Abstract
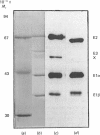
Pyruvate dehydrogenase (PDH) deficiency has been described in many patients with primary lactic acidosis. However, there are very few cases in which a structural defect in the complex has been clearly demonstrated. Measurement of the activity of the PDH complex in cultured human cells has proved unreliable, and a combination of structural and functional studies are required to make a definitive diagnosis. For this reason, an immunochemical strategy has been developed to complement direct enzyme assay in the detection and further characterization of PDH deficiency. We illustrate the usefulness of this approach by describing defects in the alpha-subunit of the pyruvate decarboxylase component of the PDH complex in two patients with primary lactic acidosis. In one patient, there is no immunologically cross-reacting material corresponding to this subunit. In the second patient, there appears to be an intrinsic structural defect in the subunit which restricts dephosphorylation (and hence activation) of the inactive phosphorylated complex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Schulman J. D., Young D. S., Hom E. An inherited defect affecting the tricarboxylic acid cycle in a patient with congenital lactic acidosis. J Clin Invest. 1972 Jul;51(7):1845–1851. doi: 10.1172/JCI106986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Jennings I. G., Cotton R. G. Comparative studies of four monoclonal antibodies to phenylalanine hydroxylase exhibiting different properties with respect to substrate-dependence, species-specificity and a range of effects on enzyme activity. Biochem J. 1981 Dec 1;199(3):527–535. doi: 10.1042/bj1990527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcucci O. L., Hunter A., Lindsay J. G. Low immunogenicity of the common lipoamide dehydrogenase subunit (E3) of mammalian pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase multienzyme complexes. Biochem J. 1985 Mar 1;226(2):509–517. doi: 10.1042/bj2260509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. M. A simple incubation flask for 14CO2 collection. Anal Biochem. 1971 Jun;41(2):578–580. doi: 10.1016/0003-2697(71)90180-1. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Wagner J. D. Glucagon and the Ca2+-linked hormones angiotensin II, norepinephrine, and vasopressin stimulate the phosphorylation of distinct substrates in intact hepatocytes. J Biol Chem. 1982 Nov 10;257(21):13135–13143. [PubMed] [Google Scholar]
- Hyland K., Leonard J. V. Revised assays for the investigation of congenital lactic acidosis using 14C keto acids, eliminating problems associated with spontaneous decarboxylation. Clin Chim Acta. 1983 Sep 30;133(2):177–187. doi: 10.1016/0009-8981(83)90403-5. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuda S., Shirahama T., Saheki T., Miura S., Mori M. Purification and immunochemical studies of pyruvate dehydrogenase complex from rat heart, and cell-free synthesis of lipoamide dehydrogenase, a component of the complex. Biochim Biophys Acta. 1983 Oct 13;741(1):86–93. doi: 10.1016/0167-4781(83)90013-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Reed L. J. Pyruvate dehydrogenase complex from bovine kidney and heart. Methods Enzymol. 1982;89(Pt 500):376–386. doi: 10.1016/s0076-6879(82)89067-8. [DOI] [PubMed] [Google Scholar]
- Robinson B. H. Inborn errors of pyruvate metabolism. Biochem Soc Trans. 1983 Dec;11(6):623–626. doi: 10.1042/bst0110623. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Sherwood W. G. The genetic heterogeneity of lactic acidosis: occurrence of recognizable inborn errors of metabolism in pediatric population with lactic acidosis. Pediatr Res. 1980 Aug;14(8):956–962. doi: 10.1203/00006450-198008000-00013. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. INSTABILITY OF PYRUVATE-C-14 IN AQUEOUS SOLUTION AS DETECTED BY ENZYMIC ASSAY. Anal Biochem. 1964 Aug;8:470–476. doi: 10.1016/0003-2697(64)90244-1. [DOI] [PubMed] [Google Scholar]
- Schofield P. J., Griffiths L. R., Rogers S. H., Wise G. An improved method for the assay of platelet pyruvate dehydrogenase. Clin Chim Acta. 1980 Dec 8;108(2):219–227. doi: 10.1016/0009-8981(80)90008-x. [DOI] [PubMed] [Google Scholar]
- Sheu K. F., Hu C. W., Utter M. F. Pyruvate dehydrogenase complex activity in normal and deficient fibroblasts. J Clin Invest. 1981 May;67(5):1463–1471. doi: 10.1172/JCI110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu K. F., Kim Y. T. Studies on the bovine brain pyruvate dehydrogenase complex using the antibodies against kidney enzyme complex. J Neurochem. 1984 Nov;43(5):1352–1358. doi: 10.1111/j.1471-4159.1984.tb05394.x. [DOI] [PubMed] [Google Scholar]
- Stanley C. J., Perham R. N. Purification of 2-oxo acid dehydrogenase multienzyme complexes from ox heart by a new method. Biochem J. 1980 Oct 1;191(1):147–154. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp L. R., Pettit F. H., Yeaman S. J., Reed L. J. Purification and properties of pyruvate dehydrogenase kinase from bovine kidney. J Biol Chem. 1983 Aug 10;258(15):9454–9458. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems J. L., de Kort A. F., Vree T. B., Trijbels J. M., Veerkamp J. H., Monnens L. A. Non-enzymic conversion of pyruvate in aqueous solution to 2,4-dihydroxy-2-methylglutaric acid. FEBS Lett. 1978 Feb 1;86(1):42–44. doi: 10.1016/0014-5793(78)80094-5. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]