Abstract
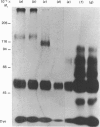
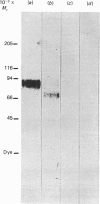
Membrane topography of the rat ovarian lutropin receptor was studied by two different approaches. Ovarian membrane preparation, labelled with 125I-labelled human choriogonadotropin in vivo, was subjected to extensive chymotryptic digestion. The soluble and membrane-bound radioactive complexes were cross-linked with glutaraldehyde, and analysed by SDS/polyacrylamide-gel electrophoresis and autoradiography. Chymotrypsin solubilized 70-75% of the radioactivity as Mr-96,000, Mr-74,000 and Mr-61,000 complexes, and decreased the size of the membrane-bound 125I-labelled human choriogonadotropin-receptor complex from Mr 130,000 to Mr 110,000. The Mr-110,000 complex was not observed when 0.1% Triton X-100 was present in the proteolytic digestion. Enrichment of inside-out-oriented plasma-membrane vesicles by concanavalin A affinity chromatography increased by 70% the fraction of radioactivity that remained in the membrane fraction after chymotrypsin treatment. Chymotrypsin also diminished the size of the membrane-bound unoccupied receptor from Mr 90,000 to Mr 70,000, as detected by ligand (125I-labelled human choriogonadotropin) blotting. These results suggest that the lutropin receptor is a transmembrane protein with a cytoplasmic domain of Mr 20,000 that is sensitive to proteolytic digestion in the inside-out-oriented plasma-membrane vesicles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dattatreyamurty B., Rathnam P., Saxena B. B. Isolation of the luteinizing hormone-chorionic gonadotropin receptor in high yield from bovine corpora lutea. Molecular assembly and oligomeric nature. J Biol Chem. 1983 Mar 10;258(5):3140–3158. [PubMed] [Google Scholar]
- Dufau M. L., Baukal A. J., Catt K. J. Hormone-induced guanyl nucleotide binding and activation of adenylate cyclase in the Leydig cell. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5837–5841. doi: 10.1073/pnas.77.10.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Ryan D. W., Baukal A. J., Catt K. J. Gonadotropin receptors. Solubilization and purification by affinity chromatography. J Biol Chem. 1975 Jun 25;250(12):4822–4824. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hwang J., Menon K. M. Characterization of the subunit structure of gonadotropin receptor in luteinized rat ovary. J Biol Chem. 1984 Feb 10;259(3):1978–1985. [PubMed] [Google Scholar]
- Ji I., Bock J. H., Ji T. H. Composition and peptide maps of cross-linked human choriogonadotropin-receptor complexes on porcine granulosa cells. J Biol Chem. 1985 Oct 15;260(23):12815–12821. [PubMed] [Google Scholar]
- Ji I., Yoo B. Y., Kaltenbach C., Ji T. H. Structure of the lutropin receptor on granulosa cells. Photoaffinity labeling with the alpha subunit in human choriogonadotropin. J Biol Chem. 1981 Nov 10;256(21):10853–10858. [PubMed] [Google Scholar]
- Kellokumpu S., Rajaniemi H. Involvement of plasma membrane enzymes in the proteolytic cleavage of luteinizing hormone receptor. Endocrinology. 1985 Feb;116(2):707–714. doi: 10.1210/endo-116-2-707. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markkanen S. O., Rajaniemi H. J. Role of internalization and degradation in the removal of receptor-bound human chorionic gonadotropin in rat luteal cells in vivo. Endocrinology. 1980 Oct;107(4):1153–1161. doi: 10.1210/endo-107-4-1153. [DOI] [PubMed] [Google Scholar]
- Markkanen S., Töllikkö K., Jäskeläinen K., Rajaniemi H. Effect of radioiodination on the structural integrity, receptor and antibody-binding activity and circulatory behavior of human choriogonadotropin. Horm Res. 1980;12(1):32–45. doi: 10.1159/000179103. [DOI] [PubMed] [Google Scholar]
- Metsikkö M. K., Rajaniemi H. J. The hormone-binding unit of luteinizing hormone receptor. Biochem J. 1982 Nov 15;208(2):309–316. doi: 10.1042/bj2080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan V. P., Menon K. M. Evidence that dissociation, not intracellular degradation, is the major pathway for removal of receptor-bound 125I-human chorionic gonadotropin in cultured rat luteal cells. Biol Reprod. 1985 Aug;33(1):60–66. doi: 10.1095/biolreprod33.1.60. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Hazum E., Zor U. Photoaffinity labeling of human chorionic gonadotropin-binding sites in rat ovarian plasma membranes. J Biol Chem. 1984 Apr 10;259(7):4267–4271. [PubMed] [Google Scholar]
- Rebois R. V., Omedeo-Sale F., Brady R. O., Fishman P. H. Covalent crosslinking of human chorionic gonadotropin to its receptor in rat testes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2086–2089. doi: 10.1073/pnas.78.4.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Barber B. H., Crumpton M. J. Preparation of inside-out vesicles of pig lymphocyte plasma membrane. Biochemistry. 1976 Aug 10;15(16):3557–3563. doi: 10.1021/bi00661a025. [DOI] [PubMed] [Google Scholar]
- Wimalasena J., Moore P., Wiebe J. P., Abel J., Jr, Chen T. T. The porcine LH/hCG receptor. Characterization and purification. J Biol Chem. 1985 Sep 5;260(19):10689–10697. [PubMed] [Google Scholar]