Abstract
Objectives
Struma ovarii (SO) accounts for approximately 1% of all ovarian tumors. The objective of our study is to contribute to the treatment algorithm by presenting our clinical experience in a comprehensive case series of patients diagnosed with SO, predominantly characterized by thyroid tissue within a monodermal teratoma.
Methods
Patients aged 17 years and older who underwent surgery due to ovarian masses and were histopathologically diagnosed with SO between January 2012 and January 2022 were included in the study. The patients' files were retrospectively reviewed. Demographic data, presenting complaints, radiological findings, tumor sizes, laboratory data, surgical procedures performed, pathology reports, additional treatments, and follow-up information were recorded.
Results
The median age of total 19 patients was 41.7 (17-74) years. Among them, malignant struma ovarii was present in 3 patients. In patients with histopathologically confirmed benign struma ovarii, no additional treatment was administered after tumor enucleation. In malignant cases, in addition to unilateral salpingo-oophorectomy, total thyroidectomy, radioactive iodine (RAI) ablation, and L-Thyroxine suppression were performed. No mortality occurred during the follow-up period.
Conclusion
Although conservative treatments are considered acceptable treatment in cases of benign struma ovarii, the management of cases with malignant struma ovarii is controversial. Fertility-sparing surgery followed by postoperative adjuvant thyroidectomy and radioactive iodine ablation may be preferred for young women.
Keywords: Ovarian cancer, ovarian disease, ovarian struma
Struma ovarii (SO) is a monodermal teratoma which arise from the first meiotic division of a single germ cell. It constitutes approximately 2-3% of all ovarian tumors.[1] There are four types of carcinoma that can contain thyroid tissue in the ovary: SO, strumal carcinoid, metastases from primary thyroid cancer, or tumors of uncertain origin. To classify a teratoma as SO, it must contain more than 50% thyroid tissue.[2] Histopathologically, these lesions can be either benign or malignant. The likelihood of malignancy varies between 0.5% and 10%, with the presence of metastasis reported in approximately 5-23% of malignant cases.[3]
The absence of distinct symptomatic features and the lack of a specific radiological appearance make preoperative diagnosis of SO cases challenging.[4] Diagnosis can be established through histopathological examination of the surgically excised mass. Clinically, patients may present with pelvic mass, lower abdominal pain, menstrual irregularities, and abdominal distension (ascites). A condition characterized by ascites and pleural effusion, known as Pseudo-Meigs’ syndrome, is observed in rare cases. High thyroglobulin (Tg) levels may be seen in some cases. Hyperthyroidism occurs in only 5%–8% of cases.[5,6] While elevated levels of the tumor marker CA 125 are observed in some cases, they do not constitute a specific indicator.[7]
Due to the rarity of SO, there still exist variations in treatment guidelines. In benign cases, tumor enucleation or unilateral oophorectomy is sufficient. Surgical treatment approaches for malignant cases remain a subject of debate.[8] The aim of this study is to contribute to the standardization of treatment approaches by presenting a decade-long experience at our center.
Methods
Patients aged 17 and above, who underwent surgery due to ovarian masses and were histopathologically diagnosed with SO, between January 2012 and January 2022 at Baskent University, were included in this study. Patient records were retrospectively reviewed to document demographic information, presenting complaints, radiological findings, laboratory data, surgical procedures performed, pathology reports, additional treatments administered, and follow-up information. Results were analyzed through a review of the literature.
This study was approved by the Baskent University Non-interventional Clinical Research Ethics Committee (Number: 23/110, Date: 21.06.2023), was designed in accordance with the principles of the Declaration of Helsinki, and was supported by the Baskent University Research Fund with project number KA23/215.
Results
Nineteen patients with a histopathological diagnosis of SO were included in the study. The average age of the patients was 41.7 (17-74) years. The most common presenting complaint among the patients was abdominal pain. The majority of patients had tumor diameters between 5-10 cm (52.6%). Laboratory data indicated that thyroid stimulating hormone (TSH) results were within normal limits, while CA 125 levels were elevated in 3 patients (15.7%).
In the treatment of benign cases, tumor enucleation was the preferred approach in the majority (52.6%) of cases. There were no patients with a preoperative diagnosis of struma ovarii. All diagnoses were made by histopathological examination. Three patients (15.7%) were diagnosed with malign struma ovarii (MSO). Since these patients were between the ages of 31 and 41, unilateral salpingo-oophorectomy (USO) was chosen to preserve fertility. Papillary carcinoma was detected in two patients, and mixed papillary and anaplastic carcinoma was detected in one patient. During the follow-up of the patient, who had mixed papillary and anaplastic cancer components, metastatic lesions were observed in the vertebra in the 3rd year and in the opposite adnexal region in the 4th year. Surgery following vertebral-directed radiotherapy included hysterectomy, contralateral salpingo-oophorectomy, omentectomy, and pelvic lymph node dissection (PLND) in the adnexal region as additional components of treatment. Patients diagnosed with MSO received treatment consisting of total thyroidectomy, radioactive iodine ablation (RAI), and TSH suppression using L-thyroxine. The follow-up period of the patients varied between 14-112 months. The average follow-up period was 56 months. There was no recurrence or mortality recorded during the follow-up period (Table 1).
Table 1.
Demographic data of patients
| Patient | Age (Year) | Complaint | Size (cm) | Tumor Marker | Pathology | Tumor Type |
|---|---|---|---|---|---|---|
| 1 | 37 | AP | 6 | N | B | - |
| 2 | 41 | AD | 10 | N | B | - |
| 3 | 20 | PM | 8 | N | B | - |
| 4 | 34 | AP | 10 | N | B | - |
| 5 | 70 | AP | 8 | N | B | - |
| 6 | 23 | AP | 8 | N | B | - |
| 7 | 61 | I | 4 | N | B | - |
| 8 | 58 | AP | 16 | N | B | - |
| 9 | 63 | AP | 15 | CA125↑ | B | - |
| 10 | 74 | I | 8 | N | B | - |
| 11 | 34 | I | 14 | CA125↑ | M | PA |
| 12 | 61 | I | 5 | CA125↑ | B | - |
| 13 | 35 | I | 4 | N | B | - |
| 14 | 31 | PM | 13 | N | M | P |
| 15 | 38 | PM | 8 | N | B | - |
| 16 | 41 | AP | 5 | N | M | P |
| 17 | 20 | PM | 15 | N | B | - |
| 18 | 17 | AP | 5 | N | B | - |
| 19 | 35 | AP | 14 | N | B | - |
AP: Abdominal pain; B: Benign; I: Incidental; M: Malignant; MI: Menstureal irregularity; N: Normal; PM: Palpable mass; P: Papillary cancer; PA: Papillary + anaplastic cancer.
Discussion
This study presents a large number of cases, including 19 patients diagnosed with struma ovarii in the last decade and treated in a single center. Despite being an ovarian tumor, the treatment of SO requires a multidisciplinary approach involving gynecology, endocrinology, endocrine surgery, nuclear medicine, and medical oncology clinics, given its inclusion of thyroid tissue. Differences in treatment approaches, especially in cases of malignant struma ovarii, increase interest in the subject. In the literature review by Leustean et al.[9] in 2021, less than 200 cases of struma ovarii were found. Therefore, the results of our study may contribute significantly to the development of follow-up and treatment guidelines. Furthermore, our study has shed light on the variability in the disease course of MSO, depending on the tumor components, which is another important finding.
SO is most commonly observed in individuals between the ages of 31 and 50, and the peak incidence occurs in women aged 40 and above; however, there have also been reported cases in the pediatric age group. Many cases of SO are asymptomatic. Various studies have indicated that the most frequently reported symptoms include lower abdominal pain, the presence of an abdominal mass, and vaginal bleeding.[10,11] Although the average age in our cases was 41.7 (17-74) years, we had 4 patients under the age of 30 and our youngest patient was 17 years old. The high size of the lesions in these patients also suggested the possibility of late diagnosis. The most common complaint was abdominal pain, consistent with the literature.
The characteristic feature of SO on ultrasonography, which is considered the most effective radiological method for detecting ovarian masses, is the presence of a smooth-edged, solid tissue mass often referred to as a "struma pearl," with vascularity on Doppler imaging.[12] Furthermore, SO appears as a multiloculated cystic mass on diffusion-weighted Magnetic Resonance Imaging (MRI). Nevertheless, the radiological diagnosis often remains unclear. In our study, we did not observe any patients with radiologically specific findings that conclusively confirmed the diagnosis. None of the patients displayed the typical "struma pearl" appearance on ultrasonography. According to the literature, SO masses can vary in size, ranging from 4 to 25 cm. It is worth noting that masses larger than 4 cm have a higher potential for malignancy.[8,11] In our patient group, all but two had a mass size exceeding 5 cm. In malignant patients, the size was over 5 cm in all patients and the average size was 9.3 cm (5 to 13 cm). It was compatible with the literature.
Cancer antigen 125 (CA125) is a high molecular weight mucinous glycoprotein overexpressed on the membrane of epithelial ovarian cancer (EOC) cells. It has clinical relevance as a standard-of-care serum biomarker for ovarian cancer surveillance despite expression in some non-gynecologic malignancies and benign conditions such as pregnancy, menstruation, endometriosis, liver disease, and congestive heart failure. Post-treatment elevation of serum CA125 level in EOC patients serves as an indicator of progressive disease and finds clinical application in the management of patients with documented evidence of ovarian cancer.[13] Patients diagnosed with SO do not have a specific tumor marker. Limited studies have shown elevated CA125 levels in some cases.[14] There is ongoing debate regarding the cause of elevated CA125 levels in SO patients. Although rare, cases of SO with Pseudo-Meigs’ syndrome findings (ovarian mass, ascites, pleural effusion) and elevated CA125 have been reported.[15-20] These are cases reported in the literature as a limited number of case reports. (Table 2). It is believed that CA125 expression originates more from mesothelial cells rather than the tumor itself. Both tumor-related and ascitic fluid-induced inflammatory responses increase CA125 expression from mesothelial cells. Despite this effect of ascites on serum CA125 level, a parallel relationship has not been clearly demonstrated.[21] In our study, CA125 levels were high in only 3 of 19 patients (15.7%), and these levels were lower than other examples in the literature. While one of these patients was pregnant, the other two patients had moderate ascites in the abdomen along with an ovarian mass. The increase in CA125 may be attributed to the inflammatory process caused by ascites in these cases.
Table 2.
Struma ovarii associated with Pseudo-Meigs' syndrome and elevated CA125 level: reported cases
| Author | Number of patients | Age (years) | CA 125 (U/mL) | Treatments |
|---|---|---|---|---|
| Huh et al.[15] | 1 | 65 | 402 | TAH+BSO |
| Mitrou et al.[16] | 1 | 58 | 1028 | TAH+BSO |
| Obeidat and Amarin[17] | 1 | 42 | 176 | TAH+BSO |
| Rana et al.[18] | 1 | 70 | 284 | TAH+BSO |
| Jiang et al.[19] | 1 | 46 | 1230 | TAH+BSO |
| Mehr et al.[20] | 1 | 54 | >500 | TAH+BSO |
TAH: Total abdominal hysterectomy; BSO: Bilateral salpingophorectomy.
Although there is no pathology in the thyroid gland, high Tg suggests ectopic Tg production. According to recent literature, the only ovarian neoplasm that secretes Tg is the SO. Tg levels are especially important in monitoring recurrence.[22] In our study, the only patient with high Tg levels during follow-up was the patient diagnosed by MSO with mixed anaplastic and papillary cancer. In this patient, Tg increased to 5000 µg/L by the occurrence of metastatic lesions in the vertebra and opposite adnexa. After surgery for these metastases, thyroglobulin levels decreased to normal values (Fig. 1).
Figure 1.
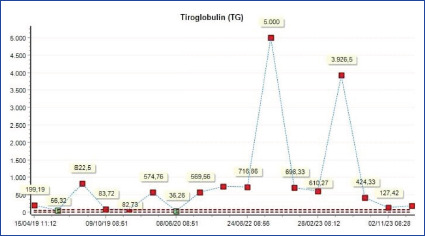
Thyroglobulin course in metastatic case.
The most common type of carcinoma that develops in the background of struma ovarii is papillary thyroid carcinoma, which accounts for approximately 40-50% of cases. Following that, follicular thyroid carcinoma, mixed follicular and papillary thyroid carcinoma, and rarely, anaplastic and medullary carcinoma can be observed.[23] In our study, the histopathological diagnosis of 16 patients was benign. Approximately 5-10% of SO cases have malignant features.[9] Our malign struma ovarii rate was above normal at 15.7%. Two of the malignant cases had histopathology consistent with papillary thyroid carcinoma, while the third case had a mixed papillary and anaplastic carcinoma (Figs. 2,3).
Figure 2.
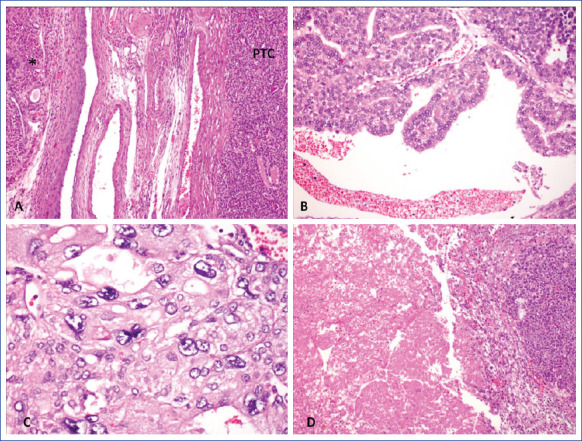
Microphotographs reveals a malignant struma ovarii. Poorly differentiated papillary carcinoma of thyroid (PTC) adjacent to luteinized cells (*) in ovary (A, HEx40). Tumor contains well differentiated areas with papillary structures (B, HE x100) and anaplastic carcinoma transformation represented with bizaar nuclei with (C, HE x200) and tumor necrosis (D, HE x40).
Figure 3.
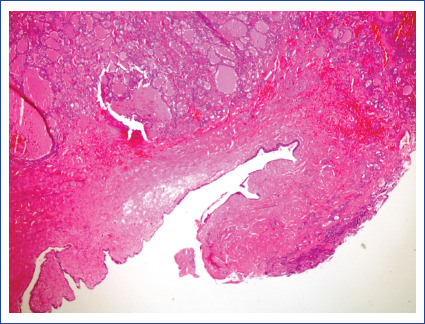
Benign thyroid tissue close to epitejial cyst in an ovarian monodermal teratoma (HE X40).
In the treatment of benign cases, tumor enucleation or unilateral oophorectomy is often sufficient. However, for malignant cases, there is still no consensus on the optimal approach. Literature data primarily consist of case reports rather than large cohort studies. In addition to considering the menopausal status of patients, the differentiation between benign and malignant lesions through frozen section examination is crucial in making surgical decisions. If the frozen section sampling result indicates a benign lesion, conservative treatment can be pursued. In case of malignancy, fertility-sparing salpingo-oophorectomy or tumor enucleation can be performed in premenopausal patients, while total abdominal hysterectomy and bilateral salpingo-oophorectomy are recommended in postmenopausal patients.[24] In our series, surgical decisions were based on the frozen section sampling results and the menopausal status of the patients. Laparoscopic tumor enucleation was predominantly performed for benign cases, whereas salpingo-oophorectomy was preferred for malignant cases (Table 3).
Table 3.
Types of surgery
| n | % | |
|---|---|---|
| Benign | ||
| Tumor enucleatıon | 10 | 52.6 |
| USO | 2 | 10.5 |
| TAH+BSO | 4 | 21 |
| Malign | ||
| Tumor enucleatıon | Ø | 0 |
| USO | 2 | 10.5 |
| TAH+BSO+Omentectomy+PLND | 1 | 5.2 |
| Thyroidectomy | 3 | 15.7 |
TAH: Total abdominal hysterectomy; USO: Unilateral salpingophorectomy; BSO: Bilateral salpingophorectomy; PLND: Pelvic Lymph node dissection.
There is also controversy regarding adjuvant therapy in MSO patients, similar to the uncertainty in surgical treatment. The recommended adjuvant treatment to prevent recurrence and metastasis in MSO cases is RAI treatment with total thyroidectomy.[8,23,25] This approach helps exclude thyroid cancer with thyroidectomy, allows for RAI for potential micrometastases, and enhances the reliability of Tg for follow-up.[26]
Synchronous thyroid cancer is rare in MSO patients. Sisti et al.[27] reported two cases of thyroid malignancies in 21 MSO patients, one synchronous and one metachronous, while Goffredo et al.[28] mentioned six cases of thyroid cancer coexistence in 68 MSO patients. While the reasons for such coexistence are not fully understood, it is presumed that the probability of representing a synchronous tumor is higher than metastasis. Therefore, clinical examination and ultrasound evaluation of the thyroid gland should be performed in these patients.
Although elevated Tg and TSH levels have been previously considered as high-risk indicators for the presence of synchronous thyroid carcinoma, numerous studies have yielded different results.[2,29,30] Euthyroid and subclinical hyperthyroid patients have been reported (Table 4). While it is recommended to perform thyroidectomy followed by RAI ablation for the reliability of Tg as a marker in the monitoring of metastasis and recurrence, this issue remains controversial. Li et al.[31] concluded that RAI treatment, while reducing the recurrence in non-metastatic cases, does not contribute to overall survival and may not be mandatory. In the same study, they showed that MSO is a cancer type with a low risk of death, similar to thyroid cancer. They also noted that the prognosis is influenced by patient age, the presence of metastatic disease, and tumor type. Hinshaw et al.[32] have also proposed guidelines for postoperative management of the thyroid. They suggested that for low-risk tumors, defined as those smaller than 2 cm and with low differentiation, TSH suppression and Tg measurements may be sufficient, but they did not specify criteria for the high-risk group. In our study, since all malignant patients had tumor sizes exceeding 5 cm, total thyroidectomy, followed by RAI ablation and L-thyroxine suppression, was performed as additional treatment regardless of tumor type. No malignancies were found in the thyroid pathology of these patients.
Table 4.
Simultaneous thyroid cancer and Tg-TSH levels
| Study | Year | Tg | TSH | Tm variant |
|---|---|---|---|---|
| Siegel et al.[2] | 2019 | N | Euthyroid | Papillary Cancer |
| Russo et al.[29] | 2016 | N | SHT | Papillary Cancer |
| Irena Batog et al.[30] | 2023 | N | Euthyroid | Papillary Cancer |
N: Normal; SHT: Subclinic hyperthyroid.
In the literature, post-treatment metastasis has been reported at a rate of 5-23%, and recurrence at a rate of 7.5-35%.[9] There was no recurrence in any patient in our study. Metastasis was observed during the follow-up of the patient with mixed anaplastic and papillary carcinoma among the patients diagnosed with MSO. The histopathology of the metastatic lesions revealed poorly differentiated thyroid carcinoma. The follow-up period of all patients was long enough. The last surgery for the lesion in the vertebra was performed in the metastatic patient 6 months ago, and disease-free follow-up continued in all patients, including this patient. There were no mortalities during the follow-up period. It is evident that the tumor type plays a significant role in the disease progression.
Our study had limitations, including its retrospective nature, the rarity of MSO and the absence of a comparison group.
Conclusion
Fertility-preserving conservative surgery is recommended for benign struma ovarii nonetheless, there are uncertainties regarding MSO. Factors such as the rarity of malignancy and the absence of a pre-surgery diagnosis contribute to these uncertainties. Although there are publications in the literature recommending aggressive treatment in cases of malignant struma ovarii, our study suggests that if the histopathological examination reveals no aggressive components in the tumor, fertility-sparing surgery, with an assessment of the risk, may be sufficient for treatment. However, future studies with larger series and comparison groups are necessary to elucidate treatment strategies
Footnotes
Please cite this article as ”Erkan S, Yabanoglu H, Avci T, Dogan Durdag G, Bolat F, Kocer NE. Struma Ovarii: Single Center Experience. Med Bull Sisli Etfal Hosp 2024;58(3):284–290”.
Disclosures
Ethics committee approval
The study was approved by the Baskent University Non-interventional Clinical Research Ethics Committee (Number: 23/110, Date: 21.06.2023).
Financial support
This study was supported by Baskent University Research Fund (KA23/215).
Conflict of Interest
Not declared.
Authorship Contributions
Concept – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Design – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Supervision – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Fundings: S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Materials – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Data collection &/or processing – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Analysis and/or interpretation – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Literature search – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Writing – S.E., H.Y., T.A., G.D., F.A.B., N.E.K.; Critical review - S.E., H.Y., T.A., G.D., F.A.B., N.E.K.
Use of AI for Writing Assistance
Artificial intelligence (AI)-enabled technologies (such as Large Language Models [LLMs], chatbots or image generators, ChatGPT) were not used in the study.
References
- 1.Boyd JC, Williams BA, Rigby MH, Kieser K, Offman S, Shirsat H, et al. Malignant Struma ovarii in a 30-year old nulliparous patient. Thyroid Res. 2017;10:3. doi: 10.1186/s13044-017-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel MR, Wolsky RJ, Alvarez EA, Mengesha BM. Struma ovarii with atypical features and synchronous primary thyroid cancer: a case report and review of the literature. Arch Gynecol Obstet. 2019;300:1693–707. doi: 10.1007/s00404-019-05329-z. [DOI] [PubMed] [Google Scholar]
- 3.Leong A, Roche PJ, Paliouras M, Rochon L, Trifiro M, Tamilia M. Coexistence of malignant struma ovarii and cervical papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:4599–605. doi: 10.1210/jc.2013-1782. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Chen P, Gao Y. Struma ovarii: a mini review Int J. Clin Exp Med. 2018;11:10364–71. [Google Scholar]
- 5.Hatami M, Breining D, Owers RL, Del Priore G, Goldberg GL. Malignant struma ovarii--a case report and review of the literature. Gynecol Obstet Invest. 2008;65:104–7. doi: 10.1159/000108654. [DOI] [PubMed] [Google Scholar]
- 6.Nagai K, Yoshida H, Katayama K, Ishidera Y, Oi Y, Ando N, et al. Hyperthyroidism due to struma ovarii: diagnostic pitfalls and preventing thyroid storm. Gynecol Minim Invasive Ther. 2017;6:28–30. doi: 10.1016/j.gmit.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamblin G, Gallice C, Bournaud C, Nadaud B, Lebail-Carval K, Chene G. Benign struma ovarii: Report of 7 cases and review of the literature. Gynecol Obstet Fertil [Article in French] 2016;44:263–8. doi: 10.1016/j.gyobfe.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Wee JYS, Li X, Chern BSM, Chua ISY. Struma ovarii: management and follow-up of a rare ovarian tumour. Singapore Med J. 2015;56:35–9. doi: 10.11622/smedj.2015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leustean L, Ungureanu MC, Preda C, Bilha SC, Obrocea F, Dănilă R, et al. Management of malignant struma ovarii: is aggressive therapy justified? Case report and literature review. Thyroid Res. 2022;15:14. doi: 10.1186/s13044-022-00132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addley S, Mihai R, Alazzam M, Dhar S, Soleymani Majd H. Malignant struma ovarii: surgical, histopathological and survival outcomes for thyroid-type carcinoma of struma ovarii with recommendations for standardising multi-modal management. A retrospective case series sharing the experience of a single institution over 10 years. Arch Gynecol Obstet. 2021;303:863–70. doi: 10.1007/s00404-021-05969-0. [DOI] [PubMed] [Google Scholar]
- 11.Nurliza Binti Md Nor, Kusumoto T, Inoue S, Nakamura K, Seki N, Hongo A, et al. Three cases of struma ovarii underwent laparoscopic surgery with definite preoperative diagnosis. Acta Med Okayama. 2013;67:191–5. doi: 10.18926/AMO/50413. [DOI] [PubMed] [Google Scholar]
- 12.Birbas E, Kanavos T, Gkrozou F, Skentou C, Daniilidis A, Vatopoulou A. Ovarian masses in children and adolescents: a review of the literature with emphasis on the diagnostic approach. Children (Basel) 2023;10:1114. doi: 10.3390/children10071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SK, Wuest M, Wang M, Glubrecht D, Andrais B, Lapi SE, et al. Immuno-PET of epithelial ovarian cancer: harnessing the potential of CA125 for non-invasive imaging. EJNMMI Res. 2014;4:60. doi: 10.1186/s13550-014-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanopol IA, Petecariu A, Baroiu L, Neagu AI, Bogdan-Goroftei RE, Nechifor A, et al. Giant benign struma ovarii with high-grade fever, elevated CA 125, and hormonal function in an adolescent patient. Children (Basel) 2023;10:856. doi: 10.3390/children10050856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh JJ, Montz FJ, Bristow RE. Struma ovarii associated with pseudo-Meigs' syndrome and elevated serum CA 125. Gynecol Oncol. 2006;86:231–4. doi: 10.1006/gyno.2002.6741. [DOI] [PubMed] [Google Scholar]
- 16.Mitrou S, Manek S, Kehoe S. Cystic struma ovarii presenting as pseudo-Meigs' syndrome with elevated CA125 levels. A case report and review of the literature. Int J Gynecol Cancer. 2008;18:372–5. doi: 10.1111/j.1525-1438.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 17.Obeidat BR, Amarin ZO. Struma ovarii with pseudo-Meigs' syndrome and elevated CA125 levels. J Obstet Gynaecol. 2007;27:97–8. doi: 10.1080/01443610601076267. [DOI] [PubMed] [Google Scholar]
- 18.Rana V, Srinivas V, Bandyopadhyay S, Ghosh SK, Singh Y. Bilateral benign non functional struma ovarii with Pseudo-Meigs' syndrome. Indian J Pathol Microbiol. 2009;52:94–6. doi: 10.4103/0377-4929.44978. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Lu X, Zhu ZL, Liu XS, Xu CJ. Struma ovarii associated with pseudo-Meigs' syndrome and elevated serum CA 125: a case report and review of the literature. J Ovarian Res. 2010;3:18. doi: 10.1186/1757-2215-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehr SGD, Ayetullahi H, Muhammedi A, Haddadi SN, Fakari FR. A Struma ovarii associated with pseudo-Meigs’ syndrome is misdiagnosed as advanced ovarian cancer: a case report. Int J Cancer Manag. 2021;14:e110036. doi: 10.5812/ijcm.110036. [DOI] [Google Scholar]
- 21.Wang S, He X, Yang H, Chen L. Struma ovarii associated with ascites and elevated CA125: two case reports and review of the literature. Int J Womens Health. 2022;14:1291–6. doi: 10.2147/IJWH.S379128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oikonomou C, Spathari N, Doumoulaki S, Koutela A, Stagkoglou C, Keramidaris D. Recurrent struma ovarii presented with high levels of thyroglobulin. Case Rep Surg. 2021;2021:8868095. doi: 10.1155/2021/8868095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Yao J, Wang S, Zhao J, Dong J, Liao L. The clinical and pathological characteristics of malignant struma ovarii: an analysis of 144 published patients. Front Oncol. 2021;11:645156. doi: 10.3389/fonc.2021.645156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanimanidis P, Chatzistamatiou K, Nikolaidou A, Kaplanis K. Struma ovarii. A case report. Hippokratia. 2014;18:357–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar SS, Rema P, R AK, Varghese BT. Thyroid type papillary carcinoma arising in a mature teratoma. Indian J Surg Oncol. 2014;5:168–70. doi: 10.1007/s13193-014-0339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzelepis EG, Barengolts E, Garzon S, Shulan J, Eisenberg Y. Unusual case of malignant struma ovarii and cervical thyroid cancer preceded by ovarian teratoma: case report and review of the literature. Case Rep Endocrinol. 2019;2019:7964126. doi: 10.1155/2019/7964126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisti A, Tassinari J, Nisi G, Grimaldi L, Sisti G, Di Tommaso M, et al. Synchronous and metachronous malignancies after malignant struma ovarii in the SEER database. In Vivo. 2016;30:713–6. [PubMed] [Google Scholar]
- 28.Goffredo P, Sawka AM, Pura J, Adam MA, Roman SA, Sosa JA. Malignant struma ovarii: a population-level analysis of a large series of 68 patients. Thyroid. 2015;25:211–5. doi: 10.1089/thy.2014.0328. [DOI] [PubMed] [Google Scholar]
- 29.Russo M, Marturano I, Masucci R, Caruso M, Fornito MC, Tumino D, et al. Metastatic malignant struma ovarii with coexistence of Hashimoto's thyroiditis. Endocrinol Diabetes Metab Case Rep. 2016;2016:160030. doi: 10.1530/EDM-16-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irena Batog W, Riain CÓ Abu Saadeh F. The dilemma of managing thyroid gland after incidental diagnosis of malignant struma ovarii. Is radical thyroidectomy and radioactive iodine necessary? A case report and literature review. Gynecol Oncol Rep. 2023;47:101189. doi: 10.1016/j.gore.2023.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Yang T, Xiang Y, Li X, Zhang L, Deng S. Clinical characteristics and survival outcomes of malignant struma ovarii confined to the ovary. BMC Cancer. 2021;21:383. doi: 10.1186/s12885-021-08118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinshaw HD, Smith AL, Desouki MM, Olawaiye AB. Malignant transformation of a mature cystic ovarian teratoma into thyroid carcinoma, mucinous adenocarcinoma, and strumal carcinoid: a case report and literature review. Case Rep Obstet Gynecol. 2012;2012:269489. doi: 10.1155/2012/269489. [DOI] [PMC free article] [PubMed] [Google Scholar]


