Abstract
The Global Burden of Disease Study 2020 ranked acne vulgaris as the eighth most common skin condition, with a global estimated prevalence of 9.4%. The clinical presentation of acne vulgaris ranges from comedones to nodules and cysts. The main treatment options for acne are retinoids, antibiotics, and benzoyl peroxide (BPO). This study aimed to evaluate the reported efficacy and safety of the combination treatment with retinoid and benzoyl peroxide.
Three databases (ScienceDirect, Google Scholar, and PubMed) were searched using keywords such as acne, adapalene/benzoyl peroxide, and randomized controlled trial, and specific search moods such as "Title and abstract" for PubMed and "Title, abstract, and keywords" for ScienceDirect. On Google Scholar, the "allintitle" option was utilized. The articles were searched for the years between 2003 and 2023.
The eight trials selected for systematic review reported randomized controlled trials in 4,596 individuals with the first trials being conducted in February 2007 and the last one in May 2022. There were 52.8% females in the trials, and the trials reported a primary reduction of acne lesions (efficacy) from 27.5% to 70.2%. The occurrence of adverse effects was also variable (ranging from 2.7% to 57.9%), but these effects were mild and vanished with time.
The results showed that the combination of adapalene and benzoyl peroxide is a safe and effective treatment.
Keywords: acne, adapalene/benzoyl peroxide, efficacy, randomized controlled trial, safety
Introduction and background
Acne is one of the most common dermatological conditions worldwide. It is caused by changes in keratinization, colonization of hair follicles with Propionibacterium acnes, inflammatory response of the skin, and increased sebum production induced by androgens. Acne scarring due to severe acne usually lasts a lifetime and has long-term psychological implications [1]. Acne vulgaris is the eighth most prevalent skin disease worldwide, with an estimated prevalence of 9.4% across all age groups, according to the 2020 Global Burden of Disease Study [2]. Acne prevalence varies by country and by age group; 35% to nearly 100% of teenagers experience acne at some stage of their life [3]. The prevalent conditions associated with acne vulgaris are comedones (blackheads and whiteheads), papules (raised lesions of less than 1 cm), and pustules (papule-like lesions that are inflammatory and filled with pus). Nodules and cysts, erythema, hyperpigmentation, and scarring can be noticed in people with acne. Patients may suffer additional detrimental effects in addition to the discomfort experienced due to acne. It has been discovered that acne negatively impacts a person’s social life, sense of self, and body image [4]. It also frequently co-occurs with psychiatric conditions including anxiety, depression, and suicidal thoughts. Furthermore, there is evidence that acne carries significant financial consequences; treating acne in Germany can cost up to 400 million euros a year, and in the US, it can cost nearly 3 billion dollars [5,6].
Multiple researchers looked at the relationship between the presence of acne or severity and several risk variables, many of them being significant like family history (pooled OR=2.91), gender (pooled OR=1.07), and body mass index (pooled OR=2.36). Diet and smoking association were less obvious, and the results of the studies themselves were inconsistent [7]. The management of acne is mainly focused on providing a targeted therapy for Propionibacterium acne. The regulation of sebocyte activity through a range of cellular complex pathways suggests a complex pathogenesis of acne. The study of cellular pathways and hormones and the influence of diet on acne has opened new gateways for research on acne treatment. Propionibacterium acne biofilm formation and antibiotic resistance emphasize the need for new options for acne management [8].
The main treatment options for acne are retinoids, antibiotics, and benzoyl peroxide (BPO). Intolerance to drugs and poor adherence to treatment are the challenges faced at the dermatology clinic. The combination of retinoids and BPO is safe and effective and does not contribute to antibiotic resistance [9]. Adapalene, tazarotene, and tretinoin are the three topical retinoids that the Food and Drug Administration (FDA) has currently approved. Treatment for acne vulgaris with topically applied retinoids is both safe and effective and improves the Investigator's Static Global Assessment and Investigator Global Assessment scores. Topical retinoid monotherapy does not have the same efficacy as topical retinoid combination therapy with an antibiotic. Among topical retinoids, adapalene exhibits a better tolerance profile [10]. Besides, adapalene and BPO combination can be helpful to treat acne as Adapalene is more stable and effective in either the presence or absence of light while combined with BPO [11].
Additionally, a wide range of novel treatments that target the inflammatory cascade of acne pathogenesis are presently being researched. These treatments include, among others, small molecule inhibitors that target sebaceous glands and enzymes as well as phytochemicals that are sebo-suppressive and anti-inflammatory. A combination of hoover help and metal nano-shells is being used in laser and light therapy to treat acne. As the effects of the microbiome on many organ systems become clearer, probiotics have become more and more prominent in medicine. Research is still being conducted to better understand how specific bacterial strains that produce ammonia can help regulate the inflammatory response of the skin [12].
Review
Materials and methods
Search Techniques
Three databases (ScienceDirect, Google Scholar, and PubMed) were searched using the keywords acne adapalene/benzoyl peroxide, randomized controlled trial, and some other following keywords using the Boolean operating system and specific search moods such as "Title and abstract" for PubMed and "Title, abstract, and keywords" for ScienceDirect. On Google Scholar, the "allintitle" option was utilized. For the PubMed search, the following MeSH terms were built: ((((("Acne Vulgaris/diagnosis"[MeSH] OR "Acne Vulgaris/drug therapy"[MeSH] OR "Acne Vulgaris/epidemiology"[MeSH] OR "Acne Vulgaris/etiology"[MeSH] OR "Acne Vulgaris/pathology"[MeSH] OR "Acne Vulgaris/therapy"[MeSH])) AND ("Adapalene, Benzoyl Peroxide Drug Combination/administration and dosage"[MeSH] OR "Adapalene, Benzoyl Peroxide Drug Combination/adverse effects"[MeSH] OR "Adapalene, Benzoyl Peroxide Drug Combination/therapeutic use"[MeSH])) AND "Randomized Controlled Trial" [Publication Type]) AND "Benzoyl Peroxide"[MeSH]) AND "Adapalene"[MeSH], acne adapalene/benzoyl peroxide, and randomized controlled trial. A detailed search strategy is mentioned in the Appendices.
Inclusion and Exclusion Criteria
Full-length research articles were targeted as per the inclusion criteria, especially where the articles reported randomized controlled trials, with blinding preferably double-blind studies, and the intervention was the application of adapalene 0.3% or 0.1% combined with 2.5% benzoyl peroxide (A/BPO) topical gel. Other articles such as reviews, systematic reviews, meta-analyses, letters to the editor, editorials, correspondence, case studies, and articles written in non-English language were excluded as per the exclusion criteria.
Extracting Data
Every study that qualified the inclusion and exclusion criteria provided the following data, which was recorded in an MS Excel Spreadsheet (Microsoft Corp., Redmond, WA): first author, year, country, sex ratio, communication media, treatment benefits, treatment concerns, level of satisfaction, age, number of patients, study timeline, summary goal and outcome, quality assessment score, and study type. The systematic review was done manually including the extraction of data.
Evaluating Study Quality
Studies that answered less than 50% of the checklist's questions were deemed low-quality studies, while those that answered between 50% and 70% of the questions were deemed moderate- and high-quality studies, respectively, according to the Joanna Briggs Institute's (JBI) critical appraisal tools [13]. The low-, moderate-, and high-quality studies imply high, moderate, and low risk of bias, respectively [14].
The systematic review rigorously followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure comprehensive and transparent reporting of the review process [15].
Results
Study Selection
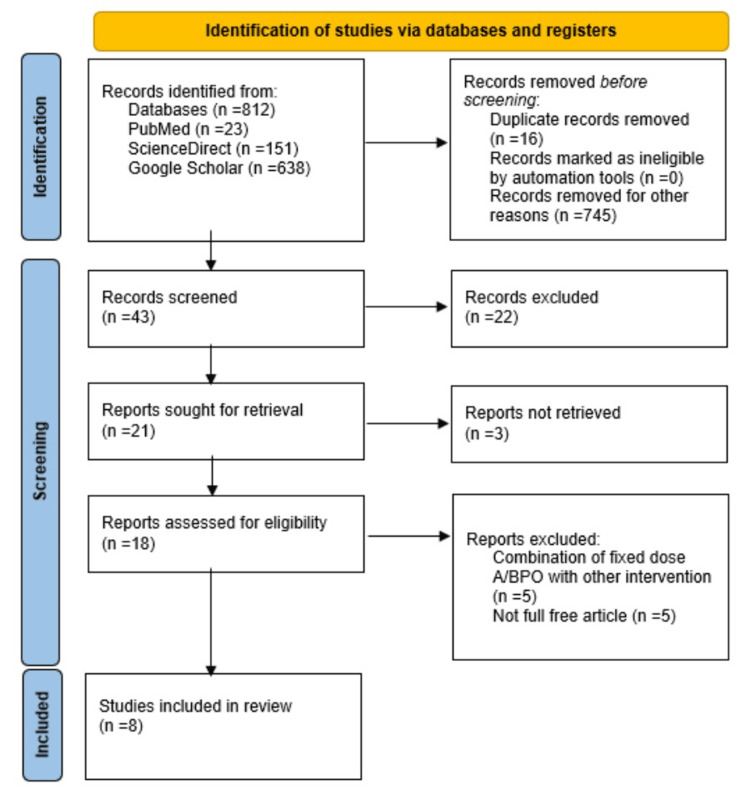
We initially found 812 articles (PubMed: 23, ScienceDirect: 151, and Google Scholar: 638) in total from three databases, and finally, after sequential screening, eight studies were selected for this study. The details are shown in the PRISMA flow diagram (Figure 1).
Figure 1. PRISMA flow diagram.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, A/BPO: adapalene/benzoyl peroxide
Acne Scoring
The score for acne severity is as follows: 0, "clear" (erythema and residual hyperpigmentation may be present (complete improvement)); 1, "almost clear" (a couple of straggling comedones and a few little papules); 2, "mild" (less than half of the face is affected and easily identifiable, and certain papules and pustules, along with certain comedones, are present); 3, "moderate" (there is involvement over half the face; numerous pustules, papules, and comedones are present; and there could be just one nodule); and 4, "severe" (there are comedones all over the face, along with many papules and pustules, a few nodules, and cysts (worse)).
Timeline of Studies
We searched articles dated between 2003 and 2023 and obtained published articles dated between 2007 and 2023. The clinical trials were performed from February 2007 [11] to the last trial in May 2022 [16].
Data Extraction
Several data were extracted from the selected studies. The total number of participants in the eight studies was 4,596. The age of the participants was 12 years and older, and the mean age ranged between 16.4 years and 23.4 years. The overall percentage of female gender was 52.87% (2,430/4,596). The studies included were from different locations, such as the United States of America (USA), Puerto Rico, Canada, France, and Europe. Acne type, comparator, criteria for study participant inclusion and exclusion, application type, and total application period were extracted as well.
Drug Applied
The drug used was adapalene 0.1% or 0.3% plus benzoyl peroxide 2.5%. The drug was applied as a once-daily dose.
Comparator
The comparators were vehicle plus monotherapy either with adapalene or benzoyl peroxide [11,17,18], vehicle only [19-22], and retinoid [16].
Outcome Variables
The outcome variables were as follows: primary efficacy (PE); lesion count reduction from baseline (inflammatory lesion (IL), non-inflammatory lesion (NIL), and total lesion (TL) count); success rate; median percentage change in IL, NIL, and total count; subjective assessment; safety; tolerability (adverse events (AEs)); and Scar Cosmesis Assessment and Rating Scale (SCAR-S), Scar Global Assessment (SGA) score, Investigator Global Assessment (IGA) score subjective assessment (skin texture and roughness), retinoid-induced skin discomfort (RISD) composite score, and Global Acne Evaluation (GEA) score.
Quality of Studies
All the studies were high-quality studies (Table 1).
Table 1. Quality assessment of the included study.
Questions: 1. Was the research question appropriate? 2. Was the study population clearly defined? 3. Were controls selected from the same or similar population including the same timeframe? 4. Did the author follow proper inclusion and exclusion criteria regarding case-control selection? 5. Were the cases clearly defined and differentiated from controls? 6. Were the assessors of exposure/risk blinded to the case or control status of participants? 7. Were the methods of quantity determination clearly defined? 8. Did the authors use statistical analyses? 9. Were the measurements/tools valid to conduct the study?
Y: yes (score=1), N: no (score=0), P: partially (score=0.5), NR: not reported (no score)
| Study ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Overall score |
| Thiboutot et al. (2007) [11] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Stein Gold et al. (2009) [17] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Gollnick et al. (2009) [18] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Stein Gold et al. (2016) [19] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Dreno et al. (2016) [20] | Y | Y | Y | NR | Y | Y | Y | Y | Y | 8 |
| Dreno et al. (2018) [21] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Dreno et al. (2019) [22] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Khammari et al. (2023) [16] | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
Discussion
Acne is a chronic inflammatory condition affecting the pilosebaceous unit of the skin. There is a dearth of high-quality evidence to support the use of topical or systemic therapies available for acne. The majority of the current recommendations for treating acne are based on professional consensus, and they recommend using topical treatments in mild to moderate cases and systemic medicines in moderate to severe or resistant cases. When topical and general treatments have failed, there is a compelling need for early, efficient treatment with systemic medication due to the psychological effects of acne [1].
Topical treatments include retinoids (e.g., adapalene, isotretinoin (ISO), tazarotene, motretinide, and tretinoin), antibiotics (e.g., erythromycin and clindamycin), and miscellaneous such as benzoyl peroxide, salicylic acid, and azelaic acid. Systemic treatments include retinoids (e.g., isotretinoin), antibiotics (e.g., erythromycin, azithromycin, doxycycline, and clindamycin), and sometimes contraceptives [21]. The last option is physical treatment; some examples include electrocauterization, cryotherapy, extraction of comedones, and intralesional corticosteroids [23]. The present review was meant to discuss the efficacy and safety of the topical benzoyl peroxide (BPO) gel when used in combination with adapalene (A) (Table 2 and Table 3). It also discusses the factors that enhance the effect of the combination (A/BPO) (Table 4).
Table 2. Characteristics of the study.
IGA: Investigator Global Assessment, GEA: Global Acne Evaluation, IL: inflammatory lesion, NIL: non-inflammatory lesion, ISO: isotretinoin, RC: routine care
| Thiboutot et al. (2007) [11] | Stein Gold et al. (2009) [17] | Gollnick et al. (2009) [18] | Stein Gold et al. (2016) [19] | Dreno et al. (2016) [20] | Dreno et al. (2018) [21] | Dreno et al. (2019) [22] | Khammari et al. (2023) [16] | |
| Study type | Randomized, double-blinded, multicenter, parallel-group, controlled trial | Double-blinded, multicenter, randomized, parallel-group, controlled trial | Double-blinded, randomized, multicenter, parallel-group, controlled trial | Double-blinded, multicenter, randomized, parallel-group | Investigator-blinded, multicenter, randomized, vehicle-controlled, international | Investigator-blinded, multicenter, randomized, vehicle-controlled | Randomized, open-labeled extension phase of Dreno et al. (2018) | Double-blinded, randomized, comparative, clinical trial |
| Location | 36 centers in the USA | USA, Puerto Rico, Canada | USA, Canada, and Europe | Canada and USA | Canada and France | Canada | Canada and France | France |
| Dates | February 17, 2007 to December 21, 2007 | 2009 | 2009 | July 2013 to March 2014 | August 2012 to September 2013 | May 2016 to November 2017 | NR | September 2021 to May 2022 |
| Time for evaluation | 1, 2, 4, and 12 weeks | 1, 2, 4, 8, and 12 weeks | 1, 2, 4, 8, and 12 weeks | 4, 8, and 12 weeks | NR | 1, 2, 4, 8, 12, 20, and 24 weeks | 24, 36, and 48 weeks | NR |
| Participants | 517 | 1,668 | 1,670 | 503 | 38 | 67 | 45 | 88 |
| Mean age | 16.4 years | 18.2 years | 19 years | 19.3±6.7 years | 23.4±3.6 years | 21.5 years | 21.8 years | 21.3±4 years |
| Gender % | 40.2% female | 51.3% female | 56.2% female | 52.3% female | 36.8% female | 65.7% female | 71.1% female | 84% female |
| Acne type | Moderate (80.07%) | IGA 3 (moderate) | IGA 3 (moderate) | Moderate to severe (50/50) | Moderate | Moderate to severe | Moderate to severe | Mild to moderate |
| Comparator | Vehicle and monotherapy | Vehicle and monotherapy | Vehicle and monotherapy | Vehicle | Vehicle | Vehicle | None | RC |
| Inclusion | ≥12 years age, 20-50 IL and 30-100 NIL, no nodules or cysts | ≥12 years age, 20-50 IL, 30-100 NIL, ≤1 nodule, no cyst | ≥12 years age, 20-50 IL, 30-100 NIL, ≤1 nodule, no cyst | 20-100 IL, 30-150 NIL, ≤2 nodules, no cyst | 18-35 years, 20-40 IL, ≤1 nodule, no cyst, ≥10 atrophic scars (≥1.5 mm) | 18-35 years, ≥25 IL, ≤2 nodules, ≥10 atrophic scars, IGA 3 and 4 | 18-35 years, ≥25 IL, ≤2 nodules, ≥10 atrophic scars, IGA 3 and 4 | ≥16 years age, GEA II and III, ≥15 IL lesions |
| Exclusion | Pregnancy, nursing, men with facial hair, severe acne requiring ISO | Systemic treatment, acne other than acne vulgaris | Pregnancy, nursing, men with facial hair, severe acne requiring ISO | Systemic treatment, acne other than acne vulgaris, pregnancy | NR | Systemic treatment, acne other than acne vulgaris | Systemic treatment, acne other than acne vulgaris | Pre-menstrual, pregnant, breastfeeding, intolerance to treatment |
| Application | Once daily | Daily, topical | Once daily | Daily, topical | Daily, split face, topical | Daily, split face, topical | Daily | Daily, split face, topical |
| Total time | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 6 months | 24 weeks | 48 weeks | 12 weeks |
Table 3. Variables measured in each trial and outcomes.
A/BPO: adapalene/benzoyl peroxide, V: vehicle, A: adapalene, BPO: benzoyl peroxide, IGA: Investigator Global Assessment, GEA: Global Acne Evaluation, SGA: Scar Global Assessment, RISD: retinoid-induced skin discomfort, RC: regular cure, DC: dermo-cosmetic, IL: inflammatory lesion, NIL: non-inflammatory lesion, TL: total lesions, AEs: adverse events, NR: not reported, PE: primary efficacy
| Thiboutot et al. (2007) [11] | Stein Gold et al. (2009) [17] | Gollnick et al. (2009) [18] | Stein Gold et al. (2016) [19] | Dreno et al. (2016) [20] | Dreno et al. (2018) [21] | Dreno et al. (2019) [22] | Khammari et al. (2023) [16] | |
| PE (%) | A/BPO (27.5), A (15.5), BPO (15.4), V (9.9) | A/BPO (70.2), A (60.1), BPO (61.1), V (49.6) | A/BPO (22.9), A (37.3), BPO (40.5), V (45.7) | NR | Acne scar: A/BPO (11.1 to 11.6), V (10.9 to 13.6) | A0.3/BPO2.5 (15.5), V (14.4) | Acne scar: A/BPO (9.2 at 24 weeks), V (13.9 at 12 weeks) | NR |
| Success rate (%) | A/BPO (30.1), A (18.1), BPO (16.5), V (13.7) | A/BPO (30.1), A (19.8), BPO (22.2), V (11.3) | A/BPO (37.9), A (21.8), BPO (26.7), V (17.9) | Moderate: A0.3/BPO2.5 (33.7), V (11.0); severe: A0.3/BPO2.5 (31.9), V (11.8) | NR | NR | A0.3/BPO2.5 (44.4), A0.3/BPO2.5 + V (28.9) | NR |
| Acne severity score | IGA score: A/BPO (70.5), A (54.1), BPO (53.7), V (47.9) | NR | IGA score: A/BPO (75.0), A (63.0), BPO (59.0), V (53.0) | NR | SGA count: A/BPO (9.7 to 45.2), V (97 to 6.5) | SGA count: A0.3/BPO2.5 (34.5), V (60.4) | SGA: A0.3/BPO2.5 (48.9 at 24 weeks, 55.6 at 48 weeks), V (24.4 at 24 weeks, 46.7 at 48 weeks) | GEA score: DC 2.75±0.44 versus RC 2.68±0.47 |
| Percentage reduction in acne (%) | NR | IL: A/BPO (62.1), A (50.0), BPO (55.6), V (34.3); NIL: A/BPO (53.8), A (49.1), BPO (44.1), V (29.5) | IL: A/BPO (70.3), A (57.1), BPO (61.9), V (45.5); NIL: A/BPO (62.2), A (50.4), BPO (48.8), V (36.7); TL: A/BPO (65.4), A (52.3), BPO (48.2), V (37.1) | Moderate IL: A0.3/BPO2.5 (27.0), V (14.4); NIL: A0.3/BPO2.5 (40.2), V (18.5); severe IL: A0.3/BPO2.5 (35.17), V (15.46); NIL: A0.3/BPO2.5 (45.61), V (17.25) | IL: A/BPO (72.0), V (40.0); NIL: A/BPO (58.0), V (31.0) | IL: A0.3/BPO2.5 (86.7), V (57.9); NIL: A0.3/BPO2.5 (59.5), V (41.4); TL: A0.3/BPO2.5 (73.3), V (50.9) | IL: A0.3/BPO2.5 (90.7 at 24 weeks, 86.7 at 48 weeks), V (12.8 at 48 weeks); TL: A0.3/BPO2.5 (82.2 at 24 weeks, 77.8 at 48 weeks), V (53.1 at 24 weeks, 74.5 at 48 weeks) | NIL, IL, TL (23.45±6.97, 20.23±4.15, 43.68±9.37 in DC group) |
| Subjective improvement (%) | A/BPO (42.5), A (34.8), BPO (30.6), V (14.5) | A/BPO (73.5), A (65.6), BPO (66.7), V (55.0) | A/BPO (50.0), A (44.0), BPO (39.0), V (29.0) | A0.3/BPO2.5 (90.7), V (40.0); satisfaction: A0.3/BPO2.5 (71.6), V (32.3) | NR | A0.3/BPO2.5 (64.2), V (19.4); satisfaction: A0.3/BPO2.5 (90.1), V (59.0) | NR | NR |
| Safety and tolerability (AEs) | 1 AE: A/BPO (38.3%), A (42.6%), BPO (29.5%), V (26.8%) | 1 AE: A/BPO (2.7%), A (1%), BPO (1.2%), V (0.5%) | 1 AE: A/BPO (48%), A (39%), BPO (33%), V (28%) | No irritation: A0.3/BPO2.5 (53%), V (76%) | Total AE: A/BPO (57.9), V (26.3) | A0.3/BPO2.5 (20.9), V (9.0) | NR | RISD: RC 0.84±0.96 versus DC 0.75±0.78 |
Table 4. Interventions or combinations that enhance the action of the combination A/BPO.
A/BPO: adapalene/benzoyl peroxide, IL: inflammatory lesion, NIL: non-inflammatory lesion, TL: total lesions, AEs: adverse events, NR: not reported, ISO: isotretinoin, IGA: Investigator Global Assessment, SEM: supplementary education material, SCOPE: standard of care patient education, QoL: quality of life, CADI: Cardiff Acne Disability Index, PDRDS: Patient-Doctor Relationship Depth-of-Relationship Scale
| Dreno et al. (2011) [24] | Tan et al. (2014) [25] | Myhill et al. (2017) [26] | Donnarumma et al. (2019) [27] | Stein Gold et al. (2021) [28] | |
| Study type | Double-blind, randomized, controlled, parallel-group | Non-inferiority, investigator-blinded, multicenter, randomized, controlled | Randomized controlled trial, multicenter | Randomized controlled trial, multicenter | Double-blind, multicenter, randomized |
| Participants | 378 | 266 | 97 | 126 | 741 |
| Age | 12-35 years | 12-35 years | 22.5 years | 19.5 years | 19.56 years |
| Gender (%) | 44.7% female | NR | 69.1% female | 68.2% female | 61.1% female |
| Acne type | Moderate to severe | Severe, nodular | Mild to moderate (87.6%) | Mild to severe | Moderate to severe |
| Combination and comparison | A0.1/BPO2.5 + lymecycline versus vehicle + lymecycline | A0.1/BPO2.5 + doxycycline, comparison with oral isotretinoin | SEM, additional visits, SCOPE | G1: Informative pamphlet, G2: SCOPE + SMS reminder, G3: SCOPE | A/BPO + clindamycin versus vehicle or combination of any two drugs |
| Inclusion and exclusion | Inclusion: 20 IL, 30-120 NIL, ≤3 nodules; exclusion: acne other than acne vulgaris and pregnancy | Inclusion: age 12-35 years, IGA score ≥ 4, ≥20 papules/pustules, ≥5 nodules | Inclusion: ≥12 years of age, acne grade II and III | Inclusion: 14 and 28 years, IGA 3 and 4, owning a mobile phone capable of receiving text (SMS); exclusion: linguistic or psychiatric issues, non-consent, pregnant or breastfeeding | Inclusion: ≥12 years of age, moderate to severe acne |
| Application | A0.1/BPO2.5 + oral lymecycline or vehicle + oral lymecycline daily | Oral ISO + vehicle gel, oral doxycycline + A/BPO gel, once daily | Daily, topical | Daily, topical | Once daily |
| Time | 12 weeks | 20 weeks | 12 weeks | 12 weeks | 12 weeks |
| Outcomes | Success rate (47.6% versus 33.7%), lesion reduction (29.9% and 35.5% for IL and NIL) versus 19.6-26.8 and 21.8-30, 70% reduction in total lesion, superior efficacy and well tolerated | A0.1/BPO2.5 + doxycycline had early onset to reduce nodules/pustules and total lesions at two weeks, while ISO was superior in reducing all; 33 adverse events with A/BPO + doxycycline, while 73 AEs with ISO | >75% adherence, SEM more helpful in adherence, use of the drug and management of skin irritation, safety, and physician satisfaction (90%) | According to CADI and PDRDS scores, QoL improvement in SMS and leaflet groups > G3; IGA score reduction was significant in G1 versus G2 and G2 versus G3, but non-significant in G1 versus G3 | Success rate (52.5% versus 8.1%), lesion reduction (-74.1% versus -56.8%), well-tolerated combination, satisfaction was more in clindamycin + A/BPO groups |
Benzoyl peroxide gel comes in two doses (2.5% and 5%). Both are safe and effective for the long-term treatment of acne vulgaris. The median score for the reduction in inflammatory and non-inflammatory lesion count was 65% on the 12th week and 80% on the 52nd week when these gels were applied in a randomized controlled trial in 458 patients. There were mild to moderate adverse events (AEs) in the first month, but they were transitory and manageable (greater AEs with 5% BPO than 2.5% BPO) [29]. Adapalene is a synthetic topical retinoid that comes in two strengths (0.1% and 0.3%) and is used in a single fixed daily dose with 2.5% benzoyl peroxide. Adapalene has exhibited good tolerability and efficacy in both doses and in combination with BPO as a fixed dose. A0.3/BPO2.5 is used for a range of acne severity and different patient characteristics (age, gender, and ethnicity) for the treatment of moderate to severe acne. A0.3/BPO2.5, when used as a fixed dose daily, offers efficacy and good patient satisfaction by providing flexible dosing instructions. It is safe and tolerable; however, some patients can develop temporary intolerance, which can be managed by patient education and ways to reduce irritation [30]. The combination (A/BPO) not only continues to reduce acne when given over an extended period of six months but also prevents relapse in cases of severe acne. This was proved in a randomized, multicenter, controlled, double-blind study involving 243 subjects having severe acne vulgaris [31].
Treatment Adherence Issue
Discontinuation of treatment causes treatment failures in acne patients mostly due to lack of adherence to treatment. An observation over two months of 1,160 subjects who were receiving five different kinds of treatment showed that the probability of discontinuation was 50%. The reason for discontinuation was adverse effects (AEs) in 9%, ineffectiveness (52%), combination of AEs and ineffectiveness (3%), controlled acne (9%), and other (1%) [32].
Poor adherence to topical treatment for acne is an issue that leads to expenditures and poor quality of life. To improve adherence, supplementary education material (SEM) or extra visits were applied apart from the standard treatment (standard of care patient education (SCOPE)). Subjective assessment was done on patients on a 12-item questionnaire and physicians on a 14-item questionnaire regarding the use of SEM. Safety of treatment was measured in terms of adverse events. Both patients and physicians were satisfied with the SEM along with SCOPE [26]. A randomized trial that combines text message and leaflet services along with A/BPO administration exhibited that this combination is affordable and simple to operate. To improve patient adherence, satisfaction, and treatment outcomes, doctors can think about implementing these procedures in their practices [27]. Another method to enhance adherence is the modulation of the treatment regimen. In a cohort of 120 patients, the A/BPO was either administered daily overnight (A/BPO-EN), for three hours (A/BPO-3h), daily overnight with moisture (A/BPO-moisture), and every other night (A/BPO-EoN). The adherence to treatment, safety, and tolerability of these regimens is improved if implemented for the first four weeks of the treatment [33]. For the treatment of severe acne, isotretinoin (ISO) has been administered orally as a gold standard treatment regimen for the past 30 years. For cases that experience ISO intolerance or side effects and in case of pregnancy, ISO cannot be administered, so an alternative regimen is required. A/BPO + doxycycline carries a favorable profile and can be used in cases of severe and nodular acne [28,25].
There has been a lot of research on the etiology of acne in the past three years that clarified the pathogenesis of acne. The research covers various aspects including sebaceous gland biology, bacteriology, immunology, cell differentiation, sebum production, keratinization, and wound healing. Bacteriology, hyperkeratinization, and inflammation were the targets of previous acne treatments. The biology of the sebaceous glands and immunology are the new aspects that should be explored for acne therapy. The closely linked factors are interleukins (IL-1β, IL-17, and IL-23) and tumor necrosis factor-alpha (TNF-α), offering new therapeutic targets for treating severe acne. The risk factors for developing acne scars include genetics, systematic and local conditions, and social factors. Research has demonstrated that pro-inflammatory cytokines are essential for the formation of hypertrophic scars after acne. It encompasses transforming growth factor-β (TGF-β), IL-6, matrix metalloproteinase (MMP), IGF-1, and B cells. This kind of scar might be easier to avoid and treat in the future if biological antibodies are made that target these cytokines. Future treatments for acne should include methods that focus on the main causes of acne. To reduce the possibility of scarring and other clinical sequelae, such as pigmentary alterations, it would be extremely ideal to place a special emphasis on intensive therapy during the acute inflammatory phase [34]. Studies included in the present review demonstrated that there is good efficacy and tolerability of fixed-dose combination of A/BPO for acne and acne scars. However, the patients included in the trials have mild to moderate or moderate acne, not severe acne. The review emphasizes that the fixed-dose combination can be used in most of the cases but also suggests that the true etiology of acne should be targeted for effective control.
Study limitation
We could not search other databases as we did not have full access to them.
Conclusions
The results of the studies indicate that the combination therapy of adapalene and benzoyl peroxide (A/BPO) possesses favorable characteristics for acne vulgaris. The primary efficacy of the drug (reduction of lesion count from baseline), reduction in scar count, improved IGA score, and percentage decrease in the lesion count are the outcomes that favor the drug. The drug is also safe as it causes mild to moderate side effects that vanish with time. The drug efficacy can be enhanced by the use of certain interventions such as SMS and literature or by combining with a once-daily dose of an oral drug.
Appendices
The search strategy used in the present study is shown in Table 5.
Table 5. Search strategy.
MeSH: Medical Subject Headings
| Databases | Search strategy |
| PubMed | ((((("Acne Vulgaris/diagnosis"[MeSH] OR "Acne Vulgaris/drug therapy"[MeSH] OR "Acne Vulgaris/epidemiology"[MeSH] OR "Acne Vulgaris/etiology"[MeSH] OR "Acne Vulgaris/pathology"[MeSH] OR "Acne Vulgaris/therapy"[MeSH])) AND ("Adapalene, Benzoyl Peroxide Drug Combination/administration and dosage"[MeSH] OR "Adapalene, Benzoyl Peroxide Drug Combination/adverse effects"[MeSH] OR "Adapalene, Benzoyl Peroxide Drug Combination/therapeutic use"[MeSH])) AND "Randomized Controlled Trial" [Publication Type]) AND "Benzoyl Peroxide"[MeSH]) AND "Adapalene"[MeSH]. acne adapalene/benzoyl peroxide, randomized controlled trial |
| ScienceDirect | Title, abstract, keywords: Acne Vulgaris therapy randomized controlled trial Title, abstract, keywords: Acne Vulgaris randomized controlled trial Title, abstract, keywords: Acne Vulgaris treatment randomized controlled trial Title, abstract, keywords: Acne Vulgaris drug randomized controlled trial Title, abstract, keywords: Acne Vulgaris drug combination randomized controlled trial |
| Google Scholar | allintitle: Acne Vulgaris randomized controlled trial allintitle: Acne Vulgaris etiology allintitle: Acne Vulgaris epidemiology allintitle: Acne Vulgaris treatment trial allintitle: Acne Vulgaris therapy trial allintitle: Acne Vulgaris diagnosis allintitle: Acne Vulgaris therapy combination |
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Amna Akbar, Sarosh Mumtaz, Syeda Sakina, Sabahat Tasneem, Faiza Behram
Drafting of the manuscript: Amna Akbar, Komal Sattar, Sarosh Mumtaz, Syeda Sakina, Faiza Behram
Concept and design: Komal Sattar, Sarosh Mumtaz, Syeda Sakina, Sarosh Khan Jadoon, Sabahat Tasneem, Faiza Behram
Critical review of the manuscript for important intellectual content: Sarosh Mumtaz, Syeda Sakina, Sarosh Khan Jadoon, Sabahat Tasneem, Faiza Behram
Supervision: Syeda Sakina, Sabahat Tasneem, Faiza Behram
References
- 1.Modern management of acne. Cooper AJ, Harris VR. Med J Aust. 2017;206:41–45. doi: 10.5694/mja16.00516. [DOI] [PubMed] [Google Scholar]
- 2.The prevalence, risk factors, and psychosocial impacts of acne vulgaris in medical students: a literature review. Sachdeva M, Tan J, Lim J, Kim M, Nadeem I, Bismil R. Int J Dermatol. 2021;60:792–798. doi: 10.1111/ijd.15280. [DOI] [PubMed] [Google Scholar]
- 3.Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Vos T, Flaxman AD, Naghavi M, et al. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quality-of-life research in acne vulgaris: current status and future directions. Marron SE, Chernyshov PV, Tomas-Aragones L. Am J Clin Dermatol. 2019;20:527–538. doi: 10.1007/s40257-019-00438-6. [DOI] [PubMed] [Google Scholar]
- 5.Acne vulgaris. Williams HC, Dellavalle RP, Garner S. Lancet. 2012;379:361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- 6.Acne vulgaris: diagnosis and treatment. Oge' LK, Broussard A, Marshall MD. https://pubmed.ncbi.nlm.nih.gov/31613567/ Am Fam Physician. 2019;100:475–484. [PubMed] [Google Scholar]
- 7.Systematic review of the epidemiology of acne vulgaris. Heng AH, Chew FT. Sci Rep. 2020;10:5754. doi: 10.1038/s41598-020-62715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acne vulgaris: new evidence in pathogenesis and future modalities of treatment. Hazarika N. J Dermatolog Treat. 2021;32:277–285. doi: 10.1080/09546634.2019.1654075. [DOI] [PubMed] [Google Scholar]
- 9.An overview of adapalene and benzoyl peroxide once-daily topical gel as a therapeutic option for acne. Emmerich VK, Purvis CG, Feldman SR. Expert Opin Pharmacother. 2021;22:1661–1667. doi: 10.1080/14656566.2021.1939678. [DOI] [PubMed] [Google Scholar]
- 10.Topical retinoids in acne vulgaris: a systematic review. Kolli SS, Pecone D, Pona A, Cline A, Feldman SR. Am J Clin Dermatol. 2019;20:345–365. doi: 10.1007/s40257-019-00423-z. [DOI] [PubMed] [Google Scholar]
- 11.Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. Thiboutot DM, Weiss J, Bucko A, et al. J Am Acad Dermatol. 2007;57:791–799. doi: 10.1016/j.jaad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Emerging therapies for acne vulgaris. Trivedi MK, Bosanac SS, Sivamani RK, Larsen LN. Am J Clin Dermatol. 2018;19:505–516. doi: 10.1007/s40257-018-0345-x. [DOI] [PubMed] [Google Scholar]
- 13.Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Int J Evid Based Healthc. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 14.Association between glomerular filtration rate and β-thalassemia major: a systematic review and meta-analysis. Khandker SS, Jannat N, Sarkar D, et al. Thalass Rep. 2023;13:195–205. [Google Scholar]
- 15.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A dermocosmetic regimen is able to mitigate skin sensitivity induced by a retinoid-based fixed combination treatment for acne: results of a randomized clinical trial. Khammari A, Kerob D, Demessant AL, Nioré M, Dréno B. J Cosmet Dermatol. 2024;23:1313–1319. doi: 10.1111/jocd.16120. [DOI] [PubMed] [Google Scholar]
- 17.A North American study of adapalene-benzoyl peroxide combination gel in the treatment of acne. Stein Gold L, Tan J, Cruz-Santana A, et al. https://pubmed.ncbi.nlm.nih.gov/19746769/ Cutis. 2009;84:110–116. [PubMed] [Google Scholar]
- 18.Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Gollnick HP, Draelos Z, Glenn MJ, et al. Br J Dermatol. 2009;161:1180–1189. doi: 10.1111/j.1365-2133.2009.09209.x. [DOI] [PubMed] [Google Scholar]
- 19.Moderate and severe inflammatory acne vulgaris effectively treated with single-agent therapy by a new fixed-dose combination adapalene 0.3%/benzoyl peroxide 2.5% Gel: a randomized, double-blind, parallel-group, controlled study. Stein Gold L, Weiss J, Rueda MJ, Liu H, Tanghetti E. Am J Clin Dermatol. 2016;17:293–303. doi: 10.1007/s40257-016-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adapalene 0.1%/benzoyl peroxide 2.5% gel reduces the risk of atrophic scar formation in moderate inflammatory acne: a split-face randomized controlled trial. Dreno B, Tan J, Rivier M, Martel P, Bissonnette R. J Eur Acad Dermatol Venereol. 2017;31:737–742. doi: 10.1111/jdv.14026. [DOI] [PubMed] [Google Scholar]
- 21.Prevention and reduction of atrophic acne scars with adapalene 0.3%/benzoyl peroxide 2.5% gel in subjects with moderate or severe facial acne: results of a 6-month randomized, vehicle-controlled trial using intra-individual comparison. Dréno B, Bissonnette R, Gagné-Henley A, Barankin B, Lynde C, Kerrouche N, Tan J. Am J Clin Dermatol. 2018;19:275–286. doi: 10.1007/s40257-018-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long-term effectiveness and safety of up to 48 weeks’ treatment with topical adapalene 0.3%/benzoyl peroxide 2.5% gel in the prevention and reduction of atrophic acne scars in moderate and severe facial acne. Dréno B, Bissonnette R, Gagné-Henley A, et al. Am J Clin Dermatol. 2019;20:725–732. doi: 10.1007/s40257-019-00454-6. [DOI] [PubMed] [Google Scholar]
- 23.Treatment modalities for acne. Fox L, Csongradi C, Aucamp M, et al. Molecules. 2016;21 doi: 10.3390/molecules21081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Combination therapy with adapalene-benzoyl peroxide and oral lymecycline in the treatment of moderate to severe acne vulgaris: a multicentre, randomized, double-blind controlled study. Dréno B, Kaufmann R, Talarico S, et al. Br J Dermatol. 2011;165:383–390. doi: 10.1111/j.1365-2133.2011.10374.x. [DOI] [PubMed] [Google Scholar]
- 25.A treatment for severe nodular acne: a randomized investigator-blinded, controlled, noninferiority trial comparing fixed-dose adapalene/benzoyl peroxide plus doxycycline vs. oral isotretinoin. Tan J, Humphrey S, Vender R, et al. Br J Dermatol. 2014;171:1508–1516. doi: 10.1111/bjd.13191. [DOI] [PubMed] [Google Scholar]
- 26.Use of supplementary patient education material increases treatment adherence and satisfaction among acne patients receiving adapalene 0.1%/benzoyl peroxide 2.5% gel in primary care clinics: a multicenter, randomized, controlled clinical study. Myhill T, Coulson W, Nixon P, et al. Dermatol Ther (Heidelb) 2017;7:515–524. doi: 10.1007/s13555-017-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.How to increase adherence and compliance in acne treatment? A combined strategy of SMS and visual instruction leaflet. Donnarumma M, Fattore D, Greco V, et al. Dermatology. 2019;235:463–470. doi: 10.1159/000502575. [DOI] [PubMed] [Google Scholar]
- 28.Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Stein Gold L, Baldwin H, Kircik LH, et al. Am J Clin Dermatol. 2022;23:93–104. doi: 10.1007/s40257-021-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Open-label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long-term use in patients with acne vulgaris: a secondary publication. Kawashima M, Nagare T, Katsuramaki T. J Dermatol. 2017;44:635–643. doi: 10.1111/1346-8138.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adapalene/benzoyl peroxide gel 0.3%/2.5% for acne vulgaris. Dréno B, Layton AM, Troielli P, et al. European Journal of Dermatology. 2022;32:445–450. doi: 10.1684/ejd.2022.4275. [DOI] [PubMed] [Google Scholar]
- 31.A 6-month maintenance therapy with adapalene-benzoyl peroxide gel prevents relapse and continuously improves efficacy among patients with severe acne vulgaris: results of a randomized controlled trial. Poulin Y, Sanchez NP, Bucko A, et al. Br J Dermatol. 2011;164:1376–1382. doi: 10.1111/j.1365-2133.2011.10344.x. [DOI] [PubMed] [Google Scholar]
- 32.Real-world adherence to topical therapies in patients with moderate acne. Lam Hoai XL, De Maertelaer V, Simonart T, et al. JAAD Int. 2021;2:109–115. doi: 10.1016/j.jdin.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The safety and efficacy of four different fixed combination regimens of adapalene 0.1%/benzoyl peroxide 2.5% gel for the treatment of acne vulgaris: results from a randomised controlled study. Tan J, Bissonnette R, Gratton D, Kerrouche N, Canosa JM. Eur J Dermatol. 2018;28:502–508. doi: 10.1684/ejd.2018.3367. [DOI] [PubMed] [Google Scholar]
- 34.Updated treatment for acne: targeted therapy based on pathogenesis. Kurokawa I, Layton AM, Ogawa R. Dermatol Ther (Heidelb) 2021;11:1129–1139. doi: 10.1007/s13555-021-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]