Abstract
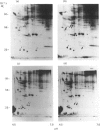
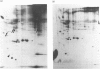
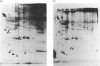
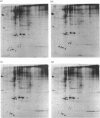
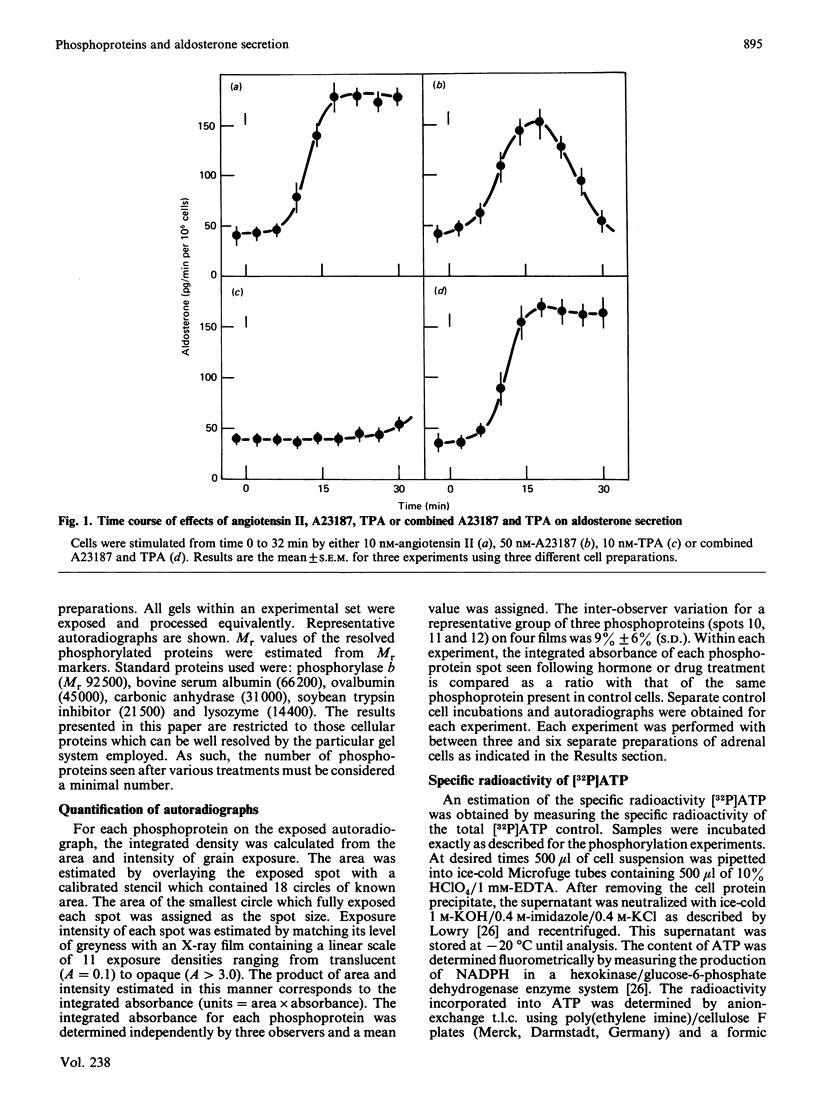
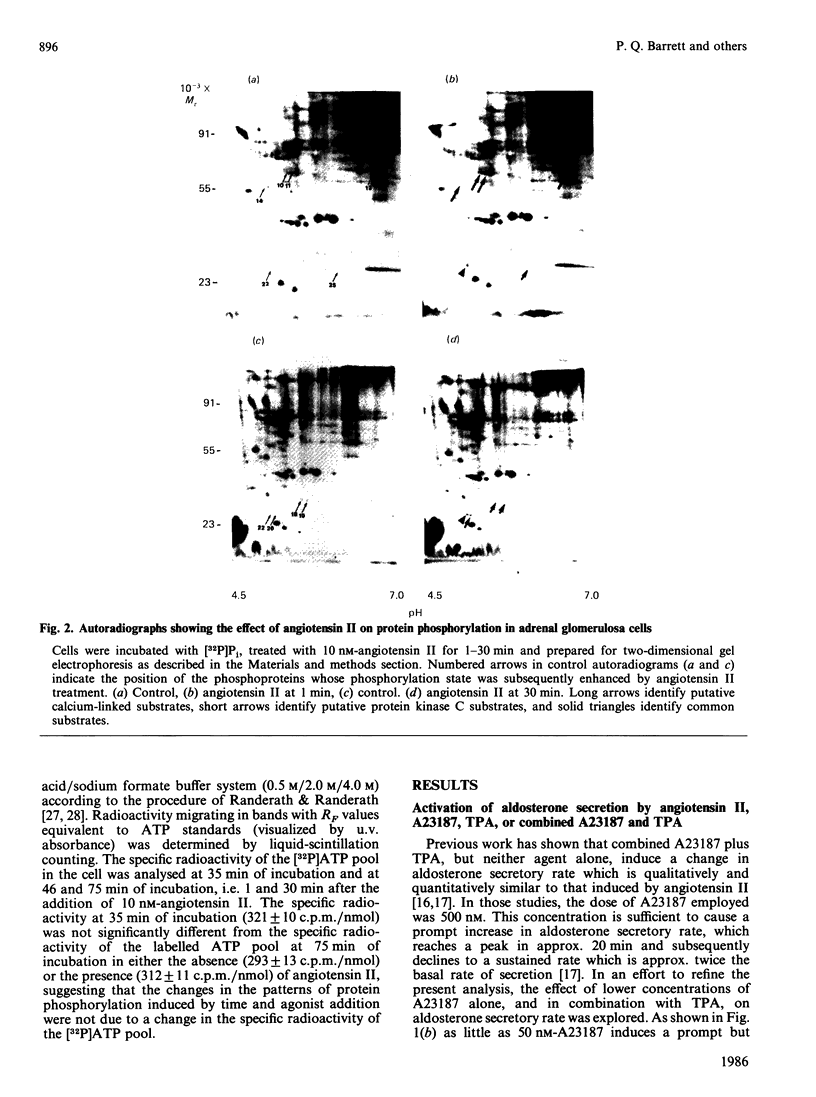
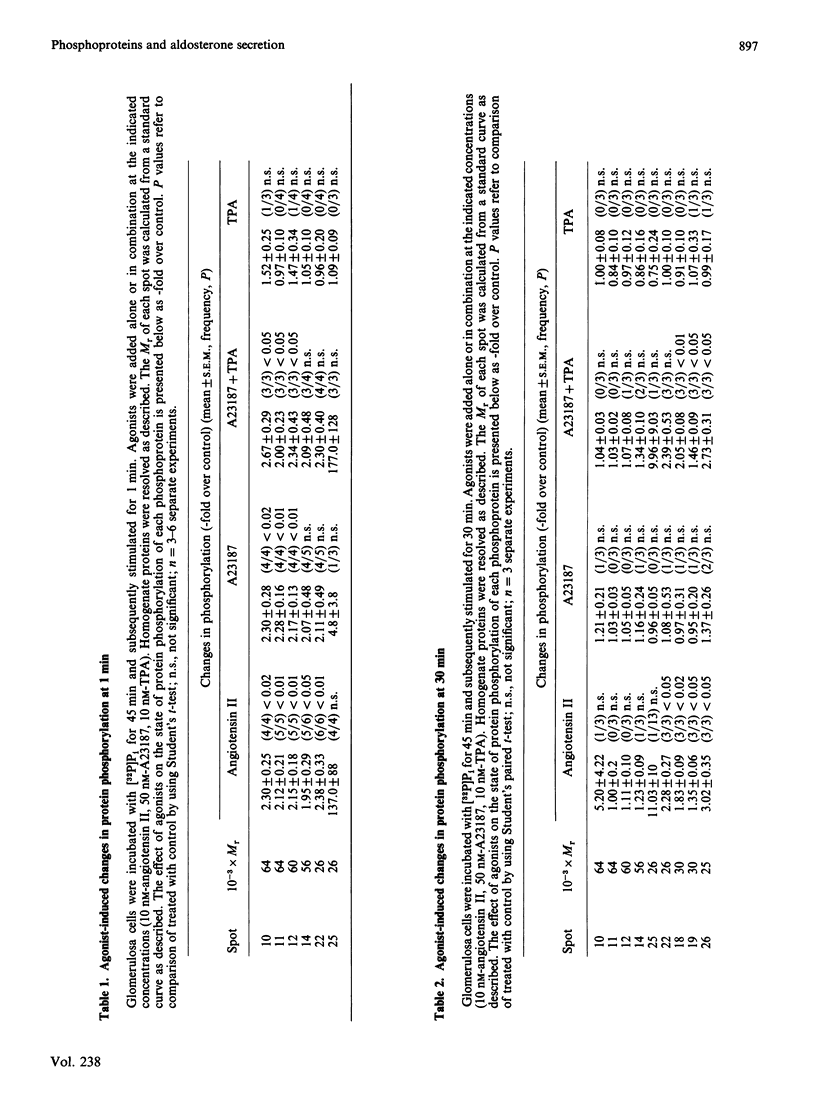
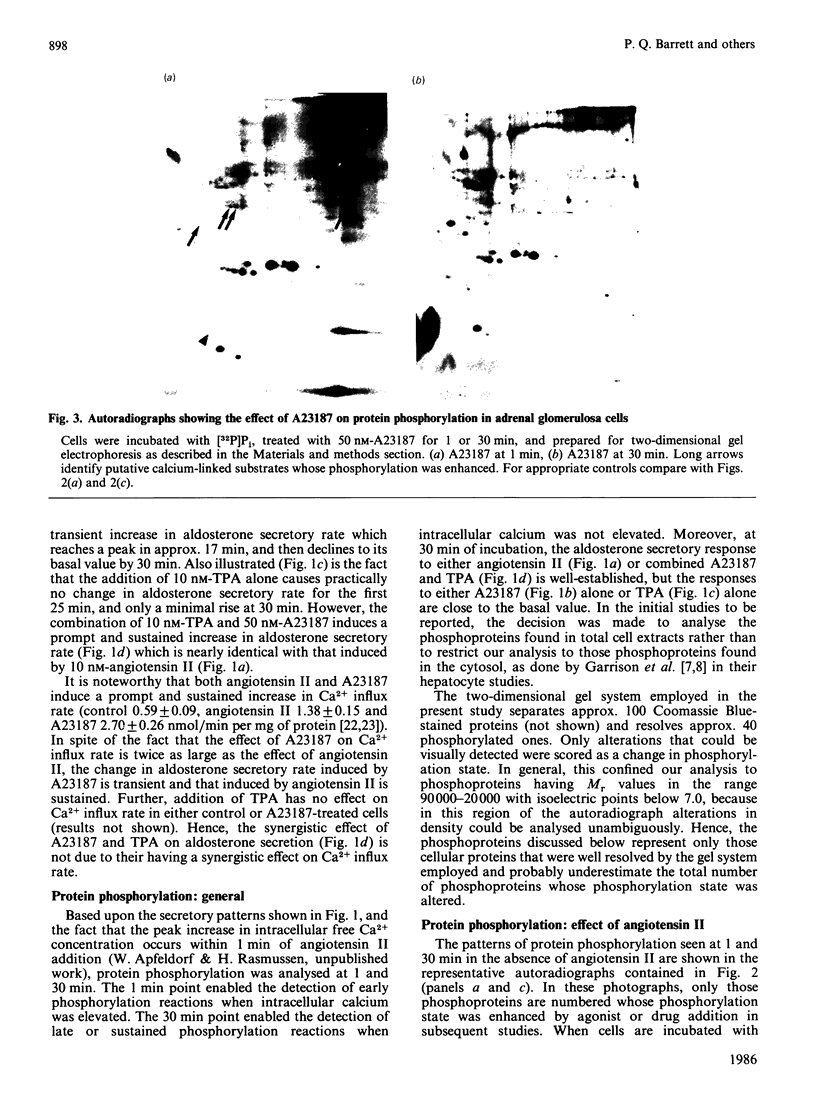
The temporal patterns of protein phosphorylation in the adrenal glomerulosa cell were analysed by two-dimensional electrophoresis after stimulation with 10 nM-angiotensin II or various agents [10 nM-12-O-tetradecanoylphorbol 13-acetate (TPA), 50 nM-A23187, 1 microM-nitrendipine], administered singly or in combination. These patterns were compared with the temporal patterns of aldosterone secretion induced by the same agonists and antagonists. After 1 and 30 min of stimulation with angiotensin II, different patterns of protein phosphorylation were observed. A comparison of these patterns reveals that: the phosphorylation of only one protein was persistently enhanced during the continuous incubation with angiotensin II; the phosphorylation of five proteins was transiently enhanced (at 1 min but not 30 min); and the phosphorylation of three proteins did not occur at 1 min but was seen at 30 min. Addition of the phorbol ester TPA alone, which at 30 min is without effect in enhancing aldosterone production, has no effect on protein phosphorylation. The combined addition of TPA and the Ca2+ ionophore, A23187, which, like angiotensin II, evokes a sustained increase in aldosterone production, reproduced the temporal patterns of protein phosphorylation seen after angiotensin II action. Manipulations (A23187 alone, angiotensin II plus nitrendipine) which evoke only a transient rise in aldosterone production rate induce a transient rise in cellular protein phosphorylation. The 1 min patterns of phosphorylation seen after A23187 or combined angiotensin II and nitrendipine (a Ca2+ channel antagonist) are similar to those observed after 1 min of angiotensin II stimulation. These results suggest that, when angiotensin II acts, the initial cellular response is mediated by a different mechanism than that responsible for the sustained response.
Full text
PDF

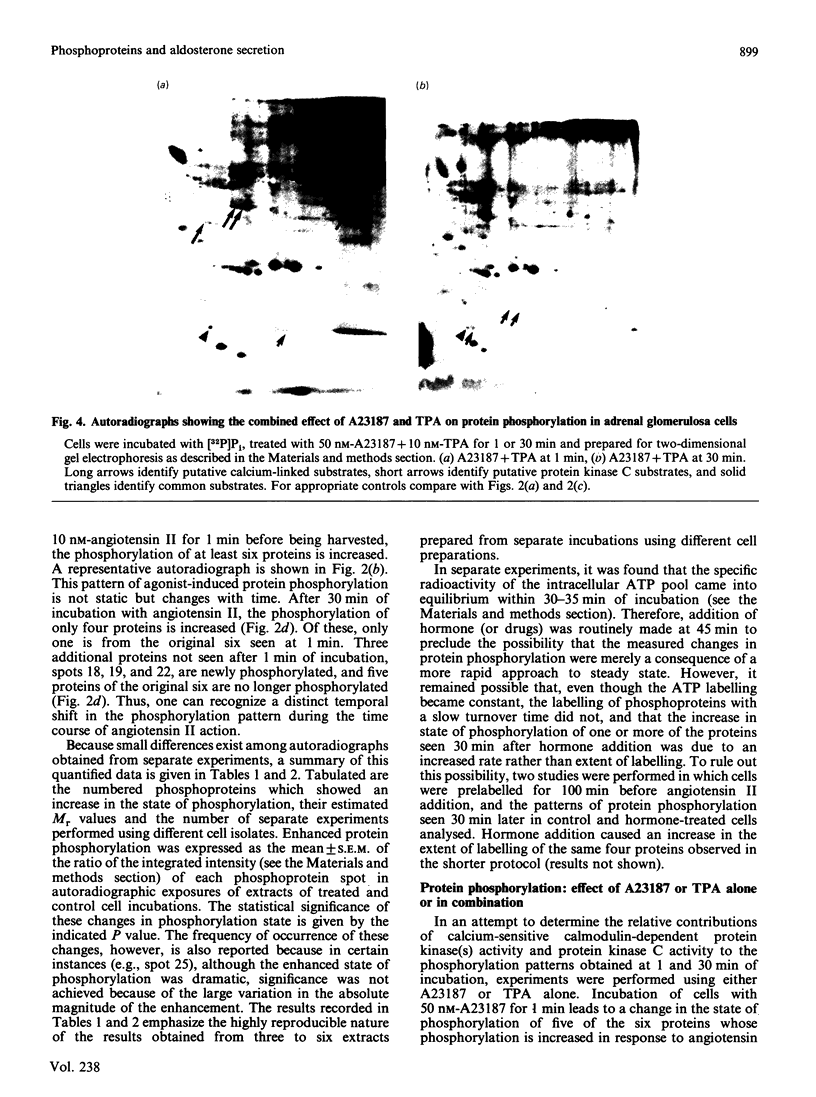
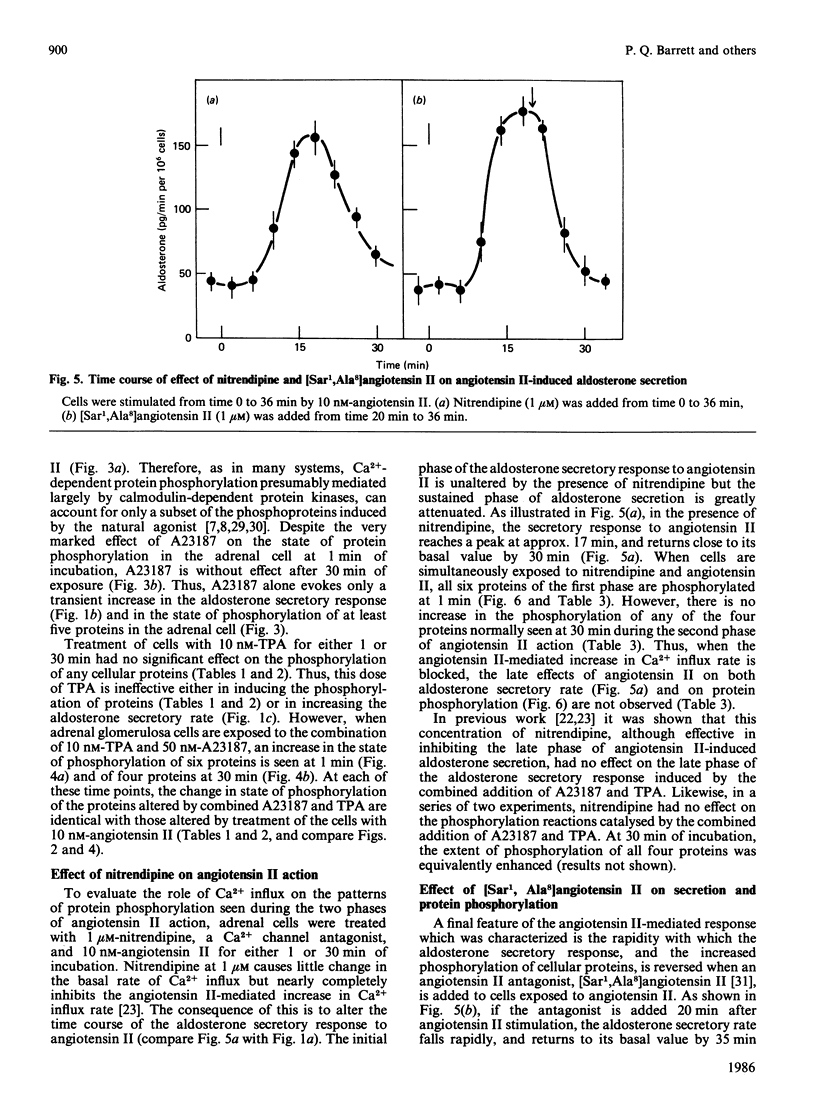
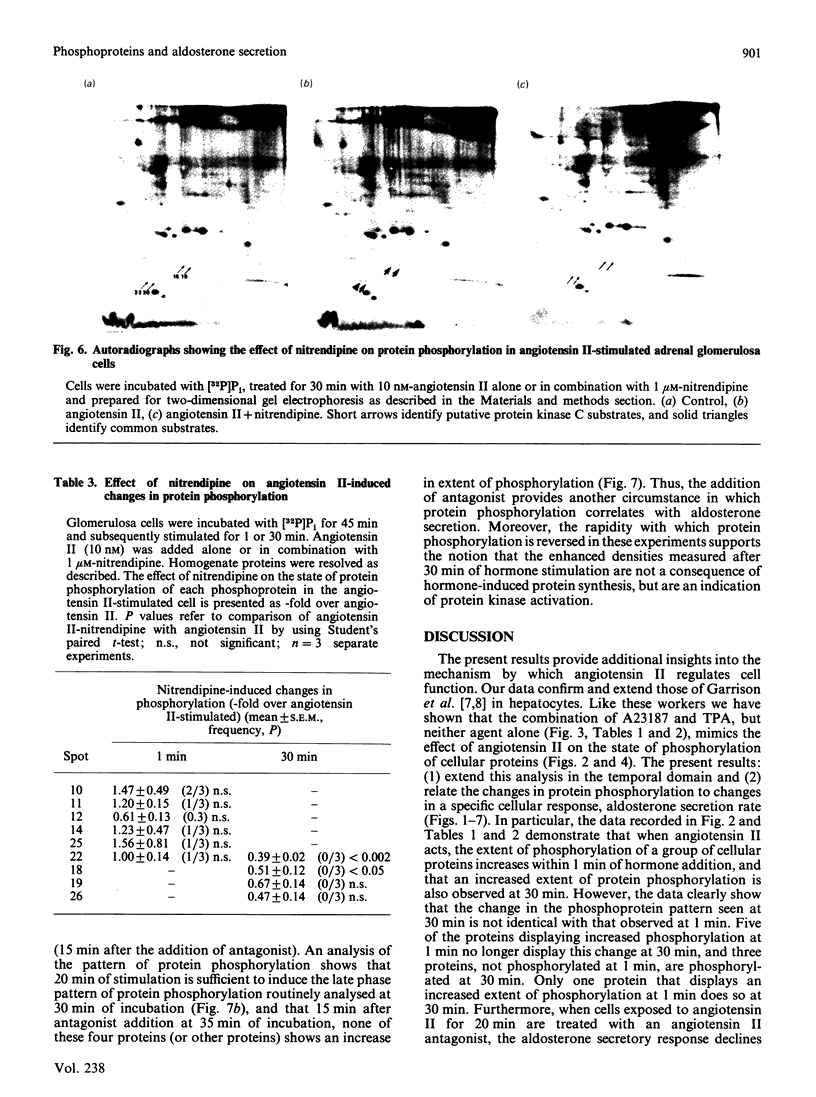
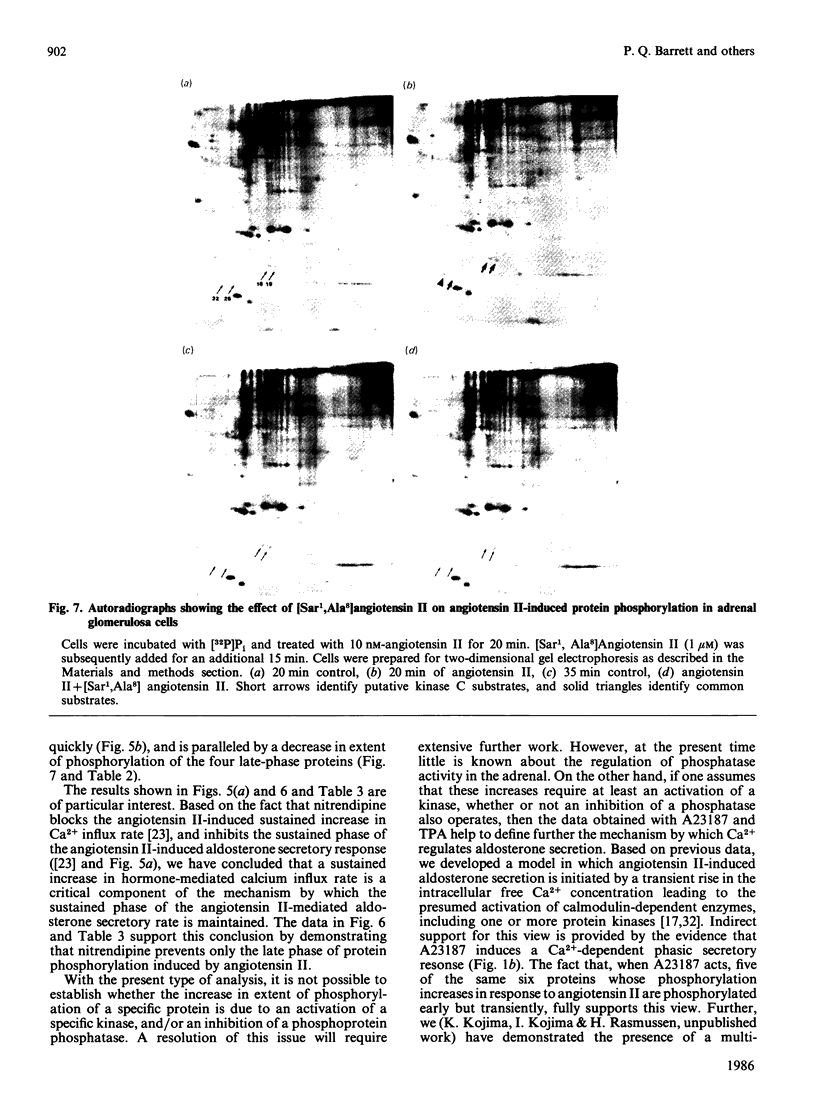

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Catt K. J. Loci of action of regulators of aldosterone biosynthesis in isolated glomerulosa cells. Endocrinology. 1979 Apr;104(4):1046–1052. doi: 10.1210/endo-104-4-1046. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Thyrotropin-releasing hormone rapidly activates protein phosphorylation in GH3 pituitary cells by a lipid-linked, protein kinase C-mediated pathway. J Biol Chem. 1984 Dec 10;259(23):14520–14530. [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Thyrotropin-releasing hormone rapidly and transiently stimulates cytosolic calcium-dependent protein phosphorylation in GH3 pituitary cells. J Biol Chem. 1982 Jul 10;257(13):7566–7573. [PubMed] [Google Scholar]
- Fakunding J. L., Catt K. J. Dependence of aldosterone stimulation in adrenal glomerulosa cells on calcium uptake: effects of lanthanum nd verapamil. Endocrinology. 1980 Nov;107(5):1345–1353. doi: 10.1210/endo-107-5-1345. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Chow R., Catt K. J. The role of calcium in the stimulation of aldosterone production by adrenocorticotropin, angiotensin II, and potassium in isolated glomerulosa cells. Endocrinology. 1979 Aug;105(2):327–333. doi: 10.1210/endo-105-2-327. [DOI] [PubMed] [Google Scholar]
- Foster R., Lobo M. V., Rasmussen H., Marusic E. T. Calcium: its role in the mechanism of action of angiotensin II and potassium in aldosterone production. Endocrinology. 1981 Dec;109(6):2196–2201. doi: 10.1210/endo-109-6-2196. [DOI] [PubMed] [Google Scholar]
- Fraser R., Brown J. J., Lever A. F., Mason P. A., Robertson J. I. Control of aldosterone secretion. Clin Sci (Lond) 1979 May;56(5):389–399. doi: 10.1042/cs0560389. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K., Florio V. A., Twible D. A. The role of calcium ion as a mediator of the effects of angiotensin II, catecholamines, and vasopressin on the phosphorylation and activity of enzymes in isolated hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7147–7156. [PubMed] [Google Scholar]
- Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J Biol Chem. 1984 Mar 10;259(5):3283–3292. [PubMed] [Google Scholar]
- Garrison J. C., Wagner J. D. Glucagon and the Ca2+-linked hormones angiotensin II, norepinephrine, and vasopressin stimulate the phosphorylation of distinct substrates in intact hepatocytes. J Biol Chem. 1982 Nov 10;257(21):13135–13143. [PubMed] [Google Scholar]
- Irita K., Takeshige K., Minakami S. Protein phosphorylation in intact pig leukocytes. Biochim Biophys Acta. 1984 Sep 14;805(1):44–52. doi: 10.1016/0167-4889(84)90035-1. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. The origin, quantitation, and kinetics of intracellular calcium mobilization by vasopressin and phenylephrine in hepatocytes. J Biol Chem. 1983 Sep 10;258(17):10425–10432. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Effects of ANG II and K+ on Ca efflux and aldosterone production in adrenal glomerulosa cells. Am J Physiol. 1985 Jan;248(1 Pt 1):E36–E43. doi: 10.1152/ajpendo.1985.248.1.E36. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Intracellular calcium and adenosine 3',5'-cyclic monophosphate as mediators of potassium-induced aldosterone secretion. Biochem J. 1985 May 15;228(1):69–76. doi: 10.1042/bj2280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Kojima K., Kojima I., Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984 Nov;247(5 Pt 1):E645–E650. doi: 10.1152/ajpendo.1984.247.5.E645. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Lis M., Gutkowska J., Genest J. Role of Ca2+ in response of adrenal glomerulosa cells to angiotensin II, ACTH, K+, and ouabain. Am J Physiol. 1981 Jul;241(1):E42–E46. doi: 10.1152/ajpendo.1981.241.1.E42. [DOI] [PubMed] [Google Scholar]
- Sobel A., Tashjian A. H., Jr Distinct patterns of cytoplasmic protein phosphorylation related to regulation of synthesis and release of prolactin by GH cells. J Biol Chem. 1983 Sep 10;258(17):10312–10324. [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]
- Williams B. C., McDougall J. G., Tait J. F., Tait S. A. Calcium efflux and steroid output from superfused rat adrenal cells: effects of potassium, adrenocorticotropic hormone, 5-hydroxytryptamine, adenosine 3':5'-cyclic monophosphate and angiotensins II and III. Clin Sci (Lond) 1981 Nov;61(5):541–551. doi: 10.1042/cs0610541. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]