Abstract
Aims
The histone deacetylase 6 (HDAC6) inhibitor, tubastatin A (TubA), reduces myocardial ischaemia/reperfusion injury (MIRI) in type 1 diabetic rats. It remains unclear whether HDAC6 regulates MIRI in type 2 diabetic animals. Diabetes augments the activity of HDAC6 and the generation of tumour necrosis factor alpha (TNF-α) and impairs mitochondrial complex I (mCI). Here, we examined how HDAC6 regulates TNF-α production, mCI activity, mitochondria, and cardiac function in type 1 and type 2 diabetic mice undergoing MIRI.
Methods and results
HDAC6 knockout, streptozotocin-induced type 1 diabetic, and obese type 2 diabetic db/db mice underwent MIRI in vivo or ex vivo in a Langendorff-perfused system. We found that MIRI and diabetes additively augmented myocardial HDAC6 activity and generation of TNF-α, along with cardiac mitochondrial fission, low bioactivity of mCI, and low production of adenosine triphosphate. Importantly, genetic disruption of HDAC6 or TubA decreased TNF-α levels, mitochondrial fission, and myocardial mitochondrial nicotinamide adenine dinucleotide levels in ischaemic/reperfused diabetic mice, concomitant with augmented mCI activity, decreased infarct size, and improved cardiac function. Moreover, HDAC6 knockout or TubA treatment decreased left ventricular dilation and improved cardiac systolic function 28 days after MIRI. H9c2 cardiomyocytes with and without HDAC6 knockdown were subjected to hypoxia/reoxygenation injury in the presence of high glucose. Hypoxia/reoxygenation augmented HDAC6 activity and TNF-α levels and decreased mCI activity. These negative effects were blocked by HDAC6 knockdown.
Conclusion
HDAC6 is an essential negative regulator of MIRI in diabetes. Genetic deletion or pharmacologic inhibition of HDAC6 protects the heart from MIRI by limiting TNF-α–induced mitochondrial injury in experimental diabetes.
Keywords: Histone deacetylase 6, Ischaemia/reperfusion, Type 1 diabetes, Type 2 diabetes, Mitochondria, Tumour necrosis factor alpha
Graphical Abstract
Graphical Abstract.
Time of primary review: 32 days
See the editorial comment for this article ‘Histone deacetylase 6 suppression protects from myocardial ischaemia-reperfusion injury in diabetes: insights from genetic deletion and pharmacological inhibition’, by Y.-J. Wang and C.M. Matter, https://doi.org/10.1093/cvr/cvae145.
1. Introduction
Diabetes mellitus is a growing public health problem with a prevalence approaching 422 million worldwide. Type 1 diabetes (T1D) is currently an incurable autoimmune disease marked by progressive and eventually exhaustive destruction of insulin-producing pancreatic β cells. Type 2 diabetes (T2D) is the combination of insulin resistance in peripheral tissue, insufficient insulin secretion from pancreatic β cells, and excessive glucagon secretion from pancreatic α cells. Both T1D and T2D enhance risk for ischaemic heart disease by two- to six-fold.1 Despite current optimal therapy, the mortality rate of acute myocardial infarction in diabetic patients is more than double that of non-diabetics.2 There is an urgent need to identify new therapeutic targets for ischaemic heart disease in diabetics.
Histone deacetylase 6 (HDAC6) functions to remove acetyl groups of lysine residues from histone and non-histone proteins.3,4 Histone deacetylation plays an important role in the modification of chromatin structure and dynamics, thereby gene transcriptional expression, whereas HDAC6-mediated deacetylation of non-histone proteins on mitochondria and myofibrils regulates mitochondrial function and myocardial passive stiffness under increased tension, respectively.3,5 Both hyperglycaemia and myocardial ischaemia augment the catalytic activity of HDAC6.6,7 A study in T1D rats showed that the selective HDAC6 inhibitor, tubastatin A (TubA), reduced myocardial ischaemia/reperfusion injury (MIRI).6 However, whether changes in cardiac HDAC6 activity affect MIRI and cardiac function in T2D has not been investigated. New proteomic technologies uncover that more than 60% of mitochondrially localized proteins including mitochondrial respiratory complexes contain lysine acetylation sites.8 How augmentation of HDAC6 activity affects MIRI and mitochondrial respiratory complexes in diabetes remains unknown.
Mitochondrial complex I [mCI, nicotinamide adenine dinucleotide (NAD(H)):ubiquinone oxidoreductase] is a multi-subunit membrane complex of the mitochondrial electron transport chain. By oxidizing reduced form of NAD(H) and reducing ubiquinone, mCI physiologically regenerates NAD(H) (NAD+) to sustain the tricarboxylic acid cycle and β oxidation.9 During MIRI, mCI can also catalyse the reverse reaction, Δp-linked oxidation of ubiquinol to reduce NAD+ (or O2), known as reverse electron transfer, to generate reactive oxygen species (ROS).10,11 Individual mCI behaves as a single unit, but it frequently forms a supramolecular unit called supercomplexes with complex III dimer and complex IV monomer to increase the efficacy of the electron transport and reduce the rate of production of ROS.12 Diabetes impairs the assembly of the supercomplex, and high levels of tumour necrosis factor alpha (TNF-α) damage the integrity of the supercomplex, which results in electron leak and increased production of ROS.13,14 Whether HDAC6 is involved in the increased generation of TNF-α and impaired mCI remains unknown in ischaemic/reperfused diabetic hearts.
Here, we quantitated myocardial HDAC6 activity, cardiac and plasma TNF-α concentrations, and mCI activity to understand their relationship in T1D and T2D mice with and without MIRI. We then examined the effects of HDAC6 knockout (KO) on TNF-α concentrations, mCI activity, mitochondrial morphology and NAD(H) levels, and myocardial infarct size in T1D mice in vivo and ex vivo. Using TubA, we further investigated the effect of HDAC6 inhibition on TNF-α concentrations, mCI activity, mitochondrial morphology and function, and cardiac function in T2D mice in vivo and ex vivo. Lastly, we evaluated the effects of HDAC6 KO and TubA on survival and cardiac function of the mice with T1D or T2D 28 days after MIRI. These studies identify HDAC6 as a potent regulator of TNF-α production and mCI activity and yield a strategy to inhibit HDAC6 to protect the heart against MIRI in diabetes. Taken together, our findings provide new insights into the pathogenesis of MIRI in experimental diabetes and highlight opportunities to improve treatment.
2. Methods
2.1. Animals
C57BL/6, C57BL/6J-Hdac6em2Lutzy/J (HDAC6−/−), homozygous T2D B6.BKS(D)-Leprdb/J (db/db), and heterozygous B6.BKS(D)-Leprdb/J (db/+) mice were maintained on a normal chow diet. The animals were kept on a 12 h light–dark cycle in a temperature-controlled room. The experimental procedures were approved by both the Animal Care and Use Committee of the Medical College of Wisconsin (IACUC #689, #2484) and Northwestern University (IACUC #375, #17860, #18015) and conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, 8th edition, 2011). Mice were euthanized by exposure to CO2 except for mice from the staining of the heart with 2,3,5-triphenyltetrazolium chloride (TTC) and phthalocyanine blue, which were euthanized using pentobarbital anaesthesia (intraperitoneal 150 mg/kg in 0.9% NaCl) followed by the removal of the heart.
2.2. Measurements of systemic haemodynamics and transthoracic echocardiography
Prior to MIRI, we determined systemic haemodynamics and the structure and function of the heart. Under 1.5% isoflurane anaesthesia, the right carotid artery was cannulated with a micromanometre-tipped mouse pressure catheter (SPR-671, Millar Instruments, Houston, TX, USA). Blood pressure was continuously recorded, as described.15 Transthoracic echocardiography was performed with a VisualSonics Vevo 3100 high-resolution imaging system, as described.16
2.3. MIRI in vivo
T1D of insulin deficiency was induced in HDAC6−/− and C57BL/6 mice by daily intraperitoneal injection of 50 mg/kg/day streptozotocin (STZ) for 5 consecutive days, as described.17 MIRI was induced by ligating the left anterior descending coronary artery for 20 min followed by reperfusion for 24 h in the mice 6 weeks after administration of STZ or citrate buffer as control (see Supplementary material online, Figure S1) or in T2D db/db mice and db/+ mice as control at 12–14 weeks of age (see Supplementary material online, Figure S2) under anaesthesia of 1.5% isoflurane, as described.18 TubA is a potent and highly selective HDAC6 inhibitor with an IC50 of 15 nM and more than 1000-fold selectivity towards all other isoforms except HDAC8 (57-fold).19 It was used to examine the effect of HDAC6 inhibition on MIRI in db/db mice. The infarct area was delineated by perfusing the coronary arteries with 2,3,5-TTC via the aortic root, and the area at risk was delineated by perfusing phthalocyanine blue dye into the aortic root after tying the coronary artery at the site of previous occlusion.
2.4. Mitochondrial imaging in the myocardium
The ultrathin sections of left ventricular (LV) samples from the infarct area of mice were examined, and mitochondria were imaged to visualize their morphology with a Philips CM120 transmission electron microscope (TEM), as described.20 TEM images were acquired and analysed blindly to the treatment group. Mitochondrial surface area was measured with ImageJ, and mitochondrial volume density was calculated as the area occupied by mitochondria divided by the area occupied by the cytoplasm.
2.5. MIRI in Langendorff-perfused hearts
Mouse hearts were mounted on a Langendorff apparatus and perfused retrogradely through the aorta at a constant pressure of 80 mmHg with Krebs–Henseleit buffer at 37°C, as described.18 A fluid-filled plastic balloon was inserted into the chamber of LV via the mitral valve and connected to a pressure transducer for continuous measurement of LV pressure. All hearts were stabilized for 30 min and subjected to 30 min of no-flow global ischaemia followed by 2 h of reperfusion. Mitochondrial NAD(H) fluorescence in mouse hearts was determined on a Langendorff apparatus placed within a light-proof Faraday cage to block the light, as described.15
2.6. Hypoxia/reoxygenation injury in H9c2 cardiomyocytes
H9c2 cardiomyocytes were treated with HDAC6 Silencer Select small interfering ribonucleic acid (siRNA) to knock down HDAC6 or scrambled siRNA as a control. H9c2 cells were used in determining the effects of HDC6 KO on hypoxia/reoxygenation injury (HRI)–induced changes in TNF-α and mCI and the effects of HDAC6 KO on high-glucose (HG)– and exogenous TNF-α–induced inhibition of mCI.
2.7. Molecular biology approaches
Total RNA was extracted with TRIzol reagent, as described.20 Real-time quantitative reverse transcriptase-polymerase chain reaction was conducted using the BioRad iCycler Real-Time Polymerase Chain Reaction Detection System. The myocardium from the area at risk of mouse hearts was harvested and homogenized, and western blots were performed using standard techniques, as described.21
2.8. Biochemical assay
The activity of HDAC6 in cardiac cells isolated from mice was assayed in wavelengths with excitation at 380 nm and emission at 420 nm. The concentrations of TNF-α in serum and the heart homogenates were detected by enzyme-linked immunosorbent assay. Mitochondria were isolated from mouse hearts, as described.22 The activity of mCI was determined by measuring the rotenone-sensitive decrease in NAD(H) absorbance at 340 nm, as described.23 Myocardial adenosine triphosphate (ATP) contents were measured by using a colorimetric/fluorometric method, as described.24
2.9. Post-infarct cardiac remodelling and function
C57BL/6, HDAC6−/− mice with and without T1D, and db/db and db/+ mice with and without TubA treatment were subjected to ligation of the left anterior descending coronary artery for 20 min. LV structure and function were examined with a VisualSonics Vevo 3100 echocardiography at baseline and 28 days after MIRI surgery. After echocardiographic examination was completed, the mice were euthanized for weighing the LV and lung. Survival rate was assessed until 28 days after surgery.
2.10. Statistical analyses
Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. All statistical analyses were performed using GraphPad Prism 9. A value of P < 0.05 (two tailed) was considered statistically significant.
3. Results
3.1. Cardiac HDAC6 activity is augmented additively by diabetes and MIRI
To study the functional implications of HDAC6 in T1D and MIRI, we first examined the myocardial HDAC6 activity in T1D C57BL/6 mice with and without MIRI (see Supplementary material online, Figure S9A). C57BL/6 mice with normal blood glucose levels and T1D were subjected to coronary artery occlusion for 20 min followed by reperfusion for 24 h in vivo or sham surgery as a control (Figure 1A). Blood glucose levels at the time point of 24 h after post-ischaemic reperfusion were comparable between T1D + MRI and T1D groups (Figure 1B). TTC and phthalocyanine blue dye staining displayed that MIRI elicited a marked infarct size (infarct size/area at risk: 59 ± 3%; Figure 1C) in T1D mice. In a separate group, mouse myocardium was collected for measuring HDAC6 activity. Compared with the Ctrl group, HDAC6 activity was significantly augmented by T1D (Figure 1D). Furthermore, it is higher in T1D + MIRI than T1D group (Figure 1D). These data indicate that T1D and MRI additively augment myocardial HDAC6 activity.
Figure 1.
Cardiac HDAC6 activity was enhanced in T1D mice undergoing sham surgery and MIRI. (A) Experimental procedures for STZ-induced T1D mice. (B) Fasting blood glucose in T1D and Ctrl mice 24 h after ischaemia or sham surgery. (C) Top: representative heart sections stained with 2,3,5-TTC and phthalocyanine blue showing area at risk and infarct size . The area at risk was defined as regions not stained with phthalocyanine blue dye, and the infarct area was defined as regions not stained with 2,3,5-TTC. Bottom: infarct size expressed as a percentage of area at risk in T1D and Ctrl mice. (D) Cardiac HDAC6 activity was enhanced in T1D and T1D + MIRI mice. Data are presented as means ± SEM. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. Ctrl and **P < 0.05 vs. T1D (n = 9–10 mice/group). (E) Experimental procedures for obese T2D db/db mice and controls (db/+). (F) Fasting blood glucose in db/db mice and Ctrl 24 h after ischaemia or sham surgery. (G) Top: heart sections stained with TTC and phthalocyanine blue showing area at risk and infarct size. Bottom: infarct size expressed as a percentage of area at risk in T2D and Ctrl mice. (H) Cardiac HDAC6 activity was increased in T2D and T2D + MIRI db/+ mice. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. db/+ and **P < 0.05 vs. T2D (n = 9–10 mice/group). Scale bar: 0.3 cm.
We next determined myocardial HDAC6 activity in obese T2D mice with and without MIRI (see Supplementary material online, Figure S9C). Obese T2D db/db and non-obese heterozygous db/+ Ctrl mice underwent coronary artery occlusion for 20 min followed by reperfusion 24 h or sham surgery as a control (Figure 1E). Blood glucose levels were higher in T2D than db/+ groups and in T2D + MIRI than T2D groups (Figure 1F). Coronary artery occlusion for 20 min followed by reperfusion for 24 h elicited 53 ± 4% of infarct size/area at risk (Figure 1G). Myocardial HDAC6 activity was higher in T2D than db/+ groups and in T2D + MIRI than T2D groups (Figure 1H). These data suggest that T2D and MIRI additively augment myocardial HDAC6 activity.
3.2. Mitochondrial morphology and mCI are impaired jointly by diabetes and MIRI
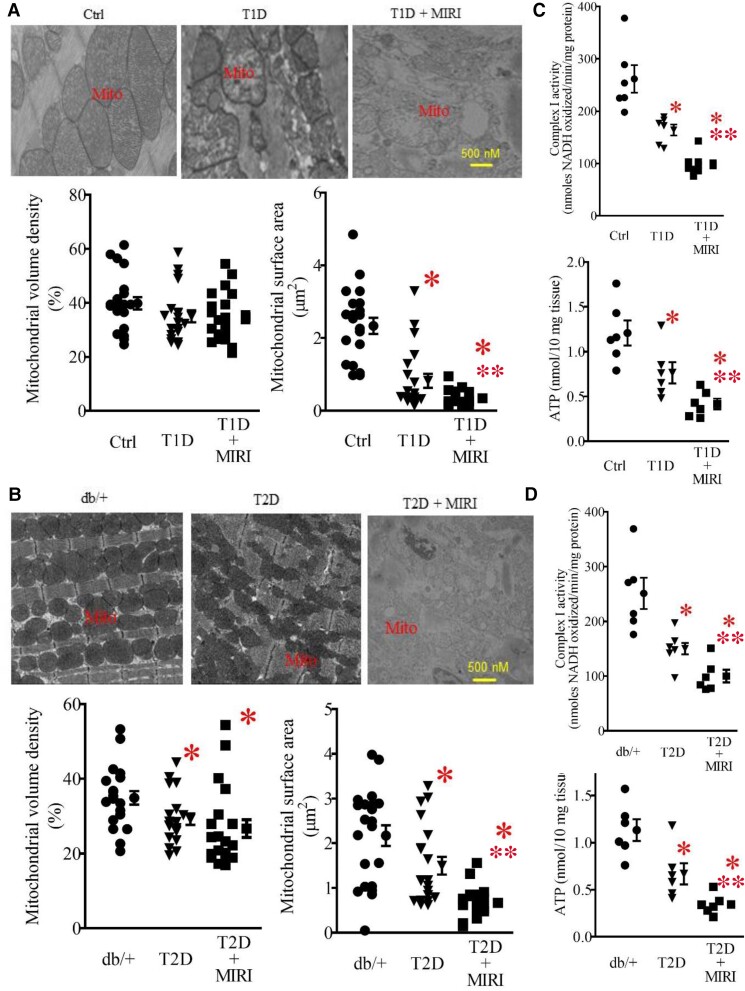
To investigate mitochondrial damage in T1D and MIRI, we used TEM to visualize subcellular structures and organization of the myocardium with a focus on the mitochondria. In the Ctrl mice, mitochondria and myofilaments were orderly arranged, and no fusion was observed between mitochondrial cristae and membranes (Figure 2A). In the T1D group, myofilaments and mitochondria were disordered, mitochondria were swollen, and mitochondrial cristae and membranes were dissolved with the presence of vacuole. Compared with the T1D group, the mitochondria in the T1D + MIRI group were highly disordered, exhibiting more oedema and dissolution with mitochondrial cristae and membrane rupture. We quantified mitochondrial volume density and mitochondrial surface area in 20–21 sections from 3 mice/group. There were no significant differences in mitochondrial volume density among the three groups (P > 0.05; Figure 2A). Mitochondrial surface area was smaller in T1D than Ctrl groups and in T1D + MIRI than T1D groups. Previous studies show that either T1D or MIRI causes mitochondrial fission through the mediation of mitochondrial fission protein, dynamin-related protein 1 (DRP1), and its adaptor protein fission 1 (FIS1).25,26 Collectively, these data demonstrate that T1D and MIRI jointly increase mitochondrial fission.
Figure 2.
Mitochondrial morphology, mCI activity, and ATP contents in T1D and T2D mice undergoing sham surgery and MIRI. (A) Top: representative electron microscope micrographs showing the changes in mitochondria of T1D mice and controls. Bottom: mitochondrial volume density (n = 20–21 sections from 3 mice/group) and mitochondrial surface area (n = 200–210 mitochondria from 3 mice/group). Ctrl, non-diabetic C57BL/6 mice were subjected to sham surgery; T1D, type 1 diabetic C57BL/6 mice underwent sham surgery; T1D + MIRI, diabetic C57BL/6 mice underwent 20 min of ischaemia followed by reperfusion for 24 h. (B) Top: representative electron microscope micrographs showing the changes in mitochondria of T2D mice and controls. Bottom: mitochondrial volume density (n = 20–21 sections from 3 mice/group) and mitochondrial surface area (n = 200–210 mitochondria from 3 mice /group). Db/+, non-diabetic db/+ mice were subjected to sham surgery; T2D, type 2 diabetic db/db mice underwent sham surgery; T2D + MIRI; db/db mice underwent 20 min of ischaemia followed by reperfusion for 24 h. (C) T1D and MIRI jointly decreased mCI activity and ATP contents. Myocardial mCI activity 5 min after post-ischaemic reperfusion (n = 6–8 hearts/group) and myocardial ATP contents (n = 6 hearts/group) in T1D mice and Ctrl mice. Data are presented as means ± SEM. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. Ctrl, **P < 0.05 vs. T1D groups. (D) T2D and MIRI jointly decreased mCI activity and ATP contents. Myocardial mCI activity 5 min after reperfusion (n = 6–8 hearts/group) and myocardial ATP contents (n = 6 hearts/group) in T2D mice and Ctrl mice. Scale bar: 500 nm.
We next examined how T2D or MIRI alone or in combination affected mitochondrial morphology. The mitochondria of the T2D mice undergoing coronary artery occlusion for 20 min followed by reperfusion for 24 h or sham surgery were imaged with a TEM. Heterozygous db/+ mice that had undergone sham surgery were used as a control (Figure 1E). The mitochondria in the Ctrl group were well organized, whereas mitochondria in the db/db mice that had undergone sham surgery (T2D) were fragmented (Figure 2B). Mitochondrial volume density was significantly lower in T2D than in Ctrl groups and in T2D + MIRI than in T2D groups, suggesting that cardiac mitochondrial biogenesis is suppressed in the db/db mice (P < 0.05, n = 20–21 from 3 mice/group; Figure 2B). Moreover, mitochondrial surface area was significantly smaller in T2D than in Ctrl groups and in T2D + MIRI than in T2D groups. Since either T2D or MIRI results in mitochondrial fission,25,27 these results suggest that T2D and MIRI jointly cause mitochondrial fission.
We have previously shown that cardiac mCI activity is noticeably lowered 5 min after reperfusion in mice subjected to MIRI.15 To measure mCI activity in diabetes with and without MIRI, we isolated mitochondria (see Supplementary material online, Figure S3) from mouse hearts at the time point of 5 min after reperfusion in T1D and T2D mice. mCI activity was significantly lower in T1D than in Ctrl groups and in T1D + MIRI than in T1D groups (P < 0.05, n = 6–8 mice/group; Figure 2C). Like the results of T1D, mCI activity was significantly lower in the T2D group than in the db/+ group and further lower in the T2D + MIRI group compared with the T2D group (P < 0.05 between T2D and T2D + MIRI groups). Furthermore, we measured myocardial ATP contents. Compared with Ctrl and db/+ groups, ATP contents were significantly decreased in T1D and T2D, which were further decreased in T1D + MIRI and T2D + MIRI (P < 0.05, n = 6 mice/group; Figure 2C and D). These results indicate that diabetes and MIRI jointly inhibit myocardial mCI activity, leading to a decrease in ATP generation.
Previous studies have demonstrated that TNF-α impairs the mitochondrial supercomplex assembly and mCI activity in cancer and dopaminergic cells,28,29 whereas both T1D and T2D elevate the generation of TNF-α.30 We used a rat IgG1 anti-TNF-α monoclonal antibody, MP6-XT22, to neutralize TNF-α in vivo (see Supplementary material online, Figure S5). Myocardial and plasma TNF-α levels were significantly increased, and mCI activity was decreased by either T1D or T2D alone and in combination with MIRI. Intriguingly, MP6-XT22 significantly decreased myocardial and plasma TNF-α levels and augmented myocardial mCI activity. Thus, increased levels of TNF-α contribute to low mCI activity in both T1D and T2D mice undergoing MIRI.
3.3. Genetic disruption of HDAC6 reduces TNF-α levels and augments mCI activity in ischaemic/reperfused T1D mice
Since myocardial HDAC6 activity is greatly augmented in ischaemic/reperfused T1D hearts, we investigated whether HDAC6 KO affected blood glucose and plasma TNF-α levels, myocardial infarct size, and mCI activity. HDAC6−/− and C57BL/6 mice were intraperitoneally injected STZ to induce T1D or citrate buffer as a control (Figure 3A). Table 1 lists the general characteristics and echocardiographic parameters (see Supplementary material online, Figure S4) of the four groups of mice: MIRI, HDAC6−/− + MIRI, T1D + MIRI, and HDAC6−/− + T1D + MIRI, prior to MIRI surgery. Fasting blood glucose levels at the time point of 24 h after post-ischaemic reperfusion were comparable between HDAC6−/− + MIRI and MIRI groups but were higher in T1D + MIRI than in MIRI and HDAC6−/− + MIRI groups (Figure 3B). Compared with the T1D + MIRI group, blood glucose levels were not significantly changed in the HDAC6−/− + T1D + MIRI group. We determined plasma TNF-α levels (see Supplementary material online, Figure S9B) and myocardial mCI activity 5 min after post-ischaemic reperfusion in HDAC6−/− and C57BL/6 mice with and without T1D (Figure 3A). Both plasma TNF-α levels and mCI activity were comparable between HDAC6−/− + MIRI and MIRI groups. Compared with the MIRI or HDAC6−/− + MIRI groups, plasma TNF-α levels were significantly increased (Figure 3C), and mCI activity was inhibited in T1D + MIRI groups (P < 0.05, n = 9–11 mice/group; Figure 3D). Interestingly, plasma TNF-α levels were lower, and mCI activity was higher in HDAC6−/− + T1D + MIRI than in T1D + MIRI groups. These results suggest that HDAC6 KO decreases plasma TNF-α levels and augments myocardial mCI activity in ischaemic/reperfused T1D mice.
Figure 3.
HDAC6 KO decreased plasma TNF-α and infarct size and augmented mCI activity during reperfusion in type 1 diabetic mice. (A) Experimental procedures. (B) Fasting blood glucose in HDAC6−/− and C57BL/6 mice. (C) HDAC6 KO decreased plasma TNF-α levels in T1D mice undergoing MIRI. (D) HDAC6 KO increased mCI activity in T1D mice undergoing MIRI (n = 9–11 hearts/group). (E) HDAC6 KO increased mitochondrial surface area and decreased the expression of cardiac DRP1 and FIS1 in T1D mice undergoing MIRI. Top: representative electron microscope micrographs of mitochondria. Bottom: mitochondrial volume density (n = 20–21 sections from 3 mice/group), mitochondria surface area (n = 200–210 mitochondria from 3 mice/group), and the expression of cardiac DRP1 and FIS1 (n = 5 hearts/group). Scale bar: 500 nm. (F) HDAC6 KO decreased myocardial infarct size in T1D mice. Top: representative heart images showing area at risk (non-black area) and infarct size. Arrows point to infarct area (white). Scale bar: 0.3 cm. Bottom: area at risk expressed as a percentage of the left ventricle and infarct size expressed as a percentage of area at risk (n = 8–10 mice/group). Data are presented as means ± SEM. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. MIRI groups, **P < 0.05 vs. HDAC6−/− + MIRI groups, ***P < 0.05 vs. T1D + MIRI groups.
Table 1.
General characteristics and echocardiographic parameters of C57BL/6 and HDAC6−/− mice prior to MIRI
| MIRI | HDAC6−/− + MIRI | T1D + MIRI | HDAC6−/− + T1D + MIRI | |
|---|---|---|---|---|
| Body weight (g) | 28.3 ± 0.3 | 28.1 ± 0.2 | 22.7 ± 0.3*,** | 24.2 ± 0.3*,**,*** |
| Heart rate (b.p.m.) | 477 ± 16 | 470 ± 19 | 452 ± 15 | 446 ± 16 |
| Blood glucose (g/dL) | 127 ± 9 | 113 ± 11 | 338 ± 15*,** | 349 ± 16*,** |
| MAP (mmHg) | 103 ± 4 | 107 ± 3 | 99 ± 2 | 106 ± 3 |
| SAP (mmHg) | 137 ± 5 | 140 ± 4 | 129 ± 4 | 138 ± 4 |
| DAP (mmHg) | 87 ± 5 | 90 ± 3 | 84 ± 2 | 89 ± 3 |
| Anterior wall at end-diastole (mm) | 1.01 ± 0.08 | 1.05 ± 0.08 | 0.87 ± 0.03 | 0.99 ± 0.05 |
| Anterior wall at end-systole (mm) | 1.51 ± 0.10 | 1.47 ± 0.11 | 1.31 ± 0.06 | 1.43 ± 0.09 |
| Posterior wall at end-diastole (mm) | 1.10 ± 0.07 | 1.02 ± 0.06 | 0.95 ± 0.03 | 0.95 ± 0.04 |
| Posterior wall at end-systole (mm) | 1.56 ± 0.08 | 1.62 ± 0.11 | 1.46 ± 0.09 | 1.48 ± 0.07 |
| Left ventricular end-diastolic volume (µL) | 64 ± 5 | 67 ± 5 | 71 ± 5 | 67 ± 5 |
| Left ventricular end-systolic volume (µL) | 17 ± 2 | 17 ± 2 | 20 ± 1 | 20 ± 3 |
| Ejection fraction (%) | 74 ± 3 | 75 ± 2 | 70 ± 2 | 71 ± 3 |
| Cardiac output (mL/min) | 19 ± 2 | 21 ± 2 | 20 ± 1 | 21 ± 2 |
| Mitral E/e′ ratio | 22 ± 1 | 23 ± 1 | 24 ± 3 | 23 ± 2 |
DAP, diastolic pressure; MAP, mean arterial pressure; MIRI, myocardial ischaemia/reperfusion injury; SAP, systolic pressure; T1D, type 1 diabetes.
* P < 0.05 vs. MIRI groups, **P < 0.05 vs. HDAC6−/− + MIRI groups, ***P < 0.05 vs. T1D + MIRI groups analysed by Kruskal–Wallis test followed by Dunn's test (n = 10–12 mice/group).
Mitochondrial morphology affects susceptibility of the heart to MIRI, and furthermore, inhibiting mitochondrial fission protects the heart against MIRI.31 We next used TEM to visualize cardiac mitochondria and quantify mitochondrial volume density and mitral surface area in HDAC6−/− and C57BL/6 mice with and without T1D. There were no significant differences in mitochondrial volume density among the four groups (P > 0.05, n = 20–21 sections from 3 hearts/group; Figure 3E). Surface area of cardiac mitochondria was comparable between HDAC6−/− + MIRI and MIRI groups but was significantly smaller in T1D + MIRI than in MIRI and HDAC6−/− + MIRI groups (P < 0.05, n = 200–210 mitochondria from 3 hearts/group). Interestingly, mitochondrial surface area was greater in HDAC6−/− + T1D + MIRI than in T1D + MIRI groups. To investigate whether changes in mitochondrial size are because of mitochondrial fission, we measured the expression of DRP1 and FIS1 (see Supplementary material online, Figure 7A). Compared with MIRI and HDAC6−/− + MIRI groups, the expression of both DRP1 and FIS1 was significantly increased in T1D + MIRI groups, which was decreased in HDAC6−/− + T1D + MIRI groups (Figure 3E). These results demonstrate that HDAC6 KO prevents mitochondrial fission during MIRI in T1D.
In separate experiments, we used phthalocyanine blue and TTC to stain mouse hearts to delineate area at risk and infarct size, respectively.15 Area at risk was comparable among the four experimental groups (P > 0.05). Compared with the MIRI groups, infarct size was not significantly altered in the HDAC6−/− + MIRI group but increased in the T1D + MIRI group (Figure 3F; P < 0.05, n = 8–10 mice/group). Interestingly, infarct size was significantly decreased in the HDAC6−/− + T1D + MIRI group compared with the T1D + MIRI group. These results indicate that HDAC6 KO protects T1D hearts from MIRI.
3.4. Pharmacological inhibition of HDAC6 reduces TNF-α levels and augments mCI activity in ischaemic/reperfused T2D mice
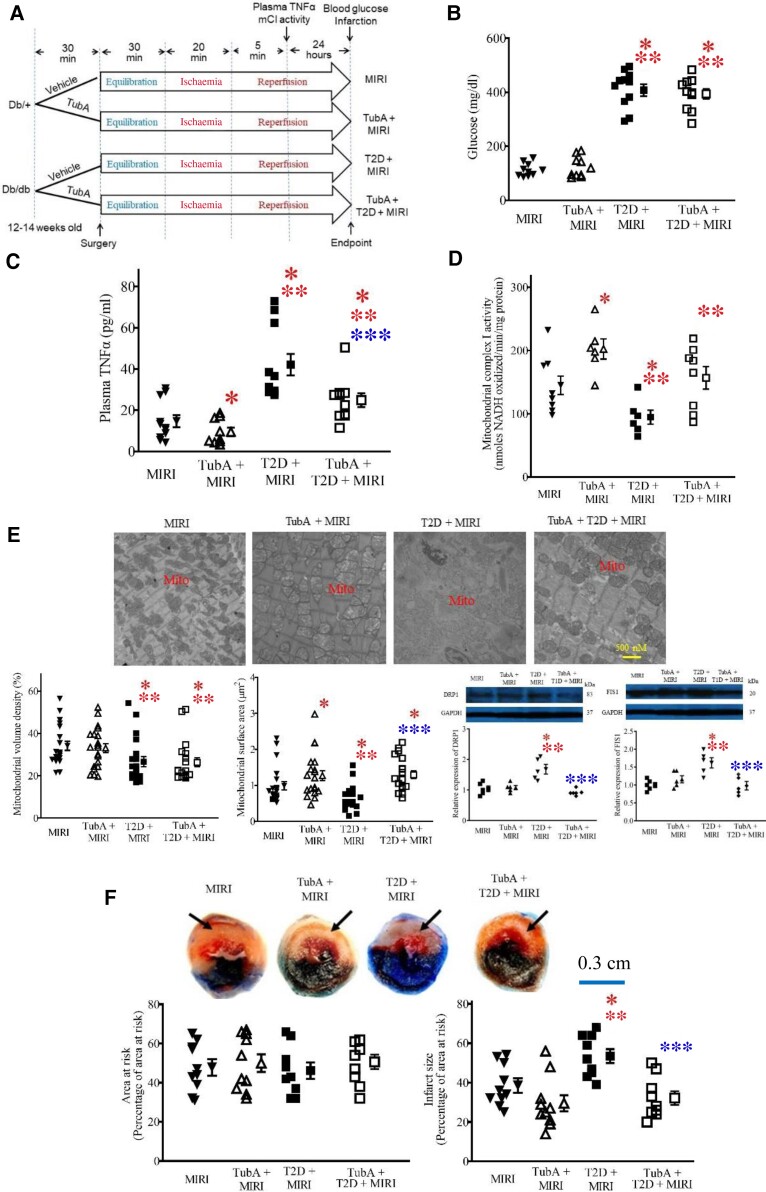
We further explored whether pharmacological inhibition of HDAC6 affected plasma TNF-α levels (see Supplementary Figure online, Figure S9D) and myocardial mCI activity in T2D mice that had undergone MIRI. db/db and db/+ mice were pre-treated with TubA 30 min before surgery for MIRI or vehicle as a control (Figure 4A). Table 2 lists the general characteristics and echocardiographic parameters (see Supplementary material online, Figure S4) of the four groups of mice: MIRI, TubA + MIRI, T2D + MIRI, and TubA + T2D + MIRI, prior to MIRI surgery. Fasting blood glucose levels were comparable between TubA + MIRI and MIRI groups and higher in both T2D + MIRI and TubA + T2D + MIRI groups than in both MIRI and TubA + MIRI groups (Figure 4B). There were no significant differences in blood glucose levels between TubA + T2D + MIRI and T2D + MIRI groups. At the time point of 5 min post-ischaemic reperfusion, plasma TNF-α levels were significantly lower, and myocardial mCI activity was higher in TubA + MIRI than in MIRI groups (Figure 4C and D). Compared with the MIRI and TubA + MIRI groups, plasma TNF-α levels were enhanced, and myocardial mCI activity was inhibited in T2D + MIRI groups. Interestingly, TubA treatment significantly decreased plasma TNF-α levels and augmented mCI activity in TubA + T2D + MIRI groups (P < 0.05, n = 9–11 mice/group). These data suggest that TubA decreases plasma TNF-α and augments mCI activity in the ischaemic/reperfused T2D mice.
Figure 4.
Inhibition of HDAC6 decreased plasma TNF-α and infarct size and augmented mCI activity during reperfusion in T2D mice. (A) Experimental procedures. (B) Fasting blood glucose in db/db mice and db/+ control mice. (C) TubA decreased plasma TNF-α levels in T2D mice undergoing MIRI. (D) TubA enhanced myocardial mCI activity in T2D mice undergoing MIRI. (E) TubA increases mitochondrial size and decreased the expression of cardiac DRP1 and FIS1 in T2D mice undergoing MIRI. Top: representative electron microscope micrographs of mitochondria. Bottom: mitochondrial volume density (n = 20–21 sections from 3 mice/group), mitochondria surface area (n = 200–210 mitochondria from 3 mice/group), and the expression of cardiac DRP1 and FIS 1 (n = 5 hearts/group). Scale bar: 500 nm. (F) TubA decreased myocardial infarct size in T2D mice. Top: representative heart images showing area at risk and infarct size. Arrows point to infarct area. Scale bar: 0.3 cm. Bottom: area at risk expressed as a percentage of the left ventricle and infarct size expressed as a percentage of area at risk (n = 8–10 mice/group). Data are presented as means ± SEM. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. MIRI groups, **P < 0.05 vs. TubA + MIRI groups, ***P < 0.05 vs. T2D + MIRI groups.
Table 2.
General characteristics and echocardiographic parameters of db/+ and db/db mice prior to MIRI
| MIRI | TubA + MIRI | T2D + MIRI | TubA + T2D + MIRI | |
|---|---|---|---|---|
| Body weight (g) | 27.4 ± 0.4 | 27.9 ± 0.4 | 49.0 ± 1.3*,** | 47.6 ± 1.4*,** |
| Heart rate (b.p.m.) | 405 ± 13 | 395 ± 15 | 377 ± 9 | 446 ± 16 |
| Blood glucose (mg/dL) | 109 ± 8 | 106 ± 9 | 379 ± 14*,** | 364 ± 20*,** |
| MAP (mmHg) | 102 ± 4 | 106 ± 3 | 112 ± 4 | 108 ± 4 |
| SAP (mmHg) | 132 ± 6 | 135 ± 4 | 145 ± 5 | 144 ± 6 |
| DAP (mmHg) | 87 ± 3 | 92 ± 3 | 95 ± 3 | 89 ± 4 |
| Anterior wall at end-diastole (mm) | 0.88 ± 0.08 | 0.87 ± 0.03 | 0.98 ± 0.04 | 0.93 ± 0.05 |
| Anterior wall at end-systole (mm) | 1.31 ± 0.09 | 1.40 ± 0.05 | 1.47 ± 0.07 | 1.38 ± 0.06 |
| Posterior wall at end-diastole (mm) | 0.84 ± 0.04 | 0.82 ± 0.03 | 0.95 ± 0.05 | 0.89 ± 0.06 |
| Posterior wall at end-systole (mm) | 1.17 ± 0.05 | 1.23 ± 0.09 | 1.36 ± 0.07 | 1.28 ± 0.08 |
| Left ventricular end-diastolic volume (µL) | 66 ± 4 | 66 ± 5 | 73 ± 4 | 70 ± 5 |
| Left ventricular end-systolic volume (µL) | 25 ± 2 | 23 ± 3 | 30 ± 4 | 25 ± 2 |
| Ejection fraction (%) | 62 ± 2 | 65 ± 3 | 59 ± 4 | 63 ± 3 |
| Cardiac output (mL/min) | 19 ± 2 | 21 ± 2 | 21 ± 2 | 20 ± 2 |
| Mitral E/e′ ratio | 23 ± 1 | 25 ± 1 | 27 ± 2 | 26 ± 1 |
DAP, diastolic pressure; MAP, mean arterial pressure; MIRI, myocardial ischaemia/reperfusion injury; SAP, systolic pressure; T2D, type 2 diabetes; TubA, tubastatin A.
* P < 0.05 vs. MIRI, **P < 0.05 vs. TubA + MIRI analysed by Kruskal–Wallis test followed by Dunn's test (n = 10–12 mice/group).
The myocardium at 24 h after post-ischaemic reperfusion was imaged with a TEM to visualize mitochondria (Figure 4E). In the MIRI and TubA + MIRI groups, myofilaments and mitochondria were disordered, mitochondria were swollen, and mitochondrial cristae and membranes were dissolved with the presence of vacuoles, compared with the T2D group in Figure 2B. Compared with the MIRI group, the mitochondria in the T2D + MIRI group were highly disordered, exhibiting more oedema and dissolution with mitochondrial cristae and membrane rupture. In contrast, the hearts of the TubA + T2D + MIRI group had preserved mitochondrial morphology, exhibiting moderate oedema and cristae dissolution. We quantified mitochondrial volume density and mitochondrial surface area from the 20–21 sections of 3 mice in each group. Mitochondrial volume density was comparable between TubA + MIRI and MIRI groups. Compared with MIRI and TubA + MIRI groups, it was significantly decreased in T2D + MIRI and TubA + T2D + MIRI groups. There were no significant differences in mitochondrial volume density between TubA + MIRI groups and between TubA + T2D + MRI and T2D + MIRI groups (Figure 4E). Mitochondrial surface area was greater in TubA + MIRI than in MIRI groups and smaller in T2D + MIRI than in MIRI and TubA + MIRI groups. Interestingly, compared with the T2D + MIRI group, mitochondrial surface area was significantly increased in the TubA + T2D + MIRI group. Compared with MIRI and TubA + MIRI groups, the expression of both DRP1 and FIS1 (see Supplementary material online, Figure 7B) was significantly increased in T2D + MIRI groups, which was decreased in TubA + T2D + MIRI (Figure 4E). These results indicate that HDAC6 inhibition prevents cardiac mitochondrial fission in ischaemic/reperfused T2D mice.
The hearts were stained with phthalocyanine blue dye and TTC to delineate area at risk and infarct size, respectively (Figure 4F). There were no significant differences in area at risk among the four experimental groups. Coronary occlusion for 20 min followed by reperfusion for 24 h resulted in infarct size of 39 ± 4% of area at risk (n = 10 mice) in Ctrl mice, which significantly increased to 53 ± 4% (n = 10 mice, P < 0.05 vs. Ctrl groups) in the T2D group (Figure 4F). TubA treatment did not significantly change infarct size in db/+ mice. Interestingly, T2D-induced increase in infarct size was blocked by TubA (n = 9 mice, P < 0.05 vs. T2D; Figure 4F). These results indicate that selective inhibition of HDAC6 protects T2D mice from MIRI.
3.5. HDAC6 KO limits mitochondrial NAD(H) release and ameliorates cardiac dysfunction in ischaemic/reperfused T1D mice
mCI oxidizes NAD(H) from the tricarboxylic acid cycle and β oxidation of fatty acids, reduces ubiquinone, and transports protons across the inner membrane, contributing to the proton-motive force.32 Since HDAC6 KO augments mCI activity in ischaemic/reperfused T1D hearts, we dynamically determined the levels of NAD(H) online during ischaemia and reperfusion in isolated Langendorff-perfused hearts (Figure 5A).15 Mitochondrial NAD(H) levels from Langendorff-perfused hearts at baseline were higher in T1D and HDAC6−/− + T1D groups than in MIRI groups (P < 0.05, n = 8 hearts/group; Figure 5B). During ischaemia, the NAD(H) signal initially increased and peaked 5 min after ischaemia followed by a gradual decline in MIRI groups. NAD(H) fluorescence was significantly lower in the HDAC6−/− + MIRI group than in the MIRI group 3–5 min after ischaemia and greater in either T1D + MIRI or HDAC6−/− + MIRI groups during a period of 30 min ischaemia than in Ctrl group (P < 0.05, n = 8–9 hearts/group; Figure 5B). During reperfusion, the NAD(H) signal remained relatively stable in the four experimental groups. NAD(H) levels were higher in T1D + MIRI groups than in MIRI, HDAC6−/− + MIRI, or HDAC6−/− + T1D + HDAC6 groups during the period of 120 min reperfusion (P < 0.05, n = 8–9 hearts/group). No significant differences were found among HDAC6−/− + T1D + MIRI, HDAC6−/− + MIRI, and MIRI groups. Thus, HDAC6 KO preserved mitochondrial NAD+ metabolism during ischaemia and reperfusion in T1D.
Figure 5.
HDAC6 KO reduced T1D-elicited increases in mitochondrial NAD(H) levels and improved cardiac function during reperfusion in Langendorff-perfused hearts. (A) Schematic presentation of experimental procedure of ex vivo experiments for measurements of NAD(H) and cardiac function of HDAC6−/− and C57BL/6 mice undergoing ischaemia/reperfusion injury (MIRI; n = 8–9 mice/group). (B) The dynastic changes in NAD(H) fluorescence of Langendorff-perfused mouse hearts at baseline, ischaemia, and reperfusion. (C) LV end-diastolic function. (D) LVDP. (E) +dP/dt (maximum rate of increase of LVDP). (F) −dP/dt (maximum rate of decrease of LVDP). Data are presented as means ± SEM. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. MIRI groups, **P < 0.05 vs. HDAC6−/− + MIRI groups, ***P < 0.05 vs. T1D + MIRI.
Since the ratio of NAD+/NAD(H) is crucial for cardiac energy and redox homeostasis,33,34 we determine cardiac function in isolated Langendorff-perfused mouse hearts during ischaemia and reperfusion. The dynamic changes in LV pressure and derivatives of HDAC6−/− and C57BL/6 mouse hearts undergoing MIRI are shown in Figure 5. Left ventricular end-diastolic pressure (LVEDP) at baseline was comparable among the four groups (P > 0.05; Figure 5C), whereas the values of left ventricular developed pressure (LVDP) and ±dP/dt (+dP/dt, maximum rate of increase of LVDP; −dP/dt, maximum rate of decrease of LVDP) at baseline were smaller in T1D + MIRI and HDAC6−/− + T1D + MIRI than in MIRI groups (P < 0.05, n = 8 mice/group; Figure 5D–F). Global ischaemia for 30 min resulted in the cessation of the contraction and relaxation of the hearts and an increase in LVEDP. With reperfusion, contraction and relaxation were gradually restored in all mouse hearts. There were no significant differences in LVEDP between T1D + MIRI and MIRI groups during ischaemia and reperfusion (P > 0.05; Figure 5C). Compared with MIRI groups, LVEDP was significantly decreased in HDAC6−/− + MIRI groups 1–2 h (P < 0.05, n = 8 mice/group) after reperfusion but not in HDAC6−/− + T1D + MIRI groups. The values of LVDP and ±dP/dt were significantly smaller in T1D + MIRI than in MIRI groups from 30 min to 2 h after reperfusion (P < 0.05; Figure 5D–F). Compared with MIRI groups, the values of LVDP and −dP/dt were significantly increased in HDAC6−/− + MIRI groups from 60 min to 2 h and 30 to 60 min after reperfusion, respectively (Figure 5D and F). There were significant differences in the values of LVDP and ±dP/dt at the time points of 60, 90, and 120 min after reperfusion between HDAC6−/− + T1D + MIRI and T1D + MIRI groups (Figure 5D–F). These results indicate that HDAC6 KO ameliorates cardiac diastolic and systolic dysfunction caused by T2D and MIRI.
3.6. TubA attenuates mitochondrial NAD(H) levels and improves cardiac function in T2D mice
We examined the effects of HDAC6 inhibition on mitochondrial NAD(H) and cardiac dysfunction during ischaemia and reperfusion in T2D and db/+ Ctrl mice. Langendorff-perfused mouse hearts were subjected to 30 min of global ischaemia followed by 120 min of reperfusion ex vivo. TubA or vehicle was perfused into the heart for 20 min prior to ischaemia and continuously throughout 120 min of reperfusion (see Supplementary material online, Figure S11A). NAD(H) fluorescence during 30 min of ischaemia was comparable between TubA + MIRI and MIRI groups and much stronger in both T2D + MIRI and TubA + T2D + MIRI groups than in MIRI groups (see Supplementary material online, Figure S11B). Compared with the T2D + MIRI group, NAD(H) levels during 30 min of ischaemia and 120 min of reperfusion were significantly decreased in the TubA + T2D + MIRI group. These results suggest that HDAC6 inhibition potently decreases mitochondrial NAD(H) levels in T2D mice undergoing MIRI.
We next quantified LVEDP, LVDP, and ±dP/dt obtained from Langendorff-perfused hearts.15 LVEDP at baseline was comparable among the four groups (P > 0.05; see Supplementary material online, Figure S11C), whereas the values of LVDP and ±dP/dt at baseline were smaller in both T2D + MIRI and TubA + T2D + MIRI groups than in both MIRI and TubA + MIRI groups (P < 0.05, n = 8 mice/group; see Supplementary material online, Figure S11D–F). Global ischaemia for 30 min resulted in the cessation of the contraction and relaxation of the hearts and an increase in LVEDP. With reperfusion, contraction and relaxation were gradually restored in all mouse hearts. There were no significant differences in LVEDP between TubA + MIRI and MIRI groups during ischaemia and reperfusion (P > 0.05; see Supplementary material online, Figure S11C). Compared with MIRI groups, LVEDP was significantly increased in T2D + MIRI groups from 30 min to 2 h (P < 0.05, n = 8 mice/group) after reperfusion but not in TubA + T2D + MIRI groups (n = 9–10 mice/group). The values of LVDP and ±dP/dt were significantly smaller in T2D + MIRI than in MIRI groups from 30 min to 2 h after reperfusion (P < 0.05; see Supplementary material online, Figure S11D–F). Compared with MIRI groups, the values of LVDP and ±dP/dt were not significantly altered in TubA + MIRI groups and decreased in T2D + MIRI groups from 30 min to 2 h after reperfusion (see Supplementary material online, Figure S11D–F). All three values were greater in TubA + T2D + MIRI than in T2D + MIRI groups from 30 to 120 min. These results indicate that HDAC6 inhibition improves cardiac diastolic and systolic function in ischaemic/reperfused T2D mice.
3.7. HDAC6 knockdown blocks the TNF-α–induced inhibition of mCI activity in vitro
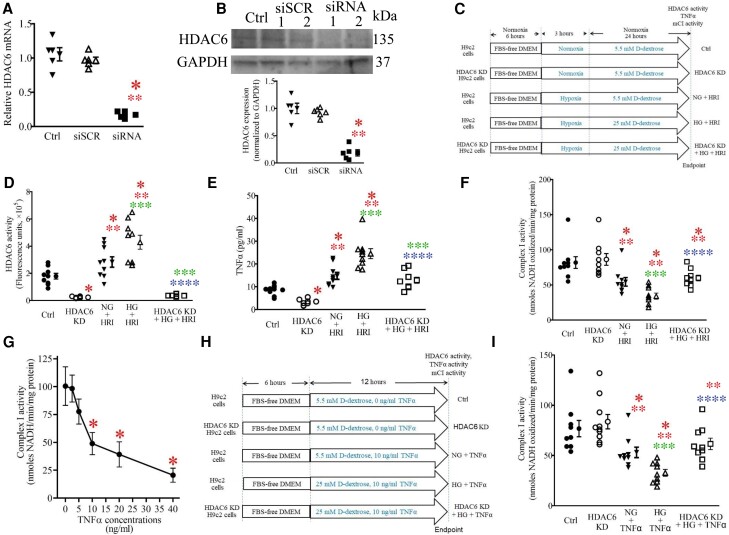
Both cardiomyocytes and inflammatory cells generate TNF-α, which impairs the electron transport of mitochondrial respiratory chain.13,35 To obtain insights into the role of HDAC6 in cardiomyocyte-derived TNF-α, we investigated the effects of HDAC6 knockdown (KD) in H9c2 cardiomyocytes on TNF-α levels and mCI activity by culturing H9c2 cells under 25.0 mM D-dextrose (HG) in vitro (Figure 6C). The HDAC6-siRNA significantly decreased HDAC6 mRNA levels and HDAC6 protein expression in H9c2 cells (P < 0.05, n = 5 dishes/group; Figure 6A and B). D-Glucose at a concentration of 25.0 mM and hypoxia/reoxygenation together significantly augmented HDAC6 activity, increased TNF-α levels, and inhibited mCI activity (Figure 6D–F). Intriguingly, HDAC6 KD decreased TNF-α and augmented mCI activity of H9c2 cells in HG and HRI.
Figure 6.
HDAC6 KD blocked TNF-α–induced inhibition of mCI activity in cardiomyocytes. (A) Quantitative reverse transcriptase-polymerase chain reaction analysis of HDAC6 mRNA levels in H9c2 cardiomyocytes. (B) Western blot analysis of HDAC6 protein expression in H9c2 cardiomyocytes. Top: representative western blot bands showing the expression of HDAC6 and GAPDH as control. The complete unedited gels are shown in Supplementary material online, Figure S6. Bottom: expression of HDAC6 proteins normalized to GAPDH in H9c2 cardiomyocytes. Ctrl, control; siSCR, scrambled siRNA; siRNA 1, HDAC6-siRNA 1; siRNA 2, HDAC6-siRNA 2. (C) Experimental procedures showing the effects of HDAC6 KD on HRI-induced changes in TNF-α and mCI in H9c2 cardiomyocytes. HG, high glucose; NG, normal glucose. (D) HDAC6 KD lowered HG- and HRI-induced increases in HDAC6 activity of H9c2 cardiomyocytes. (E) HDAC6 KD lowered HG- and HRI-induced increases in TNF-α concentrations in H9c2 cardiomyocytes. (F) HDAC6 KD enhanced HG- and HRI-induced decreases in mCI activity. *P < 0.05 vs. Ctrl, **P < 0.05 vs. HDAC6 KD, ***P < 0.05 vs. NG + HRI, ****P < 0.05 vs. HG + HRI group (n = 9–10 /group). (G) Dose-dependent effects of TNF-α on mCI activity under 25 mg/dL of glucose. *P < 0.05 vs. 0 ng/mL TNF-α groups (n = 6/group). (H) Experimental procedures showing the effects of HDAC6 KD on HG and exogenous TNF-α–induced inhibition of mCI in H9c2 cardiomyocytes. (I) HDAC6 KD antagonized TNF-α–elicited inhibition of mCI activity. Data are presented as means ± SEM. Kruskal–Wallis test followed by Dunn's test was used to analyse multiple group comparisons. *P < 0.05 vs. Ctrl, **P < 0.05 vs. HDAC6 KD, ***P < 0.05 vs. NG + TNF-α, ****P < 0.05 vs. HG + TNF-α group (n = 9–10/group).
Next, we investigated the effects of HDAC6 KD on exogenous TNF-α–induced damage to mCI in H9c2 cardiomyocytes in the presence of 5.5 mM (normal glucose) and 25.0 mM (HG) D-dextrose (Figure 6G). Under 5.5 mM D-dextrose, TNF-α from 0 to 40 ng/mL did not alter mCI activity (see Supplementary material online, Figure S10). However, under 25.0 mM D-dextrose (HG), TNF-α dose-dependently decreased CI activity (P < 0.05, n = 6 dishes/group; Figure 6G). TNF-α at 20 and 40 ng/mL elicited significant cell necrosis. Since TNF-α at 10 ng/mL did not cause cell death, TNF-α at a dose of 10 ng/mL was chosen to treat H9c2 cells (Figure 6H). Interestingly, 10 ng/mL TNF-α–induced decreases in mCI activity were blocked by HDAC6 KO (P < 0.05 between TNF-α + HDAC6 KO and TNF-α groups, n = 9–10 dishes/group; Figure 6I). These data suggest that augmentation of HDAC6 activity increases TNF-α production in cardiomyocytes and augmentation of both internal and exogenous TNF-α directly inhibits mCI activity.
3.8. KO or pharmacologic inhibition of HDAC6 improves post-infarct cardiac function in diabetes
Diabetic patients surviving myocardial infarction have substantially higher mortality due to the more frequent development of subsequent pathological cardiac remodelling and concomitant functional deterioration.36 We first examined the effect of HDC6 KO on mortality and LV systolic function during the period of 28 days after MIRI in T1D. There were no significant differences in per cent survival of mice (n = 14 mice/group) among MIRI, HDAC6−/− + MIRI, T1D + MIRI, and HDAC6−/− + T1D + MIRI groups (Figure 7A). Compared with MIRI and HDAC6−/− + T1D groups, left ventricular internal diameters at end-diastole (LVIDd) and left ventricular internal diameters at end-systole (LVIDs) were longer, and LV fractional shortening was smaller in T1D + MIRI groups (P < 0.05, n = 8–11 mice/groups). Interestingly, both LVIDd and LVIDs were shorter, whereas LV fractional shortening was greater in HDAC6−/− + T1D + MIRI than in T1D + MIRI groups (Figure 7B). Additionally, both LV weight and lung weight normalized to body weight were greater in T1D + MIRI groups than in both MIRI and HDAC6−/− + MIRI groups (P < 0.05, n = 8–11 mice/groups). There was a significant decrease in LV weight and lung weight normalized to body weight in HDAC6−/− + T1D + MIRI groups compared with T1D + MIRI groups (Figure 7C) (see Supplementary material online, Figures 8A & 8B). Collectively, these data indicate that HDAC6 KO ameliorates post-infarct LV systolic dysfunction in T1D.
Figure 7.
KO or inhibition of HDAC6 reduced LV dilation and improved LV systolic function 28 days after MIRI in diabetes. (A) Kaplan–Meier survival curves for control and HDAC6 KO mice with and without T1D 28 days after MIRI (top panel) and for control and TubA-treated mice with and without T2D 28 days after MIRI (bottom panel). (B) Representative short-axis two-chamber view–guided M-mode images and quantitative analysis of cardiac parameters, including LVIDd, LVIDs, and LV fractional shortening at 28 days after MIRI. (C) LV weight and lung weight normalized to body weight of mice 28 days after MIRI. *P < 0.05 vs. MIRI groups in T1D or in T2D, **P < 0.05 vs. HDAC6−/− + MIRI groups in T1D or TubA + MIRI groups in T2D, ***P < 0.05 vs. T1D + MIRI groups in T1D or T2D + MIRI groups in T2D analysed by Kruskal–Wallis test followed by Dunn's test (n = 8–12 mice/group).
Next, we investigated the effect of TubA on mortality and LV systolic function after MIRI in T2D. Mortality rate during the period of 28 days after MIRI was similar among MIRI, TubA + MIRI, T2D + MIRI, and TubA + T2D + MIRI groups (P > 0.05, n = 14 mice/group; Figure 7A). LV fractional shortening was smaller in T2D + MIRI groups than in MIRI and TubA + MIRI groups and greater in TubA + T2D + MIRI than in T2D + MIRI groups (P < 0.05, n = 8–11 mice/group; Figure 7B). LV weight and lung weight normalized to body weight were smaller in TubA + T2D + MIRI groups than in db/+ MIRI, TubA + MIRI, and T2D + MIRI groups (Figure 7C) (see Supplementary material online, Figures 8C & 8D). These data suggest that inhibition of HDAC6 improves post-infarct LV systolic function in T2D.
4. Discussion
By dissecting the effects of HDAC6 activity and HDAC6 KO and inhibition on TNF-α, mitochondria morphology, mCI, mitochondrial NAD(H), infarct size, and cardiac function, we found evidence of an essential contribution of HDAC6 in the pathogenesis of MIRI in experimental diabetes. First, by determining HDAC6 activity in T1D and T2D alone and in combination with MIRI, we have found that MIRI and diabetes additively augment myocardial HDAC6 activity and TNF-α levels and decrease mCI activity. Secondly, by using HDAC6 KO mice and a highly selective HDAC6 inhibitor, TubA, we demonstrate that HDAC6 inhibition decreased the production of TNF-α and NAD(H) and augmented myocardial mCI activity in ischaemic/reperfused diabetic mice, along with marked amelioration of cardiac dysfunction. Thirdly, in cultured cardiomyocytes, we demonstrated that HDAC6 KD blocked HG- and TNF-α–elicited suppression of mCI activity. Lastly, although HDAC KO and TubA treatment did not significantly affect mouse survival after MIRI, they decreased LV dilation and improved cardiac systolic function 28 days after MIRI. These findings indicate that HDAC6 activity is closely associated with MIRI in experimental diabetes.
4.1. HDAC6 regulates generation of TNF-α in ischaemic/reperfused diabetic mice
In animal experiments and cultured cardiomyocytes, we have shown that HDAC6 activity and plasma TNF-α levels are augmented by hyperglycaemic stress and MIRI/HRI. Moreover, HDAC6 KO or pharmacological inhibition markedly decreases production of TNF-α. These findings are supported by a previous study showing that augmentation of HDAC6 activity increased the expression of TNF-α gene by the ROS-MAPK-NF-κB/AP-1 pathway-dependent mechanism in macrophages.37 Taken together, HDAC6 critically affects TNF-α production in ischaemic/reperfused diabetic hearts.
Mitochondria do not only co-ordinate cellular metabolism and regulate calcium homeostasis and ROS production; they are also determinants of cell death.38 In response to hyperglycaemic and hypoxic stresses, mitochondria undergo fusion and fission.39 Growing evidence suggests that morphological changes in mitochondria by fusion and/or fission play a critical role in protecting mitochondria from metabolic stresses.40 In healthy individuals, cardiac TNF-α concentrations are low and do not affect mitochondrial function. In the current study, we demonstrate that MIRI in diabetic hearts results in excessive production of TNF-α and mitochondrial fission, whereas KO or pharmacological inhibition of HDAC6 reduces plasma TNF-α levels and mitochondrial fission. Excessive TNF-α induces mitochondrial dysfunction with impaired basal, ATP-linked, and maximal respiration, decreases cellular ATP synthesis, and increases mitochondrial superoxide production.41 Collectively, the augmentation of HDAC6 activity in ischaemic/reperfused diabetic hearts contributes to mitochondrial dysfunction by augmenting production of TNF-α.
HDAC6 KO or inhibition markedly decreased the levels of plasma and myocardial TNF-α, not only in non-diabetic mice undergoing MIRI but also in T1D or T2D subjected to MIRI. Interestingly, in the presence of HG, exogenous TNF-α dose-dependently attenuated the activity of mCI in H9c2 cardiomyocytes. This negative effect of TNF-α was decreased by HDAC6 KD. Previous studies showed that HDAC6 inhibition prevents TNF-α–induced caspase 3 activation and α-tubulin and β-catenin acetylation.42,43 Therefore, the cardioprotective effects of HDAC6 KO or inhibition is associated with reduction of the TNF-α–induced impairments to mCI activity.
4.2. Diabetes and TNF-α impair mCI activity
mCI is composed of 45 subunits including 14 core and 31 supernumerary subunits with lysine residues.44 It transfers electrons from NAD(H) to coenzyme Q10 (ubiquinone) pumping 4H+ into the intermembrane space, thereby contributing to the mitochondrial membrane potential.45 These electrons are sequentially transferred to complex III (coenzyme Q–cytochrome c oxidoreductase), cytochrome c, and complex IV (cytochrome c oxidase), resulting in the reduction of oxygen to water. By monitoring dynamic changes in mitochondrial NAD(H) levels, we demonstrated abnormal increases in mitochondrial NAD(H) levels of diabetic hearts at baseline and during ischaemia. Previous studies have shown that diabetes impairs the mitochondrial supercomplex assembly, which results in lowered activity of mCI.46 We speculate that in ischaemic/reperfused diabetic hearts, high levels of mitochondrial NAD(H) can be attributed to low mCI activity.
Aberrantly high levels of TNF-α impair the mitochondrial supercomplex assembly and activity.13,28 A recent study indicates that TNF-α–activated cellular glutamine uptake leads to an increased concentration of succinate, a Krebs cycle intermediate in a zebrafish model of tuberculosis infection.14 Oxidation of this elevated succinate by complex II drives reverse electron transfer, thereby generating the mitochondrial ROS superoxide anion at mCI.14 Excess TNF-α in mycobacterium-infected macrophages elevates mitochondrial ROS production by reverse electron transfer through mCI.14 In addition, TNF-α as a major pro-inflammatory mediator has many unfavourable effects on the heart, including down-regulation of myocardial contractility, ventricular remodelling, increased rate of apoptosis among endothelial cells and myocytes, and alteration in the expression and function of the enzymes regulating nitric oxide production.47
4.3. HDAC6 is an essential regulator of MIRI in diabetes
Genetic disruption of HDAC6 did not alter body weight, blood glucose levels, systemic blood pressure, myocardial HDAC6 activity at baseline, and cardiac phenotype and function of mice. These results are consistent with a previous study.48 The administration of STZ elicited a continuous increase in fasting blood glucose in both C57BL/6 and HDAC6−/− mice. There were no significant differences in blood glucose levels between C57BL/6 and HDAC6−/− mice. Thus, HDAC6 KO does not affect the deleterious effect of STZ on pancreatic β cells. The present data indicated that either diabetes or MIRI induced an increase in the activity of cardiac HDAC6. These results are consistent with previous studies showing that the activity of cardiac HDAC6 was enhanced in T1D rats or mice.6,49 HDAC6 KO does not markedly affect myocardial area at risk and infarct size in non-diabetic C57BL/6 mice. Intriguingly, T1D caused increased infarct size and impaired cardiac function in C57BL/6 mice but not HDAC6−/− mice. Emerging evidence suggests that HDAC6 is profoundly implicated in the responses of the heart to MIRI, hypertension, angiotensin II, etc.3,48,50 Collectively, HDAC6 is an essential negative regulator of MIRI in T1D.
In the present study, we observed that HDAC6 KO did not alter the systolic and diastolic function of the left ventricle in young naïve mice, which aligns with the findings from a previous study.48 However, HDAC6 KO resulted in an increase in the value of −dP/dt at 30 and 60 min after reperfusion in an isolated Langendorff apparatus. This suggests that HDAC6 KO improves cardiac diastolic function within a short period following post-ischaemic reperfusion. A recent study demonstrated that HDAC6 KO increased myofibril stiffness under increased tension or in aging mice, potentially leading to cardiac diastolic dysfunction.3 It is likely that the impact of HDAC6 KO on cardiac diastolic function varies with different heart pathological states.51–53
The db/db mouse of leptin receptor deficiency is currently the most widely used model for T2D.54 Administration of TubA did not affect myocardial area at risk and infarct size in non-diabetic db/+ mice that had undergone MIRI. Interestingly, TubA significantly blocked T2D-induced increase in infarct size. Taken together, the selective inhibition of HDAC6 with TubA protects the heart from MIRI in T2D. To the best of our knowledge, this is the first study to report that HDAC6 KO or inhibition attenuated MIRI in both T1D and T2D. It is reported that the activity of tissue HDAC6 is markedly elevated in patients with T2D and in db/db mice, concomitant with leptin hyposensitivity.4 A recent study showed that the administration of TubA for 2 weeks restores leptin sensitivity and reduces obesity in db/db mice.4,55 Thus, HDAC6 is a critical component of T2D hearts in response to MIRI.
Importantly, either genetic disruption or pharmacological inhibition of HDAC6 markedly attenuates MIRI in the mice with T1D and T2D. The cardioprotective effects of HDAC6 KO or inhibition are associated with decreased TNF-α levels and preservation of mCI activity. Interestingly, HDAC6 KO directly blocks TNF-α–elicited impairments to mCI in cardiomyocytes. These findings support the notion that HDAC6 regulates TNF-α levels and mCI activity in diabetic mice undergoing MIRI.
One limitation of this study is the usage of global KO mice of HDAC6 to examine the role of HDAC6 in MIRI in T1D mice. Previous studies showed that HDAC6 KO in mice promoted cardiomyocyte autophagy, inhibited NLRP3 inflammasome activation,51 alters gut microbiota and worsens obesity,52 and affects emotional behaviour in mice.53 It remains to be investigated how these HDAC6 KO–elicited changes in mice affect cardiac responses to MIRI in diabetes. HDAC6 KO inhibits NLRP inflammasome activation and promotes cardiomyocyte autophagy.51 Inhibition of NLRP inflammasome activation and enhancement of cardiomyocyte autophagy may be implicated in the cardioprotective effect of HDAC6 KO. Another limitation of this study is the lack of data regarding the effects of HDAC6 KD on HRI in rat primary ventricular cardiomyocytes. Like studies on H9c2 cardiomyocytes, we used siRNA to knock down HDAC6 in the freshly isolated rat ventricular cardiomyocytes to study the effect of HDAC6 KD on HRI-induced changes in TNF-α and mCI under HG and the effects of HDAC6 KD on HG- and exogenous TNF-α–induced inhibition of mCI. However, large amounts of cells were found to die during the process of hypoxia/reoxygenation in the presence of 33 mM D-dextrose or after the addition of 10 ng/mL TNF-α and 33 mM D-dextrose.
In summary, the current study demonstrates that either T1D or T2D and MIRI augment HDAC6 activity and production of TNF-α. Aberrant increases in TNF-α suppress myocardial mCI activity, leading to increases in NAD(H) levels during myocardial ischaemia in experimental diabetes. These negative changes impair cardiac systolic and diastolic function. HDAC6 KO or inhibition not only decreases the production of cardiac TNF-α but also blocks TNF-α–induced impairments to mCI. This study suggests that HDAC6 is an essential negative regulator of MIRI in experimental diabetes.
Translational perspective.
Patients with diabetes are more susceptible to MIRI than non-diabetics with greater mortality and resultant heart failure. There is an unmet medical need in diabetic patients for the treatment of acute myocardial infarction. We show that HDAC6 is an essential negative regulator of MIRI and cardiac function in experimental diabetes. Pharmacologic inhibition of HDAC6 protects the heart and mitochondria against MIRI in diabetes.
Supplementary Material
Contributor Information
Shelley L Baumgardt, Department of Anesthesiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA.
Juan Fang, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA.
Xuebin Fu, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Yanan Liu, Department of Anesthesiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA.
Zhengyuan Xia, Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong Province, The People’s Republic of China.
Ming Zhao, The Feinberg Cardiovascular and Renal Research Institute, Feinberg School of Medicine, Northwestern University, 300 E. Superior Avenue, Chicago, IL 60611, USA.
Ling Chen, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Rachana Mishra, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Muthukumar Gunasekaran, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Progyaparamita Saha, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Joseph M Forbess, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Zeljko J Bosnjak, Department of Medicine, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA; Department of Physiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA.
Amadou K S Camara, Department of Anesthesiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA.
Judy R Kersten, Department of Anesthesiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA.
Edward B Thorp, Department of Pathology, Feinberg School of Medicine, Northwestern University, 300 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 300 E. Superior Avenue, Chicago, IL 60611, USA.
Sunjay Kaushal, Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA.
Zhi-Dong Ge, Department of Anesthesiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53206, USA; Cardiovascular-Thoracic Surgery and the Heart Center, Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Feinberg School of Medicine, Northwestern University, 303 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Surgery, Feinberg School of Medicine, Northwestern University, 225 E. Chicago Avenue, Chicago, IL 60611, USA; Department of Pathology, Feinberg School of Medicine, Northwestern University, 300 E. Superior Avenue, Chicago, IL 60611, USA; Department of Pediatrics, Feinberg School of Medicine, Northwestern University, 300 E. Superior Avenue, Chicago, IL 60611, USA.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
Z.X., M.Z., J.M.F., Z.J.B., A.K.S.C., J.R.K., E.B.T., S.K., and Z.-D.G. conceived and designed the study. S.L.B., J.F., X.F., Y.L., L.C., R.M., M.G., P.S., and Z.-D.G. acquired the data. Z.X., M.Z., J.M.F., Z.J.B., A.K.S.C., J.R.K., E.B.T., S.K., and Z.-D.G. analysed and interpreted the data. Z.-D.G. drafted the manuscript. Z.X., M.Z., J.M.F., Z.J.B., A.K.S.C., J.R.K., E.B.T., and S.K. revised it critically for important intellectual content. All authors made critical revisions of the manuscript and approved the final version.
Funding
This work was supported, in part, by the National Institutes of Health research grants R01 HL063705 (to J.R.K), P01GM066730 (to Z.J.B and J.R.K), R01 HL122309 (to E.B.T), and R01 HL152712 (to M.Z.) from the United States Public Health Services, Bethesda, MD, USA; a National Science Foundation grant 81770831 (to Z.-D.G.) from China, Beijing, the People's Republic of China; and two research grants 931252 and FC930151 (to Z.-D.G.) from the Department of Surgery at Ann & Robert H. Lurie Children’s Hospital of Chicago, USA.
Data availability
The data underlying this article are available in its online supplementary material.
References
- 1. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larsson SC, Wallin A, Hakansson N, Stackelberg O, Back M, Wolk A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int J Cardiol 2018;262:66–70. [DOI] [PubMed] [Google Scholar]
- 3. Lin YH, Major JL, Liebner T, Hourani Z, Travers JG, Wennersten SA, Haefner KR, Cavasin MA, Wilson CE, Jeong MY, Han Y, Gotthardt M, Ferguson SK, Ambardekar AV, Lam MP, Choudhary C, Granzier HL, Woulfe KC, McKinsey TA. HDAC6 modulates myofibril stiffness and diastolic function of the heart. J Clin Invest 2022;132:e148333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cakir I, Hadley CK, Pan PL, Bagchi RA, Ghamari-Langroudi M, Porter DT, Wang Q, Litt MJ, Jana S, Hagen S, Lee P, White A, Lin JD, McKinsey TA, Cone RD. Histone deacetylase 6 inhibition restores leptin sensitivity and reduces obesity. Nat Metab 2022;4:44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalinski AL, Kar AN, Craver J, Tosolini AP, Sleigh JN, Lee SJ, Hawthorne A, Brito-Vargas P, Miller-Randolph S, Passino R, Shi L, Wong VSC, Picci C, Smith DS, Willis DE, Havton LA, Schiavo G, Giger RJ, Langley B, Twiss JL. Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J Cell Biol 2019;218:1871–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leng Y, Wu Y, Lei S, Zhou B, Qiu Z, Wang K, Xia Z. Inhibition of HDAC6 activity alleviates myocardial ischemia/reperfusion injury in diabetic rats: potential role of peroxiredoxin 1 acetylation and redox regulation. Oxid Med Cell Longev 2018;2018:9494052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang J, Grafton F, Ranjbarvaziri S, Budan A, Farshidfar F, Cho M, Xu E, Ho J, Maddah M, Loewke KE, Medina J, Sperandio D, Patel S, Hoey T, Mandegar MA. Phenotypic screening with deep learning identifies HDAC6 inhibitors as cardioprotective in a BAG3 mouse model of dilated cardiomyopathy. Sci Transl Med 2022;14:eabl5654. [DOI] [PubMed] [Google Scholar]
- 8. Baeza J, Smallegan MJ, Denu JM. Mechanisms and dynamics of protein acetylation in mitochondria. Trends Biochem Sci 2016;41:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bridges HR, Fedor JG, Blaza JN, Di Luca A, Jussupow A, Jarman OD, Wright JJ, Agip AA, Gamiz-Hernandez AP, Roessler MM, Kaila VRI, Hirst J. Structure of inhibitor-bound mammalian complex I. Nat Commun 2020;11:5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 2004;94:53–59. [DOI] [PubMed] [Google Scholar]
- 11. Wright JJ, Biner O, Chung I, Burger N, Bridges HR, Hirst J. Reverse electron transfer by respiratory complex I catalyzed in a modular proteoliposome system. J Am Chem Soc 2022;144:6791–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu M, Gu J, Guo R, Huang Y, Yang M. Structure of mammalian respiratory supercomplex I(1)III(2)IV(1). Cell 2016;167:1598–1609.e10. [DOI] [PubMed] [Google Scholar]
- 13. Mariappan N, Elks CM, Fink B, Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic Biol Med 2009;46:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roca FJ, Whitworth LJ, Prag HA, Murphy MP, Ramakrishnan L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science 2022;376:eabh2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ge ZD, Li Y, Qiao S, Bai X, Warltier DC, Kersten JR, Bosnjak ZJ, Liang M. Failure of isoflurane cardiac preconditioning in obese type 2 diabetic mice involves aberrant regulation of microRNA-21, endothelial nitric-oxide synthase, and mitochondrial complex I. Anesthesiology 2018;128:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ge ZD, Boyd RM, Lantz C, Thorp EB, Forbess JM. Cardio-omentopexy requires a cardioprotective innate immune response to promote myocardial angiogenesis in mice. JTCVS Open 2022;10:222–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu HE, Baumgardt SL, Fang J, Paterson M, Liu Y, Du J, Shi Y, Qiao S, Bosnjak ZJ, Warltier DC, Kersten JR, Ge ZD. Cardiomyocyte GTP cyclohydrolase 1 protects the heart against diabetic cardiomyopathy. Sci Rep 2016;6:27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baumgardt SL, Paterson M, Leucker TM, Fang J, Zhang DX, Bosnjak ZJ, Warltier DC, Kersten JR, Ge ZD. Chronic co-administration of sepiapterin and L-citrulline ameliorates diabetic cardiomyopathy and myocardial ischemia/reperfusion injury in obese type 2 diabetic mice. Circ Heart Fail 2016;9:e002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem So 2010;132:10842–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pant T, Dhanasekaran A, Zhao M, Thorp EB, Forbess JM, Bosnjak ZJ, Benjamin IJ, Ge ZD. Identification and analysis of circulating long non-coding RNAs with high significance in diabetic cardiomyopathy. Sci Rep 2021;11:2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Paterson M, Baumgardt SL, Irwin MG, Xia Z, Bosnjak ZJ, Ge ZD. Vascular endothelial growth factor regulation of endothelial nitric oxide synthase phosphorylation is involved in isoflurane cardiac preconditioning. Cardiovasc Res 2019;115:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song R, Yang Y, Lei H, Wang G, Huang Y, Xue W, Wang Y, Yao L, Zhu Y. HDAC6 inhibition protects cardiomyocytes against doxorubicin-induced acute damage by improving alpha-tubulin acetylation. J Mol Cell Cardiol 2018;124:58–69. [DOI] [PubMed] [Google Scholar]
- 23. Jang S, Javadov S. Elucidating the contribution of ETC complexes I and II to the respirasome formation in cardiac mitochondria. Sci Rep 2018;8:17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh SP, McClung JA, Bellner L, Cao J, Waldman M, Schragenheim J, Arad M, Hochhauser E, Falck JR, Weingarten JA, Peterson SJ, Abraham NG. CYP-450 epoxygenase derived epoxyeicosatrienoic acid contribute to reversal of heart failure in obesity-induced diabetic cardiomyopathy via PGC-1α activation. Cardiovasc Pharm Open Access 2018;7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan J, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 2014;28:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 2010;53:1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, Feldman EL. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 2010;53:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prajapati P, Sripada L, Singh K, Bhatelia K, Singh R, Singh R. TNFα regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim Biophys Acta 2015;1852:451–461. [DOI] [PubMed] [Google Scholar]
- 29. Shinde A, Jung H, Lee H, Singh K, Roy M, Gohel D, Kim HB, Mane M, Vasiyani H, Currim F, Seo YR, Yang S, Cho A, Yi EC, Singh R. TNF-α differentially modulates subunit levels of respiratory electron transport complexes of ER/PR +ve/-ve breast cancer cells to regulate mitochondrial complex activity and tumorigenic potential. Cancer Metab 2021;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch SP. Type 2-diabetes is associated with elevated levels of TNF-α, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine 2012;57:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010;121:2012–2022. [DOI] [PubMed] [Google Scholar]
- 32. Martinez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res 2021;128:1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berthiaume JM, Kurdys JG, Muntean DM, Rosca MG. Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid Redox Signal 2019;30:375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003;107:1418–1423. [DOI] [PubMed] [Google Scholar]
- 36. Murcia AM, Hennekens CH, Lamas GA, Jimenez-Navarro M, Rouleau JL, Flaker GC, Goldman S, Skali H, Braunwald E, Pfeffer MA. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med 2004;164:2273–2279. [DOI] [PubMed] [Google Scholar]
- 37. Youn GS, Lee KW, Choi SY, Park J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radic Biol Med 2016;97:14–23. [DOI] [PubMed] [Google Scholar]
- 38. Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, 8stress, and death. Circ Res 2012;111:1198–1207. [DOI] [PubMed] [Google Scholar]
- 39. Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 2006;103:2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012;337:1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lkhagva B, Kao YH, Lee TI, Lee TW, Cheng WL, Chen YJ. Activation of class I histone deacetylases contributes to mitochondrial dysfunction in cardiomyocytes with altered complex activities. Epigenetics 2018;13:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu J, Ma M, Ma Z, Fu J. HDAC6 inhibition prevents TNF-α-induced caspase 3 activation in lung endothelial cell and maintains cell-cell junctions. Oncotarget 2016;7:54714–54722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu J, Ma Z, Shetty S, Ma M, Fu J. Selective HDAC6 inhibition prevents TNF-α-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. Am J Physiol Lung Cell Mol Physiol 2016;311:L39–L47. [DOI] [PubMed] [Google Scholar]
- 44. Zhu J, Vinothkumar KR, Hirst J. Structure of mammalian respiratory complex I. Nature 2016;536:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther 2020;5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaludercic N, Di Lisa F. Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front Cardiovasc Med 2020;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferrari R. The role of TNF in cardiovascular disease. Pharmacol Res 1999;40:97–105. [DOI] [PubMed] [Google Scholar]
- 48. Demos-Davies KM, Ferguson BS, Cavasin MA, Mahaffey JH, Williams SM, Spiltoir JI, Schuetze KB, Horn TR, Chen B, Ferrara C, Scellini B, Piroddi N, Tesi C, Poggesi C, Jeong MY, McKinsey TA. HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling. Am J Physiol Heart Circ Physiol 2014;307:H252–H258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Y, Du J, Zhao YT, Zhang L, Lv G, Zhuang S, Qin G, Zhao TC. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc Diabetol 2015;14:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lemon DD, Horn TR, Cavasin MA, Jeong MY, Haubold KW, Long CS, Irwin DC, McCune SA, Chung E, Leinwand LA, McKinsey TA. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol 2011;51:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pang X, Guan Q, Lin X, Chang N. Knockdown of HDAC6 alleviates ventricular remodeling in experimental dilated cardiomyopathy via inhibition of NLRP3 inflammasome activation and promotion of cardiomyocyte autophagy. Cell Biol Toxicol 2022;39:2365–2379. [DOI] [PubMed] [Google Scholar]
- 52. Lieber AD, Beier UH, Xiao H, Wilkins BJ, Jiao J, Li XS, Schugar RC, Strauch CM, Wang Z, Brown JM, Hazen SL, Bokulich NA, Ruggles KV, Akimova T, Hancock WW, Blaser MJ. Loss of HDAC6 alters gut microbiota and worsens obesity. FASEB J 2019;33:1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fukada M, Hanai A, Nakayama A, Suzuki T, Miyata N, Rodriguiz RM, Wetsel WC, Yao TP, Kawaguchi Y. Loss of deacetylation activity of Hdac6 affects emotional behavior in mice. PLoS One 2012;7:e30924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 2014;10:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liang T, Qi C, Lai Y, Xie J, Wang H, Zhang L, Lin T, Jv M, Li J, Wang Y, Zhang Y, Chen Z, Qiu X, Li R, Li Z, Ye Z, Liu S, Liang X, Shi W, Wang W. HDAC6-mediated alpha-tubulin deacetylation suppresses autophagy and enhances motility of podocytes in diabetic nephropathy. J Cell Mol Med 2020;24:11558–11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in its online supplementary material.