Abstract
OBJECTIVE
A protocol was developed for neonatal intensive care unit (NICU) delirium: Step 1, gabapentin for pain or melatonin for sleep; Step 2, add on other Step 1 agent; Step 3, antipsychotics. The purpose of this study was to describe the utility and dosing of gabapentin for NICU delirium.
METHODS
Retrospective evaluation of NICU patients from January 1, 2021–December 31, 2022 who received >1 dose of gabapentin based on the delirium protocol. Data collection included demographics, gabapentin regimen, and concomitant sedatives and analgesics. The primary objective was to identify the number of patients receiving gabapentin for Step 1 or Step 2. Secondary objectives included identifying the number of patients requiring antipsychotics (Step 3), the gabapentin regimen, comparison of Échelle de Douleur et d'Inconfort du Nouveau-né (EDIN), Cornell Assessment of Pediatric Delirium (CAPD), and Withdrawal Assessment Tool-1 (WAT-1) scores 72 hours pre- and post-gabapentin initiation, and comparison of opioids, clonidine, and melatonin 24 hours pre- and 72 hours post-gabapentin initiation. Wilcoxon signed rank tests were employed with significance defined at p < 0.05.
RESULTS
Twenty-nine patients were studied. The majority (n = 22; 75.9%) received gabapentin for Step 1; no patients required Step 3. The median initial dose was 14.4 mg/kg/day divided every 8 hours. Twelve (41.4%) required increase to a median of 16.9 mg/kg/day. A significant decrease in EDIN and WAT-1 scores was noted, but there was no change in CAPD scores or opioid, clonidine, or melatonin doses pre- versus post-gabapentin.
CONCLUSION
The majority received gabapentin at a median dose of 14 mg/kg/day as Step 1 for delirium. Gabapentin was associated with a significant decrease in pain and withdrawal scores.
Keywords: delirium, gabapentin, infant, neonatal intensive care, neonate
Introduction
Agitation is a common characteristic observed in the neonatal intensive care unit (NICU), which can occur secondary to pain, discomfort, or hunger. For patients who continue to have agitation despite adequate analgesia, other causes of agitation including delirium must be explored.1 Delirium is defined as an acute cerebral dysfunction caused by systemic illness.2,3 A recent study by Siegel et al2 noted that delirium occurred in 22.4% of NICU patients at term-equivalent age in their prospective pilot-study. Risk factors for delirium in the NICU setting have not been fully elucidated, but Siegel et al2 found that mechanical ventilation and neurologic disability were independently associated with a positive delirium screen. Other risk factors established in older infants and children in the pediatric or cardiac intensive care unit include age <2 years, congenital heart disease, and medications (e.g., benzodiazepines and anticholinergics).3
There are no guidelines for the management of agitation or delirium in the NICU setting. Anecdotally, medications that are used for the treatment of pediatric delirium include melatonin and antipsychotics (e.g., olanzapine, quetiapine).3–5 However, due to limited evidence with melatonin and risk of adverse events with antipsychotics in infants, another option that can be considered for NICU delirium is gabapentin. Gabapentin is a gamma-aminobutyric acid analog used to treat neuropathic pain, seizures, and postoperative pain. Gabapentin targets calcium channels, specifically the alpha-2-delta-1 subunit, modulating the release of excitatory neurotransmitters that contribute to nociception.6 Currently, 8 reports have described the use of gabapentin in 77 neonates and infants for the treatment of refractory or post-operative pain and agitation.6–13 Gabapentin was shown to result in decreased pain scores and decreased sedative and analgesic doses after initiation.
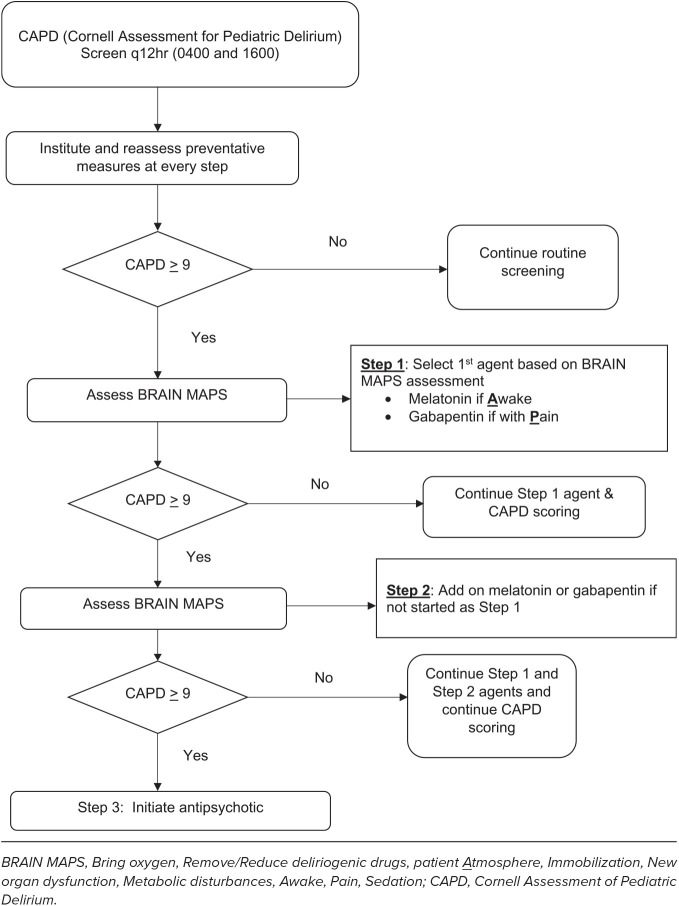
While there are few studies that evaluate the use of gabapentin for agitation, there are no studies evaluating its use to treat delirium, and there remains a paucity of data to guide selection of an initial dose. At the study institution, an interprofessional team developed and implemented a delirium protocol in 2021 that included 3 steps for management (Figure). If delirium was suspected, then providers would utilize the BRAIN MAPS acronym (Bring oxygen, Remove/Reduce deliriogenic drugs, patient Atmosphere, Immobilization, New organ dysfunction, Metabolic disturbances, Awake, Pain, Sedation) to identify potential etiologies for delirium to guide management including the addition of melatonin for sleep promotion and gabapentin for pain.3 Gabapentin or melatonin could be initiated for Step 1, depending on the BRAIN MAPS assessment. For Step 2, the alternate Step 1 agent was added. Step 3 included initiation of antipsychotics. The purpose of this study was to describe the utility of gabapentin and dosing for treatment of delirium.
Figure.
NICU delirium protocol.
Methods
This study was a retrospective, single center cohort study of neonates and infants admitted to a 96-bed NICU at a tertiary care academic medical center from January 1, 2021 through December 31, 2022. Patients were identified using the electronic medical record (EMR), Meditech (Medical Information Technology, Inc, Westwood, MA) and were included if they received >1 dose of gabapentin. Patients were excluded if they received gabapentin for seizures or for neuropathic pain managed by the pediatric pain management team. For patients who may have had >1 gabapentin course, only the first course of gabapentin was included for analysis. If gabapentin was discontinued for >48 hours, this marked the end of the first course of gabapentin.
Demographics collected included age (postnatal age, gestational age, and postmenstrual age), biological sex, and weight, and length. The underlying neurologic conditions at the time of gabapentin were collected including acute brain injury (hypoxic ischemic encephalopathy, meningitis, grade 3 or 4 intraventricular hemorrhage), congenital brain malformation (i.e., lissencephaly and hydrocephalus), and genetic syndromes associated with severe developmental delay). Data collected for the gabapentin course included dose (mg/kg), interval, and duration of treatment. During the study period, gabapentin initial dose, dose titration, and tapering were at the discretion of the provider; dosing recommendations were not part of the protocol. To assess the potential impact on enteral feeding, the total mL/kg/day of enteral nutrition were collected on the day of gabapentin initiation and 1 week after gabapentin initiation. Pain, iatrogenic withdrawal syndrome (IWS) and delirium were assessed through collection of pain [Échelle de Douleur et d'Inconfort du Nouveau-né (EDIN)], iatrogenic withdrawal syndrome [Withdrawal Assessment Tool-1 (WAT-1)], and delirium [Cornell Assessment of Pediatric Delirium (CAPD)] scores.14–16 Concomitant medications for agitation, pain, or delirium including clonidine, opioids, sedatives, melatonin, and/or antipsychotics given within 24 hours before and 72 hours after gabapentin initiation were also collected. Opioids were converted to morphine milligram equivalents (MME) to allow more direct comparison (i.e., 0.1 mg fentanyl and 1.5 mg hydromorphone = 10 mg of morphine).17 Charts were screened for mention of adverse events during gabapentin administration (e.g., over sedation, nystagmus) and after discontinuation (i.e., withdrawal).
The primary objective of this study was to identify the number of patients receiving gabapentin for Step 1 or Step 2 in a delirium protocol. Secondary objectives included identifying the number of patients requiring antipsychotics in Step 3, median gabapentin dose (mg/kg) and frequency of administration, identification of documented adverse events associated with gabapentin in provider EMR notes, comparison of enteral nutrition (mL/kg/day) the day of gabapentin initiation versus 1 week after gabapentin initiation, and comparison of EDIN, CAPD, and WAT-1 scores within the time period of 72 hours pre- and post-gabapentin initiation. An additional objective was to compare the dose per kg/day of MME, clonidine, and melatonin 24 hours pre- and the 72 hours post-gabapentin initiation.
Descriptive statistics were used to summarize patient and clinical characteristics. Wilcoxon signed rank tests were used to make comparisons for changes in melatonin, clonidine, and MME cumulative dosing and EDIN, WAT-1, and CAPD scores before and after gabapentin administration. Analysis was conducted via SAS version 9.4 (Statistical Analysis System, Cary, NC), with the a priori alpha set at <0.05.
Results
Twenty-nine patients met inclusion criteria. Baseline demographics are included in Table 1. The median gestational age was 26.3 weeks, and the median postmenstrual age at which gabapentin was initiated was 43.5 weeks. The majority (n = 17; 58.6%) were females and had an underlying neurologic condition (n = 17; 58.6%). Seven (24.1%) patients had >1 type of an underlying neurologic condition. All patients received mechanical ventilation at some point prior to gabapentin for a median (IQR) of 92 (49–109) days. However, only 16 (55.2%) were mechanically ventilated at the time of gabapentin initiation.
Table 1.
Patient Demographics and Gabapentin Regimen (n = 29)
| Variable | Median (IQR) or Number (%) |
|---|---|
| Demographics | |
| Age | |
| Gestational age, wk | 26.3 (25.3–30.7) |
| Postnatal age, days | 111.7 (70.6–140.7) |
| Postmenstrual age, wk | 43.5 (37.6–49.1) |
| Females | 17 (58.6) |
| Weight, kg | 3.7 (2.5–4.7) |
| Length, cm | 47.5 (41.0–52.0) |
| Respiratory support at time of gabapentin initiation | |
| Room air | 1 (3.4) |
| Nasal cannula | 2 (6.9) |
| Non-invasive ventilation | 10 (34.5) |
| Mechanical ventilation | 16 (55.2) |
| Underlying neurologic conditions* | |
| None | 12 (41.4) |
| Acquired brain injury† | 8 (27.6) |
| Congenital brain malformation‡ | 7 (24.1) |
| Genetic syndromes associated with severe developmental delay | 11 (37.9) |
| Gabapentin regimen | |
| Initial dose | |
| mg/kg/dose | 4.9 (4.7–5.0) |
| mg/kg/day | 14.4 (10.0–15.0) |
| Initial dose frequency | |
| Every 24 hr | 3 (10.3) |
| Every 12 hr | 4 (13.8) |
| Every 8 hr | 22 (75.9) |
| Patients requiring dose increases | 12 (41.4) |
| Maximum dose (mg/kg/day)§ | 6.9 (15.3–28.6) |
| Maximum dose frequency* | |
| Every 24 hr | — |
| Every 12 hr | 2 (16.7) |
| Every 8 hr | 10 (83.3) |
| Duration, days | 48.0 (11.0–84.9) |
| Taper plan | |
| Gabapentin taper | 15 (51.7) |
| Taper duration, days | 16.0 (9.5–25.5) |
* Numbers add up to >100% as 7 (24.1%) patients had >1 type of underlying neurologic condition.
† Includes hypoxic ischemic encephalopathy, meningitis, intraventricular hemorrhage (grade 3 or higher), and stroke.
‡ Includes lissencephaly and hydrocephalus.
§ Represents patients who required a dosage increase only.
The majority (n = 22; 75.9%) received gabapentin for their Step 1 agent. The remaining 7 (21.4%) patients received gabapentin for Step 2 after receiving melatonin for Step 1 for a median (IQR) of 42 days (18–48). For the 22 patients who received gabapentin as their Step 1 agent, 16 of these patients received melatonin for Step 2 for a median (IQR) of 30 days (16.5–67.3) after gabapentin was initiated. The other 6 patients did not require initiation of melatonin for Step 2. No patients received Step 3 therapy.
Table 1 also provides the gabapentin regimen that these patients received. The median initial dose in mg/kg/day was 14.4 mg/kg/day with most patients (n = 22; 75.9%) receiving divided doses every 8 hours. Twelve (41.4%) infants had their gabapentin dose increased to a median maximum dose of 16.9 mg/kg/day. One patient received 35 mg/kg/day divided every 8 hours. The median total duration was 48 days. Fifteen (51.7%) had their gabapentin dose tapered over a median of 16 days. No patients were noted to have adverse events while receiving gabapentin or withdrawal symptoms after gabapentin discontinuation documented in the EMR. There was also no difference in median (IQR) mL/kg/day of enteral feeds the day of gabapentin initiation versus 1 week after gabapentin therapy, 132 (109–145) versus 129 (105–140), p = 0.617.
Table 2 provides the change in EDIN, WAT-1, and CAPD scores over the 72-hour time frame prior to and 72-hour after gabapentin administration. All infants had EDIN scores documented before and after gabapentin. There was a significant decrease in the median EDIN pain scores before and after gabapentin, 3 versus 2, p = 0.029. Seventeen (60.7%) infants had WAT-1 scores documented before and after gabapentin. There was a significant decrease in the median WAT-1 score before and after gabapentin, 3 versus 2, p = 0.048. Only 8 (32.1%) infants had CAPD scores documented in the EMR. No significant change in the CAPD scores before and after gabapentin were noted.
Table 2.
Comparison of Concomitant Sedative and Analgesic Medications and Clinical Scores Before and After Gabapentin Initiation
| Change in Concomitant Sedative and Analgesic Medication Exposure | |||||
|---|---|---|---|---|---|
| Medications | 24-Hours Prior to Gabapentin Initiation | 72-Hours AfterGabapentin Initiation | p value* | ||
| Median (IQR) | N | Median (IQR) | N | ||
| Opioids, MME/kg/day | 1.09 (0.39–3.49) | 21 | 1.45 (0.56–2.24) | 21 | 0.571 |
| Clonidine, mcg/kg/day | 4.1 (2.08–8.40) | 14 | 5.81 (2.59–9.06) | 15 | — |
| Melatonin, mg/kg/day | 0.31 (0.25–0.39) | 8 | 0.35 (0.28–0.40) | 5 | — |
| Change in Pain, Sedation, and Iatrogenic Withdrawal Scores | |||||
|---|---|---|---|---|---|
| Scores | 72-Hours Prior to Gabapentin Initiation | 72-Hours After Gabapentin Initiation | p value | ||
| Median (IQR) | N | Median (IQR) | N | ||
| EDIN | 3 (2–5) | 28 | 2 (2–3) | 28 | 0.029 |
| WAT-1 | 3 (2–4) | 17 | 2 (2–3) | 17 | 0.048 |
| CAPD | 13 (12–14) | 9 | 11 (8–15) | 9 | 0.356 |
CAPD, Cornell Assessment of Pediatric Delirium; EDIN, Échelle de Douleur et d'Inconfort du Nouveau-né; MME, morphine milligram equivalents; WAT-1, Withdrawal Assessment Tool-1
* Wilcoxon signed rank test utilized for analysis.
Other concomitant opioid and sedation medications were collected during the 24 hours before and 72 hours after gabapentin initiation. Most patients (n = 21; 75.0%) were on opioids and/or clonidine (n = 17; 60.7%) before gabapentin initiation and were at risk of IWS. Table 2 provides a comparison of opioid and sedation medications. There was no significant difference in the median MME (mg/kg/day) in the 24 hours before and in the 72 hours after gabapentin initiation, 1.09 versus 1.45 mg/kg/day, p = 0.571. Fourteen (50.0%) were receiving concomitant clonidine before gabapentin initiation. One patient was initiated on clonidine in the 72 hours post-gabapentin initiation. Due to the limited sample size, statistical analysis was not performed. However, 9 (32.1%) of the infants on clonidine before gabapentin initiation did not have an increase in their clonidine dose during this 72-hour post-gabapentin time frame. Eight patients received melatonin in the 24 hours prior to gabapentin initiation and the median dose was 0.3 mg/kg/day. The weight-based dosing varied because doses were rounded to flat doses of 1 to 1.5 mg/dose. Due to the small sample size, no comparison in the cumulative melatonin dose was evaluated. However, 3 infants had their melatonin discontinued after gabapentin initiation, and the other 5 had no change in their melatonin dose.
Discussion
This study is one of the largest studies to evaluate the impact of gabapentin for delirium in the NICU. Patients included in this cohort had significant risk for delirium because the majority had underlying neurologic conditions, and all were mechanically ventilated prior to gabapentin initiation. All patients were managed according to a locally established delirium protocol. Most (75.9%) were initiated on gabapentin for Step 1 therapy according to the delirium protocol. No patients required Step 3 with initiation of an antipsychotic.
Based on the results, gabapentin does appear to aid in minimizing agitation and pain scores, but no difference in CAPD results were noted. This is also supported by Sacha et al6 who conducted a retrospective observational study in 22 infants in the NICU (median postnatal age 87 days) receiving gabapentin. They reported a median gabapentin dose of 10.2 mg/kg/day in 3 divided doses and noted a decrease in the median Neonatal Pain and Sedation Scale (N-PASS) scores from 3.1 to 0. Our study noted a significant decrease in EDIN and WAT-1 scores pre- and post-gabapentin. At the time of the study, our institution did not routinely assess N-PASS scores, so it is difficult to make direct comparisons between the studies. In the previous 8 reports evaluating gabapentin in the NICU, only 2 reports evaluated its impact on WAT-1 or Finnegan scores and CAPD scores. One report by Allen et al12 included a case series of 5 infants with single ventricle physiology, and they noted no changes in WAT-1 and CAPD scores after gabapentin initiation. It is difficult to compare this to our findings given the small sample size.
The patients in our study received a median initial dose of 14.4 mg/kg/day divided every 8 hours. This regimen appears to be within the range of the reported regimens in previous studies, ranging from 3.3 to 14 mg/kg/dose administered once daily or every 8 hours.6–13 A dose increase was required for 40% of patients up to a median of 16.9 mg/kg/day, with 1 patient receiving 35 mg/kg/day. In the previous reports evaluating gabapentin, there was wide variability in the maximum dose, but 1 study by Patz et al13 colleagues titrated gabapentin up to 43 mg/kg/day divided every 8 hours. In these previous studies, the duration of gabapentin varied as well with some patients receiving up to 116 days.6–13 Our patients had a median duration of 48 days. Four (5.6%) out of the 71 neonates and infants who have received gabapentin in other published reports have developed adverse events including nystagmus (n = 2) and bradycardia (n = 2). No patients in our study developed adverse events based on documentation in the EMR progress notes. In addition, there was no difference in enteral feeding before or after gabapentin initiation. This finding is different from what was reported by Bruce et al8 who found that initiation of gabapentin in 15 infants with congenital heart disease resulted in a significant increase in enteral feeds. It is difficult to compare with our study because our patients were receiving a median of 132 mL/kg/day of enteral nutrition when gabapentin was initiated.
In our study, 51.7% of patients had their gabapentin tapered over a period of days to weeks. Similar to most of the published studies with gabapentin in neonates and infants, no patients in our study were noted to have withdrawal symptoms after gabapentin discontinuation. Edwards et al7 noted 3 children with withdrawal attributed to gabapentin including tachycardia, emesis, and irritability upon abrupt discontinuation. Other symptoms of gabapentin withdrawal reported in the literature include confusion and delirium.7 Additionally, they noted that the symptoms of withdrawal resolved upon re-initiation of gabapentin.
Our study did not find a significant change in the mg/kg/day dosing of MME, clonidine, or melatonin in the 24 hours pre- and 72 hours post-gabapentin. Other studies evaluating gabapentin in neonates and infants reported on changes to concomitant analgesics and sedatives following gabapentin initiation.6–12 However, these studies mainly reported on the changes in the total number of doses for these concomitant agents per day. As a result, it is difficult to make direct comparisons between other published studies and our own.
Several limitations of our study were noted. First, the retrospective nature of the study made it difficult to determine the true incidence of adverse events because we relied on documentation in the provider EMR notes and events may not have been specifically attributed to gabapentin. Second, this study was conducted at a single center and may not be reproducible at other institutions. The CAPD and WAT-1 scoring tools may not be routinely used in all NICUs. The CAPD tool has been validated in children 0 to 18 years of age and the WAT-1 in patients >14 days to 18 years. It should be noted that the cohort included in this study had a median postnatal age of 111 days and postmenstrual age of nearly 44 weeks, so application of these scoring tools was appropriate. Third, only 9 (31.0%) had documentation of CAPD scores. At our institution, CAPD scoring was implemented in the NICU beginning in 2020. However, there was difficulty with documenting scores within our EMR system initially, and therefore scores for many of the patients in 2021 were not retrievable from the chart review. We believe that CAPD scoring was conducted in all patients according to the protocol, but we are limited in our analysis of these scores given the limited documentation.
Conclusion
Gabapentin was initiated in the majority of patients as Step 1 in the NICU delirium protocol at a median initial dose of 14 mg/kg/day divided every 8 hours. Upward titration of doses was required for approximately 40% of patients to a median maximum dose of 16.9 mg/kg/day. Initiation of gabapentin was associated with a significant decrease in pain and withdrawal scores, but no significant changes in the mg/kg/day of MME, clonidine, or melatonin.
Acknowledgment.
At the time of this study, Dr Chang was a PGY2 Pediatric Pharmacy Resident at The University of Oklahoma College of Pharmacy.
ABBREVIATIONS
- BRAIN MAPS
Bring oxygen, Remove/Reduce deliriogenic drugs, patient Atmosphere, Immobilization, New organ dysfunction, Metabolic disturbances, Awake, Pain, Sedation;
- CAPD
Cornell Assessment of Pediatric Delirium;
- EDIN
Échelle de Douleur et d'Inconfort du Nouveau-né;
- EMR
electronic medical record;
- IWS
iatrogenic withdrawal syndrome;
- MME
morphine milligram equivalents;
- NICU
neonatal intensive care unit;
- WAT-1
Withdrawal Assessment Tool-1
Footnotes
Disclosure. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and has been approved by our institution review board. Given the nature of this study, informed consent and assent were not required.
References
- 1.McPherson C, Miller S, El-Dib M, et al. The influence of pain, agitation, and their management on the immature brain. Pediatr Res . 2020;88(2):168–175. doi: 10.1038/s41390-019-0744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel EJ, Groves AM, Silver G, et al. Delirium in the NICU: a point prevalence study. Hosp Pediatr . 2021;11:e321–e326. doi: 10.1542/hpeds.2020-005736. [DOI] [PubMed] [Google Scholar]
- 3.Smith HAB, Berkenbosch JW, Betters KA, et al. Society of Critical Care Medicine Clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Ped Crit Care Med . 2022;2022;23(2):e74–e110. doi: 10.1097/PCC.0000000000002873. [DOI] [PubMed] [Google Scholar]
- 4.Laudone TW, Beck SD, Lahr HJ. Evaluation of melatonin practices for delirium in pediatric critically ill patients. J Pediatr Pharmacol Ther . 2021;26(4):631–365. doi: 10.5863/1551-6776-26.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capino AC, Thomas AN, Baylor S, et al. Antipsychotic use in the prevention and treatment of critically ill infants and children with delirium. J Pediatr Pharmacol Ther . 2020;25(2):81–95. doi: 10.5863/1551-6776-25.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacha GL, Foreman MG, Kyllonen K, Rodriguez RJ. The use of gabapentin for pain and agitation in neonates and infants in a neonatal ICU. J Pediatr Pharmacol Ther . 2017;22(3):207–211. doi: 10.5863/1551-6776-22.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards L, DeMeo S, Hornik CD, et al. Gabapentin use in the neonatal intensive care unit. J Pediatr . 2016;169:310–312. doi: 10.1016/j.jpeds.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce AS, Davis A, Baum CF, et al. Retrospective study of gabapentin for poor oral feeding in infants with congenital heart disease. Glob Pediatr Health . 2015;1–3 doi: 10.1177/2333794X15591565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnsed JC, Heinan K, Letzkus L, Zanelli S. Gabapentin for pain, movement disorders, and irritability in neonates and infants. Dev Med Child Neurol . 2020;62(3):386–389. doi: 10.1111/dmcn.14324. [DOI] [PubMed] [Google Scholar]
- 10.Behm MO, Kearns GL. Treatment of pain with gabapentin in a neonate. Pediatrics . 2001;108(2):482–484. doi: 10.1542/peds.108.2.482. [DOI] [PubMed] [Google Scholar]
- 11.Haney AL, Garner SS, Cox TH. Gabapentin therapy for pain and irritability in a neurologically impaired infant. Pharmacotherapy . 2009;29(8):997–1001. doi: 10.1592/phco.29.8.997. [DOI] [PubMed] [Google Scholar]
- 12.Allen CC, Canada K, Schlueter S, et al. Gabapentin can improve irritability and feeding tolerance in single ventricle interstage patients: a case series. Pediatr Cardiol . 2023;44(2):487–493. doi: 10.1007/s00246-022-03009-5. [DOI] [PubMed] [Google Scholar]
- 13.Patz C, Liviskie C, Bird M, et al. Gabapentin as adjunctive therapy in neonatal opioid withdrawal syndrome: a case series. J Pediatr Phamacol Ther . 2023;28(4):368–373. doi: 10.5863/1551-6776-28.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debillon T, Zupan V, Ravault N, et al. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal . 2001;85(1):F36–F41. doi: 10.1136/fn.85.1.F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU. Crit Care Med . 2014;42(3):656–663. doi: 10.1097/CCM.0b013e3182a66b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franck LS, Scoppettuolo LA, Wypij D, et al. Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain . 2012;153(1):142–148. doi: 10.1016/j.pain.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson PN. In: Pediatric Pharmacotherapy . 2nd ed. Nahata M, Benavides S, editors. Lenexa, KS: American College of Clinical Pharmacy; 2020. Pain management; pp. 900–943. [Google Scholar]