Abstract
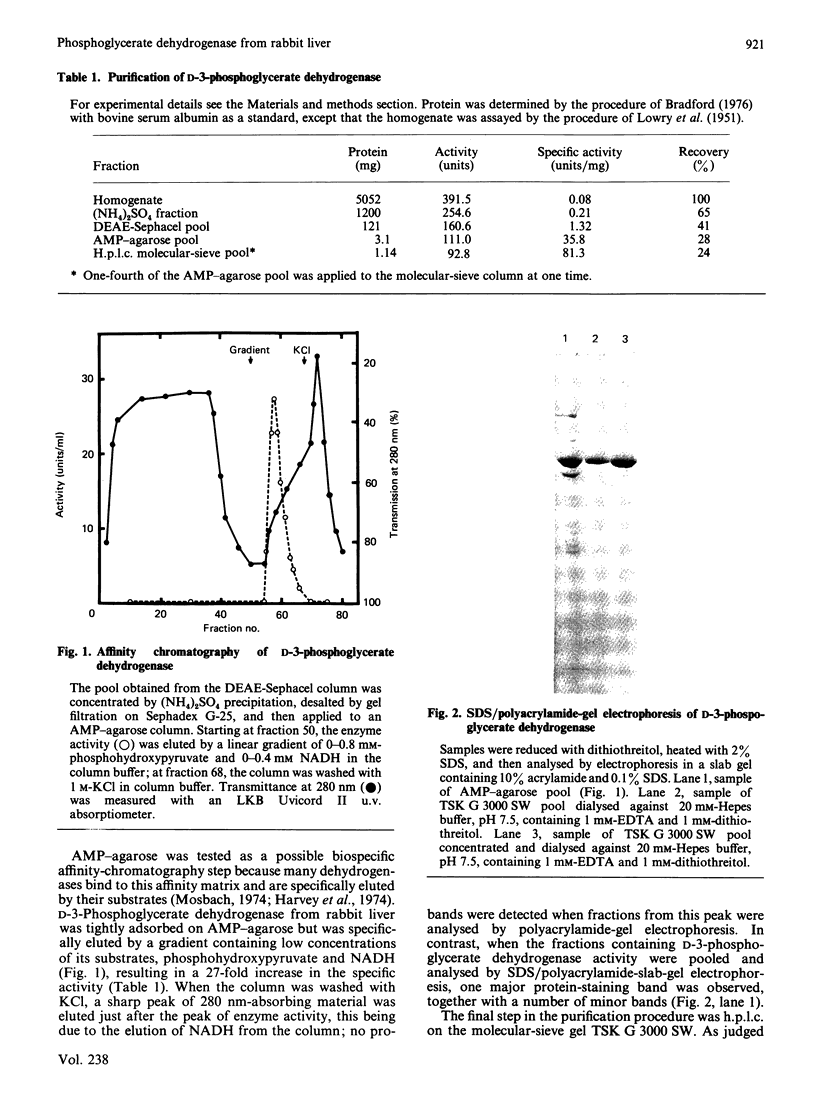
D-3-Phosphoglycerate dehydrogenase (EC 1.1.1.95) was purified from rabbit liver by (NH4)2SO4 fractionation, DEAE-Sephacel chromatography, affinity chromatography on AMP-agarose and molecular-sieve h.p.l.c. The purified enzyme was homogeneous as judged by SDS/polyacrylamide-slab-gel electrophoresis. On the basis of molecular-sieve h.p.l.c. and SDS/polyacrylamide-gel electrophoresis, the enzyme is a tetramer composed of subunits of Mr 60,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dubrow R., Pizer L. I. Transient kinetic and deuterium isotope effect studies on the catalytic mechanism of phosphoglycerate dehydrogenase. J Biol Chem. 1977 Mar 10;252(5):1539–1551. [PubMed] [Google Scholar]
- Dubrow R., Pizer L. I. Transient kinetic studies on the allosteric transition of phosphoglycerate dehydrogenase. J Biol Chem. 1977 Mar 10;252(5):1527–1538. [PubMed] [Google Scholar]
- Field R. D., Sallach H. J. D-3-phosphoglycerate dehydrogenase from hog spinal cord-1. Methods Enzymol. 1975;41:282–285. doi: 10.1016/s0076-6879(75)41064-3. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Bradshaw R. A. D-3-Phosphoglycerate dehydrogenase from chicken liver. II. Chemical and physical properties. J Biol Chem. 1978 Apr 25;253(8):2727–2731. [PubMed] [Google Scholar]
- Grant G. A., Keefer L. M., Bradshaw R. A. D-3-Phosphoglycerate dehydrogenase from chicken liver. I. Purification. J Biol Chem. 1978 Apr 25;253(8):2724–2726. [PubMed] [Google Scholar]
- Harvey M. J., Craven D. B., Lowe C. R., Dean P. D. N6-immobilized 5 -AMP and NAD+: preparations and applications. Methods Enzymol. 1974;34:242–253. doi: 10.1016/s0076-6879(74)34020-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund K., Merrill D. K., Guynn R. W. The reactions of the phosphorylated pathway of L-serine biosynthesis: thermodynamic relationships in rabbit liver in vivo. Arch Biochem Biophys. 1985 Feb 15;237(1):186–196. doi: 10.1016/0003-9861(85)90268-1. [DOI] [PubMed] [Google Scholar]
- Mosbach K. AMP and NAD as "General Ligands". Methods Enzymol. 1974;34:229–242. doi: 10.1016/s0076-6879(74)34019-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto E., Pizer L. I. The mechanism of end product inhibition of serine biosynthesis. I. Purification and kinetics of phosphoglycerate dehydrogenase. J Biol Chem. 1968 May 10;243(9):2081–2089. [PubMed] [Google Scholar]
- Walsh D. A., Sallach H. J. Comparative studies on the pathways for serine biosynthesis in animal tissues. J Biol Chem. 1966 Sep 10;241(17):4068–4076. [PubMed] [Google Scholar]
- Walsh D. A., Sallach H. J. Purification and properties of chicken liver D-3-phosphoglycerate dehydrogenase. Biochemistry. 1965 Jun;4(6):1076–1085. doi: 10.1021/bi00882a015. [DOI] [PubMed] [Google Scholar]
- Winicov I., Pizer L. I. The mechanism of end product inhibition of serine biosynthesis. IV. Subunit structure of phosphoglycerate dehydrogenase and steady state kinetic studies of phosphoglycerate oxidation. J Biol Chem. 1974 Mar 10;249(5):1348–1355. [PubMed] [Google Scholar]