/0049-3848(84)90069-0. [DOI] [PubMed] [Google Scholar]
- Bauer P. T., Machovich R., Arányi P., Büki K. G., Csonka E., Horváth I. Mechanism of thrombin binding to endothelial cells. Blood. 1983 Feb;61(2):368–372. [PubMed] [Google Scholar]
- Bleiberg I., MacGregor I., Aronson M. Heparin receptors on mouse macrophages. Thromb Res. 1983 Jan 1;29(1):53–61. doi: 10.1016/0049-3848(83)90125-1. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Quarfoot A. J., Chediak J., Stemerman M. B., Maciag T. Characterization and properties of cultured human von Willebrand umbilical vein endothelial cells. Blood. 1981 Oct;58(4):788–796. [PubMed] [Google Scholar]
- Busch C., Cancilla P. A., DeBault L. E., Goldsmith J. C., Owen W. G. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982 Nov;47(5):498–504. [PubMed] [Google Scholar]
- Busch C., Owen W. G. Identification in vitro of an endothelial cell surface cofactor for antithrombin III. Parallel studies with isolated perfused rat hearts and microcarrier cultures of bovine endothelium. J Clin Invest. 1982 Mar;69(3):726–729. doi: 10.1172/JCI110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bârzu T., Molho P., Tobelem G., Petitou M., Caen J. P. Binding of heparin and low molecular weight heparin fragments to human vascular endothelial cells in culture. Nouv Rev Fr Hematol. 1984;26(4):243–247. [PubMed] [Google Scholar]
- Bârzu T., Molho P., Tobelem G., Petitou M., Caen J. Binding and endocytosis of heparin by human endothelial cells in culture. Biochim Biophys Acta. 1985 May 30;845(2):196–203. doi: 10.1016/0167-4889(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Caranobe C., Barret A., Gabaig A. M., Dupouy D., Sié P., Boneu B. Disappearance of circulating anti-Xa activity after intravenous injection of standard heparin and of a low molecular weight heparin (CY 216) in normal and nephrectomized rabbits. Thromb Res. 1985 Oct 1;40(1):129–133. doi: 10.1016/0049-3848(85)90357-3. [DOI] [PubMed] [Google Scholar]
- Casu B., Gennaro U. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr Res. 1975 Jan;39(1):168–176. doi: 10.1016/s0008-6215(00)82654-3. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- Dawes J., Papper D. S. Catabolism of low-dose heparin in man. Thromb Res. 1979;14(6):845–860. doi: 10.1016/0049-3848(79)90004-5. [DOI] [PubMed] [Google Scholar]
- Fareed J. Development of heparin fractions: some overlooked considerations. Semin Thromb Hemost. 1985 Apr;11(2):227–236. doi: 10.1055/s-2007-1004380. [DOI] [PubMed] [Google Scholar]
- Fischer A. M., Barrowcliffe T. W., Thomas D. P. A comparison of pentosan polysulphate (SP54) and heparin. I: Mechanism of action on blood coagulation. Thromb Haemost. 1982 Apr 30;47(2):104–108. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C. M. Release of endothelial cell-derived growth factor (ECDGF) by heparin. J Cell Physiol. 1984 Oct;121(1):13–21. doi: 10.1002/jcp.1041210104. [DOI] [PubMed] [Google Scholar]
- Glimelius B., Busch C., Hök M. Binding of heparin on the surface of cultured human endothelial cells. Thromb Res. 1978 May;12(5):773–782. doi: 10.1016/0049-3848(78)90271-2. [DOI] [PubMed] [Google Scholar]
- Handin R. I., Cohen H. J. Purification and binding properties of human platelet factor four. J Biol Chem. 1976 Jul 25;251(14):4273–4282. [PubMed] [Google Scholar]
- Hatton M. W., Rollason G., Sefton M. V. Fate of thrombin and thrombin-antithrombin-III complex adsorbed to a heparinized biomaterial: analysis of the enzyme-inhibitor complexes displaced by plasma. Thromb Haemost. 1983 Dec 30;50(4):873–877. [PubMed] [Google Scholar]
- Heuck C. C., Schiele U., Horn D., Fronda D., Ritz E. The role of surface charge on the accelerating action of heparin on the antithrombin III-inhibited activity of alpha-thrombin. J Biol Chem. 1985 Apr 25;260(8):4598–4603. [PubMed] [Google Scholar]
- Hiebert L. M., Jaques L. B. The observation of heparin on endothelium after injection. Thromb Res. 1976 Feb;8(2):195–204. doi: 10.1016/0049-3848(76)90262-0. [DOI] [PubMed] [Google Scholar]
- Hiebert L. The uptake of heparin by liver sinusoidal cells in normal and atherosclerotic rabbits. 1981 Feb 15-Mar 1Thromb Res. 21(4-5):383–390. doi: 10.1016/0049-3848(81)90139-0. [DOI] [PubMed] [Google Scholar]
- Hopwood J., Hök M., Linker A., Lindahl U. Anticoagulant activity of heparin: isolation of antithrombin-binding sites. FEBS Lett. 1976 Oct 15;69(1):51–54. doi: 10.1016/0014-5793(76)80651-5. [DOI] [PubMed] [Google Scholar]
- Hurst R. E., Poon M. C., Griffith M. J. Structure-activity relationships of heparin. Independence of heparin charge density and antithrombin-binding domains in thrombin inhibition by antithrombin and heparin cofactor II. J Clin Invest. 1983 Sep;72(3):1042–1045. doi: 10.1172/JCI111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques L. B. Heparins--anionic polyelectrolyte drugs. Pharmacol Rev. 1979 Jun;31(2):99–166. [PubMed] [Google Scholar]
- Kecskés E., Büki K. G., Bauer P. I., Machovich R., Horváth I. Interaction of heparin with lipoproteins - role of the complex in the inactivation of thrombin and plasmin. Thromb Haemost. 1983 Apr 28;49(2):138–141. [PubMed] [Google Scholar]
- Lane D. A., Denton J., Flynn A. M., Thunberg L., Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725–732. doi: 10.1042/bj2180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson R., Olsson P., Lindahl U. Inhibition of thrombin on surfaces coated with immobilized heparin and heparin-like polysaccharides: a crucial non-thrombogenic principle. Thromb Res. 1980 Jul 1;19(1-2):43–54. doi: 10.1016/0049-3848(80)90402-8. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Heparin binding properties of human histidine-rich glycoprotein. Mechanism and role in the neutralization of heparin in plasma. J Biol Chem. 1983 Mar 25;258(6):3803–3808. [PubMed] [Google Scholar]
- Mahadoo J., Heibert L., Jaques L. B. Vascular sequestration of heparin. Thromb Res. 1978 Jan;12(1):79–90. doi: 10.1016/0049-3848(78)90087-7. [DOI] [PubMed] [Google Scholar]
- Marciniak E. Binding of heparin in vitro and in vivo to plasma proteins. J Lab Clin Med. 1974 Sep;84(3):344–356. [PubMed] [Google Scholar]
- Marcum J. A., McKenney J. B., Rosenberg R. D. Acceleration of thrombin-antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984 Aug;74(2):341–350. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Merton R. E., Thomas D. P., Havercroft S. J., Barrowcliffe T. W., Lindahl U. High and low affinity heparin compared with unfractionated heparin as antithrombotic drugs. Thromb Haemost. 1984 Apr 30;51(2):254–256. [PubMed] [Google Scholar]
- Naqui A. What does I50 mean? Biochem J. 1983 Nov 1;215(2):429–430. doi: 10.1042/bj2150429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogamo A., Nagai A., Nagasawa K. Binding of heparin fractions and other polysulfated polysaccharides to plasma fibronectin: effects of molecular size and degree of sulfation of polysaccharides. Biochim Biophys Acta. 1985 Jul 26;841(1):30–41. [PubMed] [Google Scholar]
- Pletcher C. H., Nelsestuen G. L. The rate-determining step of the heparin-catalyzed antithrombin/thrombin reaction is independent of thrombin. J Biol Chem. 1982 May 25;257(10):5342–5345. [PubMed] [Google Scholar]
- Rodgers G. M., Greenberg C. S., Shuman M. A. Characterization of the effects of cultured vascular cells on the activation of blood coagulation. Blood. 1983 Jun;61(6):1155–1162. [PubMed] [Google Scholar]
- Rosenberg R. D., Rosenberg J. S. Natural anticoagulant mechanisms. J Clin Invest. 1984 Jul;74(1):1–6. doi: 10.1172/JCI111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J., Morad N., Shen W. C. Heparin interaction with cultured cells: possible role of fibronectin in uncoupling surface binding and endocytosis. Cell Biol Int Rep. 1983 Nov;7(11):923–930. doi: 10.1016/0309-1651(83)90211-4. [DOI] [PubMed] [Google Scholar]
- Sache E., Maillard M., Bertrand H., Maman M., Kunz M., Choay J., Fareed J., Messmore H. Studies on a highly active anticoagulant fraction of high molecular weight isolated from porcine sodium heparin. Thromb Res. 1982 Mar 15;25(6):443–458. doi: 10.1016/0049-3848(82)90086-x. [DOI] [PubMed] [Google Scholar]
- Stern D., Nawroth P., Marcum J., Handley D., Kisiel W., Rosenberg R., Stern K. Interaction of antithrombin III with bovine aortic segments. Role of heparin in binding and enhanced anticoagulant activity. J Clin Invest. 1985 Jan;75(1):272–279. doi: 10.1172/JCI111685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler E., Schmer G. A simple two-step isolation procedure for human and bovine antithrombin II/III (heparin cofactor): a comparison of two methods. Br J Haematol. 1975 Oct;31(2):233–243. doi: 10.1111/j.1365-2141.1975.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Thomas D. P. Heparin, low molecular weight heparin, and heparin analogues. Br J Haematol. 1984 Nov;58(3):385–390. doi: 10.1111/j.1365-2141.1984.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Vairel E. G., Bouty-Boye H., Toulemonde F., Doutremepuich C., Marsh N. A., Gaffney P. J. Heparin and a low molecular weight fraction enhances thrombolysis and by this pathway exercises a protective effect against thrombosis. Thromb Res. 1983 May 1;30(3):219–224. doi: 10.1016/0049-3848(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Wessler S., Butler J. V. Plasma heparin: a unique, practical, submicrogram-sensitive assay. J Lab Clin Med. 1973 Feb;81(2):298–310. [PubMed] [Google Scholar]


