Abstract
Postural orthostatic tachycardia syndrome (POTS) is a chronic illness with unknown mortality and high morbidity, often diagnosed in the adolescent years. Published literature regarding POTS primarily focuses on the adult population, and guidance on treatment in pediatrics is sparse. The purpose of this clinical review is to evaluate the current literature on the management of POTS in pediatric patients. A search was conducted using the Cochrane database, Google Scholar, and PubMed. Studies were included if they evaluated the management of POTS, primarily in pediatric patients. Case reports and series were excluded. Eight published studies met the inclusion and exclusion criteria. To date, there are no US Food and Drug Administration-approved agents for the treatment of POTS. However, select pharmacological therapies have shown positive outcomes by addressing symptom origins, such as providing heart rate control, peripheral autonomic modulation, and targeting hypovolemia. Targeted pharmacological therapies studied in children and young adults include ivabradine, metoprolol, midodrine, pyridostigmine, intravenous crystalloid fluids, and fludrocortisone. Before adding pharmacotherapeutic interventions, non-pharmacologic interventions such as patient education, avoidance of symptom-triggering environments and medications, dietary fluid and sodium supplementation, exercise, and use of compression garments should be first attempted. Although the body of evidence for the management of POTS is expanding, additional research is needed to determine safe and efficacious dosing and establish clear guidelines for POTS in the pediatric population.
Keywords: hyperadrenergic, hypovolemia, neuropathic, orthostatic intolerance, postural, syncope
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a chronic illness of autonomic dysfunction that has gained prominence since first being recognized in 1982.1 It is characterized by orthostatic intolerances presenting as dizziness, syncope, and excessive tachycardia following postural repositioning in the absence of orthostatic hypotension.2,3 POTS has an estimated prevalence of 0.2%, with the majority of patients with POTS being white and female.2,3 It is also associated with high morbidity, with two-thirds of children with POTS reporting 10 or more symptoms.4 Although the average age range for the onset of POTS symptoms occurs between the ages of 15 and 25 years, symptoms can manifest in children as young as 6 years old.5,6 Despite the pediatric onset of the disease, management of POTS in children is primarily extrapolated from adult populations.2,3,7
Although the pathophysiology of POTS remains unclear and the etiology is multifactorial, symptoms occur due to a disruption in autonomic reflexes following repositioning of the body. In normal physiology, blood flow is redistributed when the body is repositioned from lying down to standing. Upon standing, blood volume shifts from the upper part of the body and vasculature to the lower part of the body and interstitial space.8 Other physiological changes include a temporary decline in cardiac filling and stroke volume (SV) and a decrease in arterial blood pressure (BP). In response, autonomic reflexes are activated to compensate for these changes. Cardiovascular and renal changes such as vasoconstriction, accelerated heart rate (HR), increased cardiac output (CO), and increased renal reabsorption of water and sodium leading to plasma volume expansion occur following activation of autonomic reflexes. Disruption of this compensatory mechanism causes patients to experience a variable combination of POTS symptoms, including orthostasis, lightheadedness, palpitations, blurred or tunnel vision, weakness, or syncope.2,3,7 The onset of POTS symptoms may be precipitated by or associated with various factors, including infection, menarche, autoimmune disorders, growth spurt, mitochondrial diseases, mast cell activation disorder, iron deficiency anemia, migraines, or concussion.2,8,9 About 15% of pediatric patients with POTS have underlying cardiac arrhythmias.10 Diagnostic criteria of POTS include over three months of chronic orthostatic intolerance, persistent symptomatic increase in HR over 40 beats per minute (BPM) in the first 10 minutes upright after being positioned supine, and absence of other causative etiologies.2 The stated diagnostic criteria apply to children 12 to 19 years, as diagnostic criteria for children less than 12 years of age remain undefined.2 Based on the patient’s symptoms and clinical presentation, the patient may be categorized into three main subtypes, which are neuropathic, hypovolemic, or hyperadrenergic POTS.11 The hallmark of neuropathic POTS is compromised vasoconstriction in the vessels of the lower extremities secondary to partial autonomic neuropathy. Hyperadrenergic POTS is characterized by significantly increased sympathetic nervous system activity defined as an increase of >10 mm Hg in systolic blood pressure within 10 minutes of standing or tilting and an upright position plasma norepinephrine concentration of 600 pg/mL. Hypervolemic POTS is characterized by a significant reduction in plasma volume, compromising venous return and triggering compensatory tachycardia. Other categories referenced in the literature include joint-hypermobility-related and immune-related.
An expert consensus statement published in 2015 on the diagnosis and treatment of POTS in adults and children recommended non-pharmacologic measures as a first-line approach for managing POTS (Table 1), with progression to pharmacologic agents if symptoms persist.3,12 No single therapy has been proven successful for treating POTS in adult or pediatric patients, and combination pharmacotherapy may be needed to treat different pathologies occurring within the multiple organ systems involved. This article reviews published literature evaluating pharmacological therapies for POTS in pediatric patients and summarizes non-pharmacotherapeutic modalities.
Table 1.
Summary of Non-Pharmacologic Management of Pediatric POTS
| Non-pharmacologic | Mechanism | Benefit |
|---|---|---|
| Patient education | De-stigmatization, understanding symptoms, avoiding triggers, protection when experiencing symptoms | Allows patients to attain access to therapy; allows patients to be self-advocates of their own health2,42 |
| Avoidance of symptom exacerbating medications | Avoid medications that reduce blood volume and/or decrease blood vessel tone Avoid norepinephrine reuptake inhibitors |
Decrease risk of exacerbating orthostatic tachycardia or orthostatic symptoms8,43 Decrease symptom burden and risk of increased standing HR44 |
| Management of comorbid conditions | Complex multiple mechanisms | Holistic approach to the management of POTS to decrease symptoms burden |
| Oral rehydration and salt supplementation | Increases CBFV and promotes volume expansion via fluid and salt absorption | Increases orthostatic tolerance and mitigates autonomic symptoms40 |
| Physical activities | Physical conditioning via increases VO2peak and peak stroke volume/cardiac output | Improves quality of life; decreases tachycardia related symptom burden45-47 |
| Head-up (supine) sleep positioning | Conditions the heart by activation of the RAAS and increasing total blood volume | Improves orthostatic tolerance and overall syncope burden48 |
| Compression garments | Shifts pooled blood in lower extremities back into central circulation to increase cardiac output | Reduces tachycardia and symptom burden49 |
CBFV , cerebral blood flow velocity; POTS, postural orthostatic tachycardia syndrome; RAAS, renin-angiotensin-aldosterone-system; VO2peak , peak oxygen uptake
Methods and Materials
An extensive search of the Cochrane Database of Systematic Reviews, Google Scholar, and PubMed was performed to identify articles evaluating pharmacotherapeutic agents used to treat POTS in pediatric patients. The search terms utilized focused on treating POTS specific to the pediatric population. Search terms included “postural orthostatic tachycardia syndrome or POTS,” “treatment or medication or therapy,” and “pediatric or children or adolescent.” Studies were included if they: 1) were original research studies, including randomized controlled trials, non-randomized controlled studies, and observational studies, 2) evaluated medication therapy in the management of POTS, 3) were published in the English language, and 4) were published in journals requiring peer review. Published full articles that evaluated treatment modalities in children younger than 19 years were prioritized for inclusion. The criteria was loosened to include studies that included young adults (>18–26 years) when no pediatric-exclusive study was identified. The references of articles from the initial search results were screened to identify other potential articles to include. To avoid duplication and overlap of studies, meta-analyses and systematic reviews were excluded. Lastly, to ensure the inclusion of rigorously studied treatment modalities, case reports and case series were excluded.
Results
Of the 62 articles retrieved based on the literature search methods, 8 met the inclusion and exclusion criteria for analysis—1 randomized control trial, 2 prospective studies, and 5 retrospective studies (Table 2). Medications used in POTS aim to alleviate symptoms and improve orthostatic tolerance by controlling HR, modulating peripheral autonomic stimulation, and blood volume expansion (Table 3). Currently, no FDA-approved drugs are indicated for the management of POTS, but the following medications—ivabradine, midodrine, metoprolol, pyridostigmine, fludrocortisone, and parenteral crystalloid fluids—have been studied in pediatric patients.
Table 2.
Summary of Study Outcomes for Pharmacotherapy in the Management of Pediatric POTS
| Reference | Study Design | Medication and Dose | Patient Characteristics and Follow-Up | Outcomes |
|---|---|---|---|---|
| Delle13 | Retrospective cohort | Ivabradine Starting at 2.5 mg bid up to 7.5 mg bid |
22 patients Age 11–17 yr Follow-up at 4.6 mo (range, 0.9–17 mo) |
Symptom improvement was observed in 15/22 patients (68%). |
| Towheed9 | Retrospective cohort | Ivabradine Starting dose 1.25–2.5 mg bid up to 7.5 mg bid |
27 patients Age 12–17 yr Follow-up between 3–12 mo |
Symptom improvement was observed in 18/27 patients (67%). |
| Lai14 | Retrospective review with follow-up survey | Metoprolol Midodrine (doses not provided) Other medications included: analgesics, SSRIs, PPIs, and stool softeners |
47 patients responded to the survey (metoprolol = 12; midodrine = 13) Age 11–17 yr Follow-up at 19.5 mo (range, 9–50 mo) |
Of the patients on β-blocker, 57% reported symptom improvement compared with 46% in the midodrine group. |
| Chen15 | Prospective cohort | Metoprolol 0.25 mg/kg twice daily Midodrine 2.5 mg once daily |
53 patients (metoprolol = 19; midodrine = 19; conventional treatment = 15) Age 6–17 yr Short-term follow-up at 3–6 mo Long-term follow-up at 15 mo (range, 5–24 mo) |
Midodrine outperformed metoprolol and conventional therapy groups. Cure and effective rates were found to be significantly higher in midodrine-treated patients (68% and 89%), compared with metoprolol (42% and 57%), and conventional therapy (20% and 53%) respectively. Midodrine had more symptom-free days and a shorter time to clinical improvement compared with metoprolol. Additionally, symptom recurrent rates were also significantly lower with midodrine compared with metoprolol or conventional treatment groups. |
| Ross23 | Randomized control trial | Midodrine 2.5–10 mg tid | 20 patients (neuropathic POTS = 12; Hyperadrenergic POTS = 8) Age 12–20 yr Follow-up after 35 days of study |
Midodrine decreased HR, vascular capacitance, and blood flow, and increased vascular resistance and MAP more effectively in patients classified as neuropathic POTS. |
| Kanjwal30 | Retrospective cohort | Pyridostigmine 30 mg bid increased based on tolerance to 90 mg tid or 180 mg daily of sustained release formulation | 208 patients Age 26 ± 12 yr Follow-up at 12 mo (range, 9–15 mo) |
Pyridostigmine improved symptoms of orthostatic intolerance in 51% of patients, with significant changes in standing HR, DBP, and symptoms of orthostatic intolerance. |
| Moak31 | Retrospective cohort | 1–2 L daily of IV 0.9% NaCl administered 3–7 days per week | 39 patients Age 12–26 yr Duration of 0.9% IV NaCl was 30 wk (range, 1 wk–3.8 yr) |
Supplemental IV hydration improved quality of life scores in 31/39 (79%) patients. |
| Fortunato32 | Prospective cohort | 0.1–0.2 mg of fludrocortisone | 16 patients Age 14.8 ± 2.8 yr Follow-up occurred after a minimum of 4 wk on fludrocortisone |
Fludrocortisone significantly improved overall POTS symptoms including nausea (32%), dizziness (28%), abdominal pain (32%), and flushing symptoms (33%), but did not significantly reduce syncope rates. |
DBP, diastolic blood pressure; HR, heart rate; IV, intravenous; MAP, mean arterial pressure; NaCl, sodium chloride; POTS, postural orthostatic tachycardia syndrome; PPI, proton pump inhibitors; SSRIs, selective serotonin reuptake inhibitors
Table 3.
Synopsis of Pharmacotherapy for Management of Pediatric POTS
| Drug | Mechanism | Dose | Adverse Effects |
|---|---|---|---|
| Heart rate control | |||
| Ivabradine | Selective funny channel (If) antagonist | 2.5–5 mg bid (max 7.5 mg bid)9,13 | Headache, palpitations, bradycardia, hypertension, visual disturbances |
| Metoprolol | β1-adrenergic receptor antagonist | 0.25 mg/kg/dose twice daily15 | Light-headedness, exercise intolerance, depression |
| Peripheral autonomic modulation | |||
| Midodrine | α1-adrenergic receptor agonist | 2.5 mg once daily - 10 mg tid15,23 | Scalp tingling, goosebumps, headache |
| Pyridostigmine | Acetylcholinesterase inhibitor | Adolescents and young adults: 30 mg twice daily - 90 mg tid30 | GI disturbances |
| Volume expansion | |||
| Parenteral crystalloid fluids | — | 1–2 L/day for 3–7 days/wk prn in addition to oral fluids, salt replacement, exercise, and pharmacological therapy31 | Increased risk of upper extremity DVT Must be used as adjuvant treatment |
| Fludrocortisone | Aldosterone analogue | 0.1 mg–0.2 mg daily32 | Rash, ankle swelling, headache, mood changes |
DVT, deep vein thrombosis; GI, gastrointestinal; POTS, postural orthostatic tachycardia syndrome
Review of Studies
Heart Rate Control. A cardinal symptom of POTS is tachycardia, defined in patients 12 to 19 years, as an increase in HR >40 BPM above baseline in the first 10 minutes upright after being supine. Therefore, pharmacologic agents targeting HR control may have a role in reducing symptom burden. Agents studied for heart rate control in pediatric patients with POTS include ivabradine and metoprolol.
Ivabradine. Ivabradine is a selective and specific inhibitor of hyperpolarization-activated cyclic nucleotide-gated channels, also known as the funny (I(f)) current channel. These channels are predominantly found in the sinoatrial and atrioventricular nodes. Inhibiting these channels results in the reduction of intrinsic HR without beta (β)-adrenergic receptor antagonism. In a retrospective chart review published by Delle Donne et al,13 pediatric patients aged 11 to 17 years prescribed ivabradine for POTS were evaluated. Twenty-two patients (15 females) were initiated on 2.5 mg twice daily and titrated up based on symptom control in 11 patients to a maximum of 7.5 mg twice daily. The mean daily dose achieved was 9.5 mg daily, which corresponded to 0.1 mg/kg twice daily. All patients utilized non-pharmacological therapies and were followed for a range of 0.9 to 17 months (median 4.6 months). The authors reported significant symptom improvement in 68% of patients (n = 15), presenting as reduced syncopal episodes and resolution of symptoms, including a reduction in HR (p = 0.007) on electrocardiogram (ECG) without significant corrected QT (QTc) changes (p = 0.44). However, 1 patient (4%) was reported to experience mild phosphenes (flashing lights), which improved with a slight daily dose reduction from 10 to 7.5 mg/day, while another patient discontinued the medication due to no improvement in symptoms. In another single-center retrospective study, Towheed et al9 evaluated 27 patients, ages 12 to 17 years, diagnosed with POTS. Most of the patients were females (92.5%) diagnosed with Ehlers-Danlos syndrome (40.7%), and all had been optimized on non-pharmacological methods and tried 1 other medication before initiating ivabradine. Prior to starting ivabradine, beta (β) blockers and calcium channel blockers were stopped to prevent excessive bradycardia. The patients received oral ivabradine starting at 1.25 to 2.5 mg twice daily and titrated based on therapeutic response. The maintenance dose achieved in 22 patients was 5 mg twice daily, with 4 patients reaching the maximum dose of 7.5 mg twice daily. The follow-up time ranged from 3 to 12 months. Of the patients included in the study, 67% of patients demonstrated a reduction in POTS symptoms. The authors also found that syncope/presyncope episodes were reduced by 90%, lightheadedness by 85%, and fatigue by 81%. Additionally, an overall lowering of HR was reported when comparing patients before and after ivabradine treatment during sitting and standing (change in means of HR 16.5 and 19.6 BPM, respectively; p < 0.05), without significant changes in BP. Of the 27 patients reviewed, 22% presented with adverse effects. One patient’s dose was decreased from 5 to 2.5 mg twice daily due to excessive bradycardia, while 5 patients stopped the medication. Two patients stopped due to visual disturbances, 2 patients due to severe bradycardia and excessive flushing, and 1 due to joint pain and fatigue. Four patients discontinued the medication due to no effect on their symptoms. The authors concluded that ivabradine might be useful in decreasing the burden of symptoms associated with POTS without causing hypotension, exercise intolerance, or fatigue, often seen with other medications used to control HR.
Metoprolol. Metoprolol exerts its effects on HR control in patients with POTS by selectively antagonizing β1 adrenergic receptors located in cardiac tissue, providing relief of symptoms associated with tachycardia. Lai et al14 conducted a retrospective review of charts with a follow-up survey of 121 adolescents who underwent autonomic reflex screening as part of an evaluation for POTS. Of the 121 adolescents, 47 (age range, 11–17 years) responded to the survey between 9 and 50 months (mean 19.5 months) after initial evaluation. All patients met the criteria for POTS diagnosis and utilized non-pharmacological interventions, including increased fluid and salt intake, elevating the head of the bed, consulting with a physical therapist, and wearing elastic support hoses. Of the survey responders, 81% were prescribed medications, with most patients on β-blockers (n = 14; metoprolol = 12, atenolol = 2) and midodrine (n = 13), with no overlapping use of the 2 medication classes. Other medications used include analgesics, selective serotonin reuptake inhibitors, proton pump inhibitors, and stool softeners. Of the patients prescribed a β-blocker, 64% of patients continued for at least 6 months, and 57% reported symptom improvement compared with 38% and 46% in the midodrine group, respectively. The authors reported greater improvements in quality-of-life scores (p = 0.04) and overall improvement (p = 0.016) reported by patients in the β-blockers group over midodrine. The authors did not report on doses received or adverse effects experienced by patients.
Chen et al15 compared a conventional treatment (n = 15) arm to midodrine (n = 19) and metoprolol (n = 19) in 53 children (22 males and 31 females), ages 6 to 17 years, diagnosed with POTS in a controlled trial. Patients received metoprolol 0.25 mg/kg twice daily and midodrine 2.5 mg once daily and were evaluated at 2 time points, including short-term at 3 to 6 months and long-term at 15 months (range, 5–24 months). All patients received non-pharmacological interventions, which included increased water and salt intake, avoiding triggering events or positions, techniques to counter blood pooling in lower extremities (e.g., crossing legs), and going into a supine posture to abort the episode, and reassurance regarding the condition’s non-life-threatening nature. One of the study outcomes included the calculation of symptom scores on a scale of 0 to 4 based on the frequency of symptoms (syncope, dizziness, chest tightness, palpitations, headache, blurred vision, trembling, and cold sweat) with a score of 0 representing no symptoms and a score of 4 representing more than 1 symptom episode in 1 day. While the metoprolol group showed a reduction in symptom scores compared with patients receiving conventional treatment (2.8 ± 2.4 vs 3.7 ± 2.0; p < 0.05), the group did not perform better compared with conventional treatment in short-term cure rate (57.59 vs 53.33%; p > 0.05) and long-term symptom recurrence rate, which were the primary outcomes of the study. No adverse effects were reported on patients taking metoprolol.
The unsatisfactory effectiveness of metoprolol, with approximately 57% of children with POTS experiencing a reduction in symptoms, prompted clinical investigations into factors that may predict response. Zhao et al16 compared 49 children (7–16 years) with POTS to a control group with 25 healthy children (11–13 years). The children diagnosed with POTS received metoprolol 0.5 mg/kg twice daily for 1.5 to 3 months and were evaluated for POTS symptoms and orthostatic intolerance tests at baseline and follow-up. All study patients had blood drawn to measure the variations of copeptin, a stable biomarker of arginine vasopressin (AVP), which is an important stress hormone that may be increased during excessively reduced venous return and central blood volume. The authors found higher baseline plasma copeptin concentrations in the group of children with POTS compared with the control group (10.524 ± 2.016 vs 8.750 ± 1.419 pmol/L; p < 0.001). the plasma copeptin concentration was also identified to be lower in the 28 patients who responded to metoprolol when compared with the 21 patients who did not respond (9.377 ± 1.411 vs 12.054 ± 1.662 pmol/L; p < 0.001). The authors concluded that because AVP secretion is inhibited by the increased catecholamine concentrations in hyperadrenergic POTS, copeptin measurement may indirectly reflect plasma osmotic pressure and may be used to predict the effectiveness of metoprolol in children with POTS. Subsequent studies evaluated other biomarkers that could predict response to metoprolol and showed consistent results. Patients who responded to metoprolol had higher plasma norepinephrine concentrations,17 C-type natriuretic peptide18 (a small molecule protein involved in accelerating HR and increasing the secretion of plasma catecholamine), HR differential on head-up tilt test,19 and pre-treatment baseline left ventricular ejection fraction.20 Wang et al21 also evaluated baseline QTc interval dispersion, given the laborious or costly process of obtaining the aforementioned biomarkers, and found longer pre-treatment baseline QTc interval dispersion in patients that responded to metoprolol (66.3 ± 20.3 ms vs 45.7 ± 19.9 ms; p = 0.001). Xu et al22 developed and externally validated a predictive model using ECG indicators with high sensitivity (90.9%) and specificity (95%) for the therapeutic efficacy of metoprolol in children and adolescents with POTS. These studies suggest that metoprolol may have a preferential role in patients with POTS exacerbated by hyperadrenergic pathogenesis and may be useful when patients have long baseline QTc.
Peripheral Autonomic Modulation. Symptoms of POTS may have neuropathic origin due to impaired peripheral vasoconstriction from adrenergic denervation leading to peripheral venous pooling. Therefore, the use of pharmacotherapies that modulate peripheral autonomic dysfunction may reduce symptoms of orthostatic intolerance by increasing peripheral vasoconstriction, improving venous return. Agents studied for pediatric patients include midodrine and pyridostigmine.
Midodrine. Midodrine is a prodrug that forms an active metabolite, desglymidodrine, which functions as an alpha (α)1 adrenergic receptor agonist. Desglymidodrine works through various pathways causing peripheral vasoconstriction, decreasing inappropriate sympathetic activity, and improving flow-mediated dilation. This results in increased peripheral vascular resistance and reduced venous pooling when standing. Previously discussed studies by Lai et al14 and Chen et al15 included midodrine treatment groups. The study by Lai et al14 reported fewer patients remaining on midodrine compared with β-blockers (30% vs 50%) during the follow-up period and fewer patients attributing their general improvement to the medication compared with β-blockers (36.4% vs 63%; p = 0.011).14 The authors found no difference in quality-of-life scores between patients on midodrine and patients not on midodrine or β-blockers. Unlike the results from the study by Lai et al,14 the results from the study by Chen et al15 favored midodrine 2.5 mg once daily compared with metoprolol 0.25 mg/kg twice daily and non-pharmacological intervention groups in both short-term (3–6 months) and long-term (15 months) outcomes, range of 5 to 24 months.15 Patients treated with midodrine had significantly higher cure rates (midodrine = 68.42% vs metoprolol = 42.11% and non-pharmacological interventions = 20%; p < 0.05); effective rates (89.47% vs 57.89% and 53.33%; p = <0.05) and lower symptoms scores (1.1 ± 2.2 vs 2.8 ± 2.4 vs 3.7 ± 2.0; p < 0.05). Symptom recurrence rates were also significantly lower with midodrine vs metoprolol and non-pharmacological interventions (p < 0.05). Unlike metoprolol, some patients who received midodrine experienced adverse effects, including 3 patients with increased BP (SBP increased by 5 mm Hg compared with baseline) and 1 patient with stomach pain. The authors concluded that midodrine is an effective agent for treating children with POTS.
Ross et al23 conducted a randomized, double-blinded, placebo-controlled trial (n = 20) evaluating the effectiveness of midodrine on vascular changes associated with POTS in children and young adults ages 12 to 20 years, 15 of whom were female. The authors stratified study participants into hyperadrenergic (n = 8) and neuropathic (n = 12) POTS subtypes. No differences were identified between groups at baseline. The study protocol included 2 patient groups receiving placebo or midodrine at 2.5 mg 3 times a day on days 1 to 4; 5 mg 3 times a day on days 5 to 7; 7.5 mg 3 times a day on days 8 to 10; and 10 mg 3 times a day on days 11 to 14. A 7-day drug washout occurred after the first 2 weeks, followed by a crossover treatment for 14 more days. All study subjects were maintained on a minimum of 7.5 mg 3 times a day of midodrine or placebo. Patients were not on any other medications and avoided caffeine-containing products while enrolled in the study. A separate control group with 14 healthy patients without POTS and no receipt of midodrine or placebo was also included for the purpose of comparing pre-treatment baseline hemodynamics to the subgroup with POTS. The authors identified important baseline differences, including higher HR following the head-up tilt test in the POTS group compared with controls (p < 0.001), but no difference within patients in the POTS groups (p = 0.6). Patients in the hyperadrenergic group had higher baseline supine HR than the controls (p = 0.02) but no difference between the neuropathic group and controls (p = 0.4). Also, patients with hyperadrenergic POTS had significantly less calf blood flow than control subjects and patients in the neuropathic POTS group. The authors also noted interesting differences in treatment effects between neuropathic and hyperadrenergic POTS groups treated with midodrine. Patients in the group with neuropathic POTS demonstrated significant decreases in HR, calf blood flow, and calf venous capacitance during head-up testing (supine to standing assessments) compared with placebo, while mean arterial pressure and calf vascular resistance were increased (all p values < 0.05). In contrast, patients in the hyperadrenergic POTS groups had limited changes after receiving midodrine, with no significant differences in all outcomes measured, with the exception of HR during the 35° head-up tilt test (p = 0.05). Mild adverse effects were reported, including headaches and goosebumps; however, these adverse effects did not deter patients from completing the study. The authors concluded that midodrine effectively decreased POTS symptoms in patients with neuropathic POTS but did not find outcome differences between midodrine and placebo treatment in patients with hyperadrenergic POTS.
Like metoprolol, the efficacy of midodrine is variable depending on various factors. A study evaluating predictive factors of midodrine’s efficacy in pediatric patients with POTS suggested BP changes from a supine to an upright position to be impactful.24 The authors identified midodrine to be effective when pre-midodrine treatment SBP decreased or did not change, or when diastolic blood pressure (DBP) was ≤6.5 mm Hg from supine to standing position with sensitivity and specificity of 72% and 88%, respectively. Other potential biomarkers that may be predictors of midodrine effect include erythrocytic hydrogen sulfide,25 a vasodilating gasotransmitter; midregional fragment of pro-adrenomedullin,26 a potent vasodilator; and copeptin,27 all of which were observed to be higher in midodrine responders. Response to midodrine was also greater in patients with higher flow-mediated vasodilation of the brachial artery.28
Pyridostigmine. Pyridostigmine is a peripheral acetylcholinesterase inhibitor with potential therapeutic benefits in patients with POTS. Pyridostigmine increases acetylcholine in the autonomic ganglia and enhances nerve conduction of the parasympathetic nervous system. Through modulation of peripheral autonomic control, venous pooling is decreased, thus attenuating the compensatory tachycardia that causes the symptoms associated with orthostasis.29 Pediatric-exclusive studies are lacking; however, a retrospective study evaluating treatment with oral pyridostigmine was conducted in children and young adults.30 The study reviewed 208 patients’ medical records aged 26 ± 12 years, 88% of which were females, who were refractory to nonpharmacological interventions and select medications, including fludrocortisone, midodrine, and selective serotonin reuptake inhibitors either alone or in combination. Patients were initiated on oral pyridostigmine 30 mg twice daily, and doses were increased if no therapeutic effect was noted after a treatment period of 7 days up to a maximum dose of 90 mg 3 times a day or 180 mg of the sustained release form. The authors reported improved symptoms of orthostatic intolerance in 51% of the remaining 173 patients who tolerated the drug during follow-up at 12 months (range, 9–15 months) reported symptom improvements in 88 of 203 patients (43%). The most frequently reported symptoms that were improved included fatigue, palpitations, presyncope, and syncope (48%–60%). Significant changes included a reduction in post-treatment standing HR (p < 0.05) and an increase in standing DBP (p < 0.05) from baseline were observed in the patients who reported orthostatic symptom control. However, 19% of patients reported gastrointestinal side effects, warranting discontinuation in 35 patients due to intolerance. GI adverse effects included severe abdominal cramps, severe nausea, and diarrhea. Other minor adverse effects reported included tremors, twitching and hyperhidrosis (n = 5), urinary urgency (n = 2), hypertension (n = 2), chest pain (n = 1), and hypotension (n = 1). The authors concluded that in the subgroup of patients who were refractory to other forms of therapy, treatment with pyridostigmine may provide symptomatic relief, reduction in standing HR, and improvement in standing DBP in patients who can tolerate the medication. Due to the limited data demonstrating efficacy and safety, pyridostigmine should not be used as an initial treatment for pediatric patients with POTS.
Volume Expansion. Adequate blood volume is essential in preventing the main symptoms of POTS by maintaining appropriate CO, SV, and HR. Parenteral crystalloid fluid replenishment increases blood volume and reduces the need for compensatory tachycardia, alleviating burdensome symptoms.
Parenteral Crystalloid Fluids. Intravenous (IV) normal saline supplementation may be necessary to maintain adequate blood volume in certain patients with POTS. Moak et al31 conducted a retrospective chart review evaluating IV normal saline in adolescents and young adults aged 10 to 26 years (n = 39). These patients had refractory POTS symptoms despite initial treatment with non-pharmacological therapies, including increased oral fluid intake (2–3 L/day), increased salt consumption, aerobic exercises, improved sleep efficiency, and medications including fludrocortisone, midodrine, β-blockers or octreotide. The baseline quality of life assessment based on symptoms scores was 4.2/10, indicating moderate to severe disability secondary to POTS symptoms. Patients received 1 to 2 L of normal saline daily for 3 to 7 days per week and up-titrated until clinical improvement or if the patient no longer wanted to continue with the therapy. Patients received IV hydration by peripherally inserted central catheters (PICC) lines (n = 22), peripherally inserted vascular access lines (n = 10), and surgically implanted subcutaneous ports (n = 7). The average duration of IV normal saline administration was 30 weeks and ranged from 1 week to 3.8 years. Of the patients treated, 79% (n = 31) self-reported clinically improved quality of life scores post-treatment. Six patients who had previously discontinued IV rehydration therapy restarted treatment after the recurrence of symptoms. Three patients (11%) with PICC (n = 2) or central port (n = 1) experienced upper extremity deep vein thromboses (DVT), and 4 (14%) developed infections (PICC, n = 3; port n = 1). DVT rate was considerably higher in the study population (11%) compared with the institution’s DVT rate (1.6%). The authors concluded that IV rehydration with normal saline is highly effective for medication-resistant symptoms of orthostatic intolerance. Due to the risk of serious adverse effects such as DVTs, patients with pre-established IV access for other indications may be a preferred group for parenteral IV rehydration. Strategies to mitigate the risk of DVT include performing a comprehensive thrombophilia evaluation, discouraging the use of birth control pills, or close monitoring while on IV therapy.
Fludrocortisone. Fludrocortisone is a mineralocorticoid that acts on the distal tubules of the kidney to enhance the reabsorption of sodium and water, leading to an increase in blood volume. In a prospective cohort study (n = 16), Fortunato et al32 assessed the use of fludrocortisone (0.1–0.2 mg/day) for at least 4 weeks on both orthostatic intolerance and gastrointestinal symptom management in 16 patients (all females) with unexplained nausea and orthostatic intolerance concerns. Patients with a mean age of 14.8 ± 2.8 years rated the severity of their symptoms on a scale of 0 (none) to 4 (severe) before and after fludrocortisone treatment. There was a significant reduction in overall mean symptom scores for study participants (p = 0.0046), and for specific symptoms, including nausea, dizziness, abdominal pain, flushing, and missing school improved after fludrocortisone treatment (all p < 0.05). No significant improvement was found for symptoms such as vomiting, syncope, constipation, or anorexia. The authors concluded that fludrocortisone produced symptomatic relief in patients with POTS, particularly patients with comorbid GI symptoms, such as nausea and abdominal pain, but did not actively prevent syncope episodes.
Discussion
POTS is a chronic disorder of the autonomic nervous system characterized by orthostatic intolerances without hypotension. Our review identified eight publications that evaluated using ivabradine, metoprolol, midodrine, pyridostigmine, fludrocortisone, and intravenous normal saline to treat pediatric patients with POTS. Assessment of these publications revealed varying degrees of effectiveness, likely due to the heterogeneity of POTS types and patient-reported symptoms. Individualizing treatment plans based on symptomatology and underpinning pathogenesis, that is, hypovolemic, neuropathic, and hyperadrenergic, may improve the efficacy of medications. Hyperadrenergic POTS is characterized by persistently increased plasma norepinephrine after standing, resulting from increased sympathetic activity triggered by baroreceptors sensing volume accumulation in the extremities and decreased volume in venous return to the heart.8 The consequence of unabated plasma norepinephrine increase is sustained tachycardia while in an upright position. Symptoms frequently present in hyperadrenergic POTS include tachycardia, hyperhidrosis, pallor, tremors, and pale and cold skin.33,34 Medications with clinical effects on cardiac chronotropy, blunting the effects of elevated catecholamines without impacting the BP, are desirable to manage hyperadrenergic POTS. Ivabradine and metoprolol are negative cardiac chronotropes that have been clinically evaluated in pediatric patients with POTS. Studies evaluating ivabradine reported approximately 67% of patients responded and tolerated the medication, with dose-dependent adverse effects, including bradycardia and phosphenes (transient enhanced brightness).9,13 Metoprolol has a lower response rate of 57% in pediatric studies for managing POTS. This lower efficacy rate is attributable to initial studies not delineating POTS phenotypes in their patient populations. Studies further exploring the variability in therapeutic efficacy with metoprolol focused on identifying biomarkers that predicted response.16–21 Identifiable and measurable descriptors such as plasma norepinephrine, C-type natriuretic peptide, and heart rate variability delineated hyperadrenergic POTS from other POTS phenotypes and had higher predictive sensitivity and specificity for treatment efficacy with metoprolol.33
Patients with neuropathic POTS typically have excessive pooling in the extremities due to blunted peripheral vascular sympathetic activity and abnormal lower limb vascular tension. Venous pooling in the extremities during orthostatic challenges results in decreased cardiac preload, which leads to compensatory HR increases. This pathology is commonly observed in patients with POTS related to autoimmune or post-viral autonomic neuropathy and is associated with the acute onset of symptoms such as persistent light-headedness, fatigue, postprandial bloating, and vomiting.34,35 Midodrine causes vasoconstriction within the extremities, increasing peripheral vascular resistance while reducing venous capacitance and orthostatic tachycardia. Biomarkers (e.g., erythrocyte hydrogen sulfide production, copeptin, pro-adrenomedullin, flow-mediated dilation, and blood pressure changes in standing tests) that indicate neuropathic pathophysiology had higher predictive sensitivity and specificity for treatment response to midodrine.33 In addition to this direct effect on the vascular wall, midodrine may also indirectly increase norepinephrine concentrations. Therefore, patients taking midodrine should be monitored for supine hypertension. Midodrine is contraindicated in patients with renal or cardiac disease, pheochromocytoma, or thyrotoxicosis, as increased BP can be harmful in these patients.36 Patients should also avoid over-the-counter cough and cold products that may produce additive effects on BP while taking midodrine.36
Altered central blood volume is a hallmark mechanism of hypovolemic POTS, and is observed in about 57% of children with POTS.37 Reduction in central blood volume can result from excessive venous pooling of blood in the lower extremities. Significant reduction in fluid intake is also a culprit, with POTS symptoms manifesting almost 4 times higher in children with less than 800 mL/day of water intake.38 Additionally, dysregulation of the renin-angiotensin-aldosterone system can contribute to disruptions in the body’s ability to compensate for hypovolemia.39 Non-pharmacological interventions such as oral rehydration therapy and increasing dietary sodium intake have been shown to improve orthostatic tolerance and maintain cerebral blood-flow velocity in pediatric patients with POTS.40 Patients with unresolved POTS despite oral rehydration therapy, increased salt intake, and other non-pharmacological interventions or unable to tolerate increased oral fluids and salts may benefit from short-term use of parenteral normal saline therapy. This option may be feasible for patients with pre-established IV access for other conditions, given the greater risk of DVTs in children with POTS compared with the standard DVT pediatric DVT risk.31
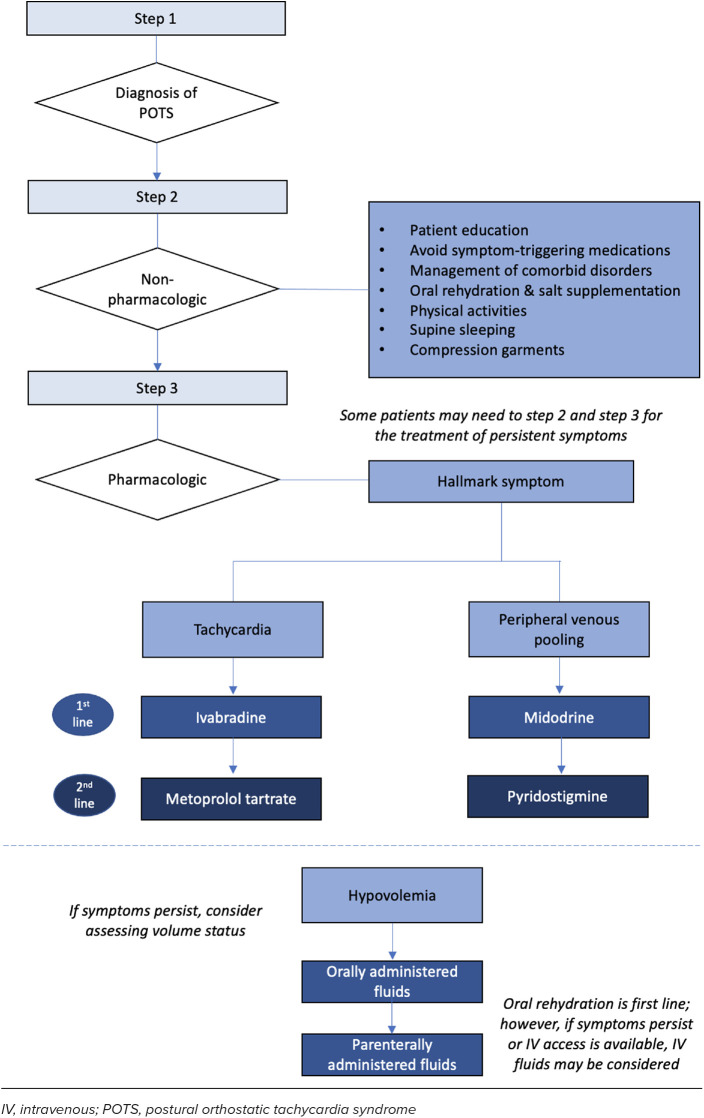
The evidence for using pyridostigmine and fludrocortisone to work for pediatric POTS is limited and conflicting. Pyridostigmine may have a role in refractory POTS but may not be well-tolerated due to GI adverse effects. Additional studies in pediatric patients with POTS are necessary to guide clinicians on its role. Fludrocortisone may have beneficial effects in patients with POTS with co-morbid functional GI disorders. Additionally, to safely use fludrocortisone, patients must be on a high-sodium diet, with appropriate monitoring of serum potassium.41 Other medications, such as clonidine, methyldopa, modafinil, methylphenidate, and desmopressin, have been studied in adult populations and reported in pediatric cases but lack rigorous studies evaluating their use in children or young adults. Based on the assessment of the available literature, we propose a treatment algorithm for managing POTS in pediatric patients specific to POTS phenotypes (Figure). Given the heterogeneity and overlapping nature, POTS subtypes are neither fully inclusive nor exclusive, and patients may exhibit characteristics of more than one mechanism.41 The clinical utility of phenotyping POTS is not fully mapped out; thus, targeting multiple subtypes (e.g., therapies targeting hypovolemia combined with either neuropathic or hyperadrenergic treatment approaches) may be warranted. Continuous follow-up for reassessment is necessary to ensure the appropriateness of treatment as data on long-term prognosis and response to therapies is limited. Further investigation into POTS, understanding the pathophysiology, and identifying effective pharmacotherapies is warranted.
Figure.
Proposed treatment algorithm for the management of POTS.
Conclusion
POTS is a complex heterogeneous disorder that requires an individualized approach involving non-pharmacologic and, in some cases, pharmacologic modalities for management. This clinical review provides insight into the management landscape of POTS in pediatric patients. Recognition of interpatient variability in pediatric POTS can guide treatment selection; however, additional studies are needed to better understand the disorder and identify medications that can provide optimal relief in pediatric patients.
Acknowledgments.
At the time of manuscript submission, Peter Huyhn and Alexandra Brown were Doctor of Pharmacy candidates at Midwestern University College of Pharmacy, Glendale Campus, Glendale, Arizona, and University of Arizona College of Pharmacy, Phoenix Campus, Phoenix, Arizona, respectively; Lauren Campisi was a first-year pharmacy resident at Phoenix Children’s; Titilola Afolabi was faculty at Midwestern University and a clinical pharmacist at Phoenix Children’s.
ABBREVIATIONS
- BP
blood pressure;
- BPM
beats per minute;
- ECG
electrocardiogram;
- HR
heart rate;
- MAP
mean arterial pressure;
- POTS
postural orthostatic tachycardia syndrome;
- QTc
corrected QT
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med . 1982;72(5):847–850. doi: 10.1016/0002-9343(82)90559-9. [DOI] [PubMed] [Google Scholar]
- 2.Boris JR, Moak JP. Pediatric postural orthostatic tachycardia syndrome: where we stand. Pediatrics . 2022;149(6):e2021054945. doi: 10.1542/peds.2021-054945. [DOI] [PubMed] [Google Scholar]
- 3.Sheldon RS, Grubb BP, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm . 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boris JR, Bernadzikowski T. Demographics of a large pediatric postural orthostatic tachycardia syndrome program. Cardiol Young . 2018;28(5):668–674. doi: 10.1017/S1047951117002888. [DOI] [PubMed] [Google Scholar]
- 5.Staples A, Thompson NR, Moodley M. Pediatric-onset postural orthostatic tachycardia syndrome in a single tertiary care center. J Child Neurol . 2020;35(8):526–535. doi: 10.1177/0883073820916260. [DOI] [PubMed] [Google Scholar]
- 6.Boris JR. Postural orthostatic tachycardia syndrome in children and adolescents. Auton Neurosci . 2018;215:97–101. doi: 10.1016/j.autneu.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res . 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Du J, Jin H, Huang Y. Postural tachycardia syndrome in children and adolescents: pathophysiology and clinical management. Front Pediatr . 2020;8:474. doi: 10.3389/fped.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towheed A, Nesheiwat Z, Mangi MA, et al. Ivabradine in children with postural orthostatic tachycardia syndrome: a retrospective study. Cardiol Young . 2020;30(7):975–979. doi: 10.1017/S1047951120001341. [DOI] [PubMed] [Google Scholar]
- 10.Hong J, Litt SJ, Moak JP. Cardiac arrhythmias in postural tachycardia syndrome and orthostatic intolerance. Cardiol Young . 2023;33(2):255–259. doi: 10.1017/S1047951122000580. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Xu B, Du J. Update of individualized treatment strategies for postural orthostatic tachycardia syndrome in children. Front Neurol . 2020;11:525. doi: 10.3389/fneur.2020.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei LY, Chew DS, Sandhu RK, et al. Non-pharmacological and pharmacological management of cardiac dysautonomia syndromes. J Atr Fibrillation . 2020;13(1):2395. doi: 10.4022/jafib.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delle Donne G, Roses NF, Till J, et al. Ivabradine in postural orthostatic tachycardia syndrome: preliminary experience in children. Am J Cardiovasc Drugs . 2018;18(1):59–63. doi: 10.1007/s40256-017-0248-x. [DOI] [PubMed] [Google Scholar]
- 14.Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol . 2009;32(2):234–238. doi: 10.1111/j.1540-8159.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Wang L, Sun J, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostasis tachycardia syndrome. Circ J . 2011;75:927–931. doi: 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Du S, Yang J, et al. Usefulness of plasma copeptin as a biomarker to predict the therapeutic effectiveness of metoprolol for postural tachycardia syndrome in children. Am J Cardiol . 2014;114(4):601–605. doi: 10.1016/j.amjcard.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Chen X, Li J, Du J. Orthostatic plasma norepinephrine level as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. J Transl Med . 2014;12:249. doi: 10.1186/s12967-014-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Han Z, Li H, et al. Plasma C-type natriuretic peptide as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. PloS ONE . 2015;10(3):e0121913. doi: 10.1371/journal.pone.0121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Zou RZ, Cai H, et al. Heart rate and heart rate difference predicted the efficacy of metoprolol on postural tachycardia syndrome in children and adolescents. J Pediatr . 2020;224:110–114. doi: 10.1016/j.jpeds.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Han Z, Wang Y, et al. Baseline left ventricular ejection fraction associated with symptom improvements in both children and adolescents with postural tachycardia syndrome under metoprolol therapy. Chin Med J . 2021;124(16):1977–1982. doi: 10.1097/CM9.0000000000001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Sun Y, Zhang Q, et al. Baseline corrected QT interval dispersion is useful to predict effectiveness of metoprolol on pediatric postural tachycardia syndrome. Front Cardiovasc Med . 2022;8:808512. doi: 10.3389/fcvm.2021.808512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu B, Zhang Q, Li X, et al. A predictive model of response to metoprolol in children and adolescents with postural tachycardia syndrome. World J Pediatr . 2023;19:390–400. doi: 10.1007/s12519-022-00677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) . 2014;126(4):289–296. doi: 10.1042/CS20130222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng W, Liu Y, Liu AD, et al. Difference between supine and upright blood pressure associates to the efficacy of midodrine on postural orthostatic tachycardia syndrome (POTS) in children. Pediatr Cardiol . 2014;35(4):719–725. doi: 10.1007/s00246-013-0843-9. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Zhao J, Du S, et al. Postural orthostatic tachycardia syndrome with increased erythrocytic hydrogen sulfide and response to midodrine hydrochloride. J Pediatr . 2013;163:1169–1173. doi: 10.1016/j.jpeds.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Li Z, Ochs T, et al. Midregional pro-adrenomedullin as a predictor for therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. J Am Coll Cardiol . 2012;60:315–320. doi: 10.1016/j.jacc.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Tang C, Jin H, Du J. Plasma copeptin and therapeutic effectiveness of midodrine on postural tachycardia syndrome in children. J Pediatr . 2014;165:290–294. doi: 10.1016/j.jpeds.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Liao Y, Yang J, Zhang F, et al. Flow-mediated vasodilation as a predictor of therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. Am J Cardiol . 2013;112:816–820. doi: 10.1016/j.amjcard.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Gales BJ, Gales MA. Pyridostigmine in the treatment of orthostatic intolerance. Ann Pharmacother . 2007;41(2):314–318. doi: 10.1345/aph.1H458. [DOI] [PubMed] [Google Scholar]
- 30.Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience. Pacing Clin Electrophysiol . 2011;34:750–755. doi: 10.1111/j.1540-8159.2011.03047.x. [DOI] [PubMed] [Google Scholar]
- 31.Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol . 2016;37(2):278–282. doi: 10.1007/s00246-015-1274-6. [DOI] [PubMed] [Google Scholar]
- 32.Fortunato JE, Wagoner AL, Harbinson RL, et al. Effect of fludrocortisone acetate on chronic unexplained nausea and abdominal pain in children with orthostatic intolerance. J Pediatr Gastroenterol Nutr . 2014;59(1):39–43. doi: 10.1097/MPG.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 33.Fan S, Cui Y, Liao Y, Jin H. Predicting therapeutic efficacy of pharmacological treatments in children with postural orthostatic tachycardia syndrome: a mini-review. Children . 2023;10:1093. doi: 10.3390/children10071093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiBaise JK, Harris LA, Goodman B. Postural tachycardia syndrome (POTS) and the GI tract: a primer for the gastroenterologist. Am J Gastroenterol . 2018;113:1458–1467. doi: 10.1038/s41395-018-0215-4. [DOI] [PubMed] [Google Scholar]
- 35.Hasan B, Almasri J, Marwa B, et al. Treatment of postural orthostatic tachycardia syndrome with medication: a systematic review. J Child Neurol . 2020;35(14):1004–1016. doi: 10.1177/0883073820948679. [DOI] [PubMed] [Google Scholar]
- 36.Midodrine product information. Shire, Inc. Jan, 2017. Accessed December 28, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019815s010lbl.pdf.
- 37.Li JW, Zhang QY, Jin HF, et al. Clinical features and management of postural tachycardia syndrome in children: a single-center experience. Chin Med J . 2014;127(21):36843689. [PubMed] [Google Scholar]
- 38.Lin J, Han Z, Li X, et al. Risk factors for postural tachycardia syndrome in children and adolescents. PloS One . 2014;9(12):e113625. doi: 10.1371/journal.pone.0113625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagiub M, Moskowitz W, Fortunato J. Systematic literature review of pathophysiology of postural orthostatic tachycardia syndrome (angiotensin II receptor subtype imbalance theory) Prog Pediatr Cardiol . 2018;50:50–61. [Google Scholar]
- 40.Medow MS, Guber K, Chokshi S, et al. The benefits of oral rehydration on orthostatic intolerance in children with postural tachycardia syndrome. J Pediatr . 2019;214:96–102. doi: 10.1016/j.jpeds.2019.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernino S, Bourne KM, Stiles LE, et al. Postural orthostatic tachycardia syndrome (POTS): state of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting-Part 1. Auton Neurosci . 2021;235:102828. doi: 10.1016/j.autneu.2021.102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Y, Du J. Pathophysiology and individualized management of vasovagal syncope and postural tachycardia syndrome in children and adolescents: an update. Neurosci Bull . 2020;36(6):667–681. doi: 10.1007/s12264-020-00497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raj SR, Black BK, Biaggioni I, et al. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation . 2005;111(21):2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 44.Green EA, Raj V, Shibao CA, et al. Effects of norepinephrine reuptake inhibition on postural tachycardia syndrome. J Am Heart Assoc . 2013;2(5):e000395. doi: 10.1161/JAHA.113.000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol . 2010;55:2856–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm . 2016;13(4):943–950. doi: 10.1016/j.hrthm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Kempert, H. Intensive rehabilitation can be tolerated by adolescents with postural orthostatic tachycardia syndrome (POTS) Pediatric Pain Letter . 2018;20:11–17. [Google Scholar]
- 48.Cooper VL, Hainsworth R. Head-up sleeping improves orthostatic tolerance in patients with syncope. Clin Auton Res . 2008;18(6):318–324. doi: 10.1007/s10286-008-0494-8. [DOI] [PubMed] [Google Scholar]
- 49.Heyer GL. Abdominal and lower-extremity compression decreases symptoms of postural tachycardia syndrome in youth during tilt table testing. J Pediatr . 2014;165(2):395–397. doi: 10.1016/j.jpeds.2014.04.014. [DOI] [PubMed] [Google Scholar]