Abstract
Objectives
Chronic Limb-Threatening Ischemia (CLTI) represents a complex manifestation of peripheral artery disease distinguished by symptoms such as ischemic rest pain, non-healing ulcers on the lower limb or foot, and the development of gangrene. CLTI is associated with a high risk of limb amputation, decreased quality of life, and substantial morbidity and mortality. The Prognostic Nutritional Index (PNI), which is calculated using albumin and lymphocyte levels, reflects the immunological and nutritional status. The objective of this study was to investigate the correlation between PNI levels and mortality among patients diagnosed with CLTI who underwent endovascular therapy.
Methods
Individuals diagnosed with CLTI who received endovascular therapy below the knee in our tertiary care center were enrolled in this retrospective study. The patients were divided into two groups: survivors and non-survivors. Logistic regression analyses were performed to detect independent predictors of mortality and using Cox regression model, we assessed the relationship between PNI and mortality. Survival curves were estimated using the Kaplan-Meier method.
Results
The study comprised 113 patients diagnosed with PAD who underwent EVT. The non-survivor group (42 patients) was older (62.9±10.9 vs. 67.7±9.9, p=0.045) and had a higher prevalence of chronic renal failure (22.5% vs. 42.9%, p=0.023) and congestive heart failure (8.5% vs. 21.4%, p:0.049) than the survivor group (71 patients). The median PNI value was lower in the non-survivor group than in the survivor group (35.9±5 vs 38.2±4.4, p=0.012). Cox regression analyses showed that Low PNI was associated with increased mortality (HR=0.931, CI=0.872-0.995, p=0.035). PNI cut-off of 37.009 showed 64.3% sensitivity, 64.8% specificity, and AUC of 0.642 for predicting all-cause mortality. Kaplan-Meier analysis supported higher PNI correlating with better survival.
Conclusion
The Prognostic Nutritional Index was independently associated with mortality among individuals diagnosed with Chronic Limb-Threatening Ischemia.
Keywords: Chronic Limb-Threatening Ischemia, mortality, peripheral artery disease, Prognostic Nutritional Index
Lower extremity artery disease (LEAD) affects approximately 230 million people worldwide.[1] It typically emerges post the age of 50, with a sharp rise after reaching 65, reaching a prevalence of 20% by the age of 80.[2] Chronic Limb-Threatening Ischemia (CTLI) represents the most severe form of Peripheral Artery Disease (PAD), characterized by persistent inadequate tissue perfusion at rest. This results in symptoms such as ischemic rest pain, non-healing ulcers on the lower limb or foot persisting for two or more weeks, or the onset of gangrene. Over a span of 5 years, 5-10% of individuals with asymptomatic PAD or intermittent claudication advance to CTLI.[3] This progression is independently linked to factors such as advanced age, smoking, diabetes mellitus, and chronic renal dysfunction.[4]
CTLI poses a considerable risk of limb amputation, a decline in the quality of life, and significant morbidity and mortality over time. Revascularization is the preferred treatment, and many specialized centers have adopted an endovascular approach first for patients with CTLI.[5,6] Endovascular therapy (EVT) is primarily advocated for individuals with short stenotic lesions or those with lengthy lesions deemed high-risk candidates for surgery. This approach is also recommended for patients lacking a suitable autologous saphenous vein graft.[7] However, notwithstanding optimal revascularization, the prognosis for patients with CTLI remains poor, with amputation or death rates of up to 20-30% at 1 year.[8-10]
Prognostic factors that may affect clinical outcomes after EVT in patients with CTLI are crucial. CTLI shares major risk factors for atherosclerosis, such as hypertension, dyslipidemia, diabetes, and smoking. Inflammation significantly contributes to the development and advancement of atherosclerosis. Various indicators of inflammation, including high-sensitivity C-reactive protein, fibrinogen, and interleukin 6, are linked to an elevated likelihood of the existence, advancement, and complications of LEAD.[11-13] Inflammation is also associated with a greater risk of malnutrition.[14,15] This heightened risk may exacerbate patient outcomes by intensifying existing inflammation, expediting atherosclerosis, and amplifying susceptibility to infections.[16,17] In patients with malnutrition, the process of wound healing may be further hindered, leading to delays. This is attributed to the prolonged inflammatory phase and diminished fibroblast proliferation and collagen synthesis.[18-20] Therefore, inflammation and nutritional factors should be investigated as potential independent prognostic indicators in patients with CTLI. Identifying all prognostic risk factors that may affect clinical outcomes after endovascular surgery is essential to provide the best medical treatment for all patients with CTLI.
Several parameters and tools have been developed to assess malnutrition, such as Global Leadership Initiative on Malnutrition (GLIM) criteria, 14 Mini-nutritional assessment (MNA)[21], and NRS 2002.[22] However, these assessments are time-consuming and subjective. Therefore, there is a need for a brief, practical, and objective tool to detect malnutrition and inflammation. Many studies have shown that malnutrition and inflammation can be evaluated using a prognostic nutritional index (PNI), which provides a simple, effective, and objective assessment. The PNI reflects the immunological and nutritional status using albumin and lymphocyte values.[23] This scoring system is one of the determinants of mortality and morbidity in patients with cardiovascular disease.[24]
Research indicates that PNI is independently associated with limb amputation in individuals with peripheral artery disease affecting the lower extremities.[25] In this study, we aim to assess the relationship between PNI levels and mortality among patients diagnosed with CTLI.
Methods
Study Population
This retrospective study focused on patients with chronic limb-threatening ischemia who received below-the-knee endovascular therapy at a tertiary care center between January 2015 and January 2020. Demographic and clinical data were obtained from the medical records of patients who underwent EVT for PAD. Mortality data were validated through death notification records from the National Social Security Administration's. Exclusion criteria for the study encompassed patients with active or chronic infections necessitating antibiotics, malignancies, chronic inflammatory diseases, end-stage liver disease, and individuals with a history of prior intervention in the same vascular bed (either EVT or surgery) and who had missing data. The study adhered to the guidelines set forth in the Declaration of Helsinki and obtained approval from the ethics committee of Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital (number: 2023.01-06, date: 10.01.2023).
Blood Sampling
Blood samples containing serum albumin, eGFR, total cholesterol, LDL, HDL were collected from each patient on the morning prior to the procedure. Complete blood count parameters, including lymphocyte count, were also noted. Albumin levels were analyzed by Cobas® 6000 c501 and hematological analysis of the blood samples was performed using automated hematology analyzer (Mindray BC-6000). The calculation of PNI involved the following formula: PNI =10 × serum albumin (g/dL) +0.005 × total lymphocyte count (per mm3).[23,26]
Treatment and Follow-up
After EVT, all participants received aspirin (100 mg) and clopidogrel (75 mg) for at least one month, and cilostazol (100 mg) twice a day for 1, 3, or 6 months based on the physician's discretion. Patients were arranged for regular follow-up appointments at our institution every 3 to 6 months.
Statistical Analysis
The normality of the variables was assessed, and for normally distributed variables, numerical values were expressed as mean±standard deviation, while skewed-distributed continuous variables were presented as median (minimum-maximum). Categorical variables were summarized using frequencies. Group comparisons were conducted through either Mann-Whitney U test or independent sample t-test as needed. For non-numerical data, 2x2 contingency tables underwent analysis using the chi-square test with Yates correction and Fisher's exact test as applicable. Correlations between numerical parameters were examined using either Pearson's or Spearman's rho correlation test, depending on the distribution of the data.
Variables that showed significant associations with mortality in the initial univariate analysis underwent further evaluation through logistic regression analysis and Cox regression analysis. To ensure the reliability of the regression analyses, potential multicollinearity among independent variables was scrutinized using Pearson, Spearman, or Kendall's tau-b correlation analyses. Variables exhibiting multicollinearity, defined by an r-coefficient value exceeding 0.7, were deliberately excluded from the same regression models. A receiver operating characteristic (ROC) curve was applied to determine the cut-off PNI values for predicting all-cause mortality. The predictive ability of PNI to indicate all-cause mortality was measured using two indicators: sensitivity, specificity. The ROC analysis was performed to determine the area under the curve (AUC). Kaplan–Meier method and log-rank test were applied to compare the survival difference between PNI groups.
A p-value below 0.05 was deemed statistically significant. Data analysis was performed using SPSS (IBM SPSS Statistics for Windows, version 21.0 released 2012, IBM Corp., Armonk, NY, USA).
Results
A comprehensive evaluation was conducted on 765 patients diagnosed with PAD, and the data of those with lower extremity PAD were reviewed retrospectively. Those with iliac and superficial femoral artery involvement and those who did not undergo EVT were excluded from the study. A total of 142 patients with PAD who underwent EVT below the knee were evaluated, and 29 patients were excluded due to exclusion criteria. Finally, 113 patients who underwent EVT and had accessible data on serum albumin and lymphocytes were included for further evaluation. Among these 113 patients, 71 (62.8%) survived and 42 (37.2%) died. After EVT, bleeding requiring transfusion was observed in three patients, with one of these patients exhibiting a retroperitoneal hematoma. All three patients were discharged following clinical follow-up. The patients were divided into two groups: survivors and non-survivors. No statistically significant disparities were observed between the two groups concerning gender, presence of hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, atrial fibrillation, history of stroke, or smoking status. However, the non-survivors group was older (67.7±9.9 vs 62.9±10.9; p=0.045) and had a higher prevalence of chronic renal failure (42.9% vs 22.5%; p=0.023) and congestive heart failure (8.5% vs 21.4%; p=0.049) than the survivors group. The GFR (62.3±33.2 vs 77.8±25.9), albumin (3.6±0.5 vs 3.8±0.4), and lymphocyte (1850±0.8 vs 2214±0.8) levels were lower in the non-survivors group. The median PNI value was observed to be lower in the group of non-survivors compared to the group of survivors. (35.9±5 vs 38.2±4.4, p=0.012). Table 1 displays the baseline characteristics and laboratory findings.
Table 1.
The baseline study parameters of patients according to limb amputation
| Parameters | Mortality (-) | Mortality (+) | p |
|---|---|---|---|
| Age, years (mean±SD) | 62.9±10.9 | 67.7±9.9 | 0.045 |
| Gender, n (%) | 0.537 | ||
| Male | 56 (78.9) | 31 (73.8) | |
| Female | 15 (21.1) | 11 (26.2) | |
| Hypertension, n (%) | 40 (56.3) | 22 (52.4) | 0.683 |
| Diabetes mellitus, n(%) | 57 (80.3) | 38 (90.5) | 0.152 |
| Hyperlipidemia, n (%) | 26 (36.6) | 10 (23.8) | 0.158 |
| Coronary artery disease, n (%) | 43 (60.6) | 23 (54.8) | 0.545 |
| Chronic renal failure, n (%) | 16 (22.5) | 18 (42.9) | 0.023 |
| Hemodialysis, n (%) | 2 (2.8) | 6 (14.3) | 0.050 |
| Congestive heart failure, n (%) | 6 (8.5) | 9 (21.4) | 0.049 |
| Atrial fibrillation, n (%) | 11 (15.5) | 6 (14.3) | 0.862 |
| Smoking, n (%) | 44 (62) | 20 (47.6) | 0.137 |
| Cerebrovascular accident, n (%) | 5 (7) | 6 (14.3) | 0.219 |
| Rutherford Classification, n (%) | 0.109 | ||
| Stage IV | 32 (45.1) | 12 (28.6) | |
| Stage V | 22 (31) | 21 (50) | |
| Stage VI | 17 (23.9) | 9 (21.4) | |
| Fontaine classification, n (%) | 0.115 | ||
| Stage III | 29 (40.8) | 11 (26.2) | |
| Stage IV | 42 (59.2) | 31 (73.8) | |
| Lesion localization n (%) | |||
| Popliteal artery | 14 (19.7) | 19 (45.2) | 0.004 |
| Anterior tibial artery | 43 (60.6) | 17 (40.5) | 0.039 |
| Tibioperoneal truncus | 5 (7) | 3 (7.1) | 1.000 |
| Posterior tibial artery | 27 (38) | 16 (38.1) | 0.994 |
| Peroneal artery | 17 (23.9) | 12 (28.6) | 0.586 |
| Combined | 23 (32.4) | 24 (57.1) | 0.010 |
| Amputation n (%) | 22 (31) | 5 (11.9) | 0.022 |
| Medication, n (%) | |||
| Aspirin | 52 (73.2) | 32 (76.2) | 0.729 |
| Clopidogrel | 7 (9.9) | 4 (9.5) | 1.000 |
| Cilastozol | 7 (9.9) | 2 (4.8) | 0.480 |
| Statin | 38 (53.5) | 13 (31) | 0.020 |
| ACEi/ARB | 15 (21.1) | 7 (16.7) | 0.630 |
| B-blocker | 32 (45.1) | 23 (54.8) | 0.319 |
| Calcium channel blocker | 20 (28.2) | 11 (26.2) | 0.820 |
| PNI (mean±SD) | 38.2±4.4 | 35.9±5 | 0.012 |
ACEi: Angiotensin-converting enzyme inhibitors; ARB: Angiotensin receptor blockers.
Multivariate logistic regression analyses were performed to ascertain independent factors correlated with mortality, utilizing the variables identified as significantly associated with mortality in the univariate analysis. Multicollinearity assessments revealed a correlation between chronic renal failure and dependency on dialysis. Consequently, the variable of dialysis dependence was excluded from the regression analyses. In regression model, mortality served as the dependent variable and the independent variables were age, chronic renal failure, congestive heart failure, amputation, statin use, PNI level. The presence of congestive heart failure, statin use, amputation and PNI level were found to be independently associated with mortality (Table 2).
Table 2.
Results of multivariate logistic regression analyses showing independent predictors of mortality
| OR | 95%CI | p | |
|---|---|---|---|
| Age | 1.033 | 0.988-1.079 | 0.149 |
| CKD | 2.315 | 0.861-6.226 | 0.096 |
| CHF | 5.057 | 1.372-18.637 | 0.015 |
| Amputation | 0.296 | 0.093-0.948 | 0.040 |
| Statin | 0.316 | 0.122-0.816 | 0.017 |
| PNI | 0.893 | 0.802-0.994 | 0.038 |
CKD: Chronic kidney disease; CHF: Chronic heart failure; PNI: Prognostic nutritional index; Dependent variable: mortality; independent variables: age, chronic kidney disease, chronic heart failure, amputation, statin use, prognostic nutritional index.
Cox regression model was used to evaluate the association between the overall survival and PNI score. The presence of heart failure, statin use and PNI level were associated with mortality (Table 3).
Table 3.
Results of cox regression analyses
| HR | 95%CI | p | |
|---|---|---|---|
| Age | 1.004 | 0.975-1.033 | 0.792 |
| CKD | 1.858 | 0.953-3.625 | 0.069 |
| CHF | 3.146 | 1.462-6.770 | 0.003 |
| Statin | 0.381 | 0.195-0.746 | 0.005 |
| PNI (continuous | 0.931 | 0.872-0.995 | 0.035 variable) |
CKD: Chronic kidney disease; CHF: Chronic heart failure; PNI: Prognostic nutritional index.
Cut-off values for PNI were determined using ROC curve analysis. Cut-off value was 37.009.
The sensitivity of the PNI for all-cause mortality was 64.3%, and its specificity was 64.8%. Additionally, the AUC value was 0.642. The ROC curve was shown in Figure 1. Kaplan-Meier survival analysis showed that higher PNI levels were associated with better survival rates (log-rank value <0.002; Fig. 2).
Figure 1.
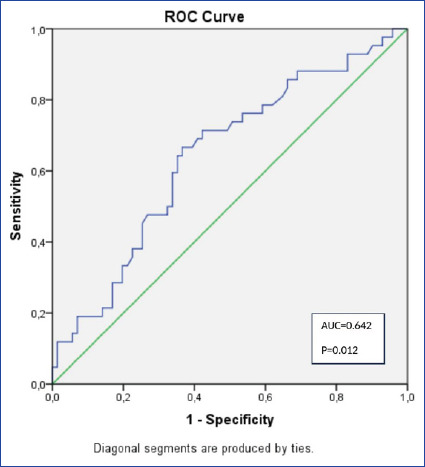
ROC curve.
Figure 2.
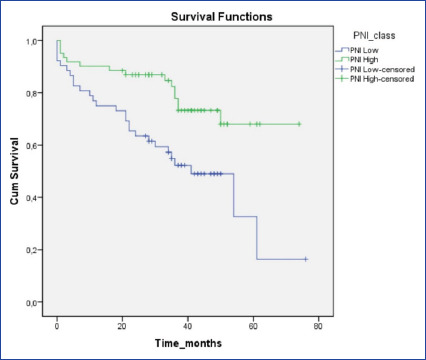
Kaplan-Meier survival analysis.
Discussion
In this study, we discovered that the immune-nutritional status assessed via PNI, exhibited independent association with mortality among patients diagnosed with CLTI who underwent EVT. CLTI, an advanced stage of PAD, is linked to a high risk of limb amputation, significant morbidity, and mortality. Several factors are associated with the poor prognosis of CLTI patients, including non-ambulatory status, dialysis dependence, heart failure history, and Rutherford classification.[8,27,28] which were also observed in our study population. Chronic renal failure, congestive heart failure, and dialysis dependence were associated with mortality in our study population. While diabetes is a major risk factor for lower limb amputation and mortality in CLTI patients[29], diabetes was not associated with mortality in our analysis, despite 95 (84.1%) patients having diabetes. Although the mortality group had a higher prevalence of diabetes mellitus (57 (80.3%) vs. 38 (90.5%) p=0.152), it was not statistically significant. In our study, statin use was independently associated with mortality, consistent with literature supporting the use of statins in PAH patients and showing their potential benefits in reducing cardiovascular morbidity and mortality as well as lower extremity complications.[30-32]
In addition to the risk factors commonly associated with CLTI, such as non-ambulatory status, dialysis dependence, heart failure history, and Rutherford classification, nutritional status is often overlooked but is a significant factor in the prognosis of CLTI patients. It's well-established that malnutrition heightens the likelihood of postoperative complications and mortality, extends hospitalization durations, and diminishes overall quality of life. It affects wound healing by reducing fibroblast proliferation, collagen formation, and angiogenesis.[33] In addition, malnutrition weakens the immune system and reduces bacteriolytic leukocyte activity, leading to a higher risk of infection and delayed wound healing. Albumin, a nutritional marker, is a predictor of poor outcomes in CLTI patients[34], and preoperative hypoalbuminemia is associated with morbidity and mortality in those who undergo lower extremity bypass surgery.[35] Lymphocyte count is also affected by malnutrition, resulting in impaired cellular immunity and decreased bacteriolytic leukocyte activity. Hence, the evaluation of nutritional status using the Prognostic Nutrition Factor (PNI), which considers both albumin and lymphocyte levels, may be more representative in predicting clinical outcomes in patients with CLTI. PNI is a simple marker that combines nutritional status and inflammation factors and can be calculated from routine biochemistry and hemogram. PNI evaluation may play a role in predicting outcomes after EVT in CLTI patients and guide clinical practice by initiating early nutritional support and intervention to improve clinical outcomes. Multidisciplinary care, including resistance training and nutritional therapy, has been shown to improve the prognosis of CLTI patients.[36], and a simple nutritional index for risk stratification and clinical management of CLTI should be determined.
In a 2022 study, Morisaki et al.[37] analyzed data from patients who underwent revascularization for CLTI and examined three nutritional indexes: the controlling nutritional status (CONUT), PNI, and geriatric nutritional risk index (GNRI). Their analysis revealed that wound, ischemia, and foot infection stage, along with each nutritional index (CONUT and PNI), emerged as independent risk factors for major amputation in the multivariate analyses. Likewise, Erken Pamukcu et al.[25] explored the correlation between PNI and extremity amputation among individuals diagnosed with lower extremity PAD and reported that PNI was independently related to amputation. In our current study, we found that amputation was associated with mortality, and in the regression analyses we performed, PNI was independently associated with mortality, even after adjusting for amputation. Therefore, we can interpret that malnutrition and inflammation assessed by PNI are associated with mortality, with or without amputation.
Limitations
It's crucial to emphasize that this study is retrospective, conducted at a single center, and of a cross-sectional nature, with a limited number of patients. However, this center is a reference center and we reviewed all PAD patients in our database. Additionally, we did not determine the relationship between PNI and the long-term prognosis of CLTI patients, so we cannot comment on whether correcting malnutrition will have a positive effect on survival.
Conclusion
This study suggests that PNI serves as an independent predictor of mortality in CLTI patients who underwent EVT. Further investigation is needed to confirm these findings, and a multicenter, prospective study with a larger number of participants is necessary. Additionally, implementing early nutritional support and intervention based on PNI evaluation may improve clinical outcomes in CLTI patients.
Footnotes
Please cite this article as ”Erdogan O, Erdogan T, Panc C, Tasbulak O, Altunova M, Yalcin AA, et al. Prognostic Nutritional Index as a New Prediction Tool for All-Cause Mortality in Patients with Chronic Limb-Threatening Ischemia Undergoing Endovascular Therapy. Med Bull Sisli Etfal Hosp 2024;58(3):346–353”.
Disclosures
Ethics Committee Approval
The study was approved by the Ethics Committee of Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital (Number: 2023.01-06, Date: 10.01.2023).
Financial support
None.
Conflict of Interest
No potential conflict of interest was reported by the authors.
Authorship Contributions
Concept – O.E.; Design – O.E.; Supervision – M.E., A.A.Y.; Materials – C.P., O.E., M.A.; Data collection &/or processing – C.P., M.A., O.T., O.E.; Analysis and/or interpretation – T.E.; Literature search – O.E., T.E.; Writing – O.E.; Critical review - O.E., T.E., C.P., O.T., M.A., A.A.Y., M.E.
Use of AI for Writing Assistance
Artificial intelligence-supported technologies were not used in the study.
References
- 1.Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, et al. American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation. 2021;144:e171–91. doi: 10.1161/CIR.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polonsky TS, McDermott MM. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: a review. JAMA. 2021;325:2188–98. doi: 10.1001/jama.2021.2126. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Howard DP, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM. Oxford Vascular Study. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132:1805–15. doi: 10.1161/CIRCULATIONAHA.115.016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasr MK, McCarthy RJ, Hardman J, Chalmers A, Horrocks M. The increasing role of percutaneous transluminal angioplasty in the primary management of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2002;23:398–403. doi: 10.1053/ejvs.2002.1615. [DOI] [PubMed] [Google Scholar]
- 6.Dosluoglu HH, O'Brien-Irr MS, Lukan J, Harris LM, Dryjski ML, Cherr GS. Does preferential use of endovascular interventions by vascular surgeons improve limb salvage, control of symptoms, and survival of patients with critical limb ischemia? Am J Surg. 2006;192:572–6. doi: 10.1016/j.amjsurg.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. ESC Scientific Document Group. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. BASIL Trial Participants. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51:52S–68S. doi: 10.1016/j.jvs.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 9.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 10.Iida O, Nakamura M, Yamauchi Y, Kawasaki D, Yokoi Y, Yokoi H, et al. OLIVE Investigators. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv. 2013;6:68–76. doi: 10.1161/CIRCINTERVENTIONS.112.975318. [DOI] [PubMed] [Google Scholar]
- 11.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–9. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 12.Stone PA, Yacoub M. Inflammatory biomarkers in peripheral arterial disease. Semin Vasc Surg. 2014;27:148–51. doi: 10.1053/j.semvascsurg.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–62. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 14.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition-a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Fatyga P, Pac A, Fedyk-Łukasik M, Grodzicki T, Skalska A. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur Geriatr Med. 2020;11:383–91. doi: 10.1007/s41999-020-00303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. 2016;23:713–27. doi: 10.5551/jat.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg. 2006;117:42S–58S. doi: 10.1097/01.prs.0000225432.17501.6c. [DOI] [PubMed] [Google Scholar]
- 19.Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care (New Rochelle) 2014;3:663–81. doi: 10.1089/wound.2014.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11:281–8. doi: 10.1097/MCO.0b013e3282fbd35a. [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56:M366–72. doi: 10.1093/gerona/56.6.M366. [DOI] [PubMed] [Google Scholar]
- 22.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–36. doi: 10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 23.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi [Article in Japanese] 1984;85:1001–5. [PubMed] [Google Scholar]
- 24.Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6:e004876. doi: 10.1161/JAHA.116.004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erken Pamukcu H, Sunman H, Taş A, Aker M, Şahan HF, Açıkel S. The role of prognostic nutritional index in predicting amputation in patients with lower extremity peripheral artery disease. J Cardiovasc Thorac Res. 2021;13:43–8. doi: 10.34172/jcvtr.2021.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon HG, Choi DK, Sung HH, Jeong BC, Seo SI, Jeon SS, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23:321–7. doi: 10.1245/s10434-015-4614-0. [DOI] [PubMed] [Google Scholar]
- 27.Biancari F, Salenius JP, Heikkinen M, Luther M, Ylönen K, Lepäntalo M. Risk-scoring method for prediction of 30-day postoperative outcome after infrainguinal surgical revascularization for critical lower-limb ischemia: a Finnvasc registry study. World J Surg. 2007;31:217–27. doi: 10.1007/s00268-006-0242-y. [DOI] [PubMed] [Google Scholar]
- 28.Soga Y, Iida O, Takahara M, Hirano K, Suzuki K, Kawasaki D, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv. 2014;7:1444–9. doi: 10.1016/j.jcin.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Ying AF, Tang TY, Jin A, Chong TT, Hausenloy DJ, Koh WP. Diabetes and other vascular risk factors in association with the risk of lower extremity amputation in chronic limb-threatening ischemia: a prospective cohort study. Cardiovasc Diabetol. 2022;21:7. doi: 10.1186/s12933-021-01441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen-Chaparro S, López-Carmona MD, Cobos-Palacios L, Sanz-Cánovas J, Bernal-López MR, et al. Statins and peripheral arterial disease: a narrative review. Front Cardiovasc Med. 2021;8:777016. doi: 10.3389/fcvm.2021.777016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westin GG, Armstrong EJ, Bang H, Yeo KK, Anderson D, Dawson DL, et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63:682–90. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters F, Kuchenbecker J, Kreutzburg T, Marschall U, Debus ES, Behrendt CA. Long-term effectiveness and safety of initiating statin therapy after index revascularization in patients with peripheral arterial occlusive disease. J Am Heart Assoc. 2020;9:e018338. doi: 10.1161/JAHA.120.018338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61–8. doi: 10.1177/0884533609358997. [DOI] [PubMed] [Google Scholar]
- 34.Shirasu T, Miyata T, Nishiyama A, Miyahara T, Hoshina K, Shigematsu K, et al. Useful predictors for critical limb ischemia in severely ischemic limbs. Int Angiol. 2016;35:460–8. [PubMed] [Google Scholar]
- 35.Peacock MR, Farber A, Eslami MH, Kalish JA, Rybin D, Doros G, et al. Hypoalbuminemia predicts perioperative morbidity and mortality after infrainguinal lower extremity bypass for critical limb ischemia. Ann Vasc Surg. 2017;41:169–75. doi: 10.1016/j.avsg.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara Y, Matsumoto T, Aoyagi Y, Tanaka S, Okadome J, Morisaki K, et al. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J Vasc Surg. 2015;61:945–50. doi: 10.1016/j.jvs.2014.10.094. [DOI] [PubMed] [Google Scholar]
- 37.Morisaki K, Matsubara Y, Kurose S, Yoshino S, Furuyama T. Evaluation of three nutritional indices as predictors of 2-year mortality and major amputation in patients with chronic limb-threatening ischemia. Vascular. 2023;31:1094–102. doi: 10.1177/17085381221102801. [DOI] [PubMed] [Google Scholar]


