Graphical Abstract
Graphical Abstract.
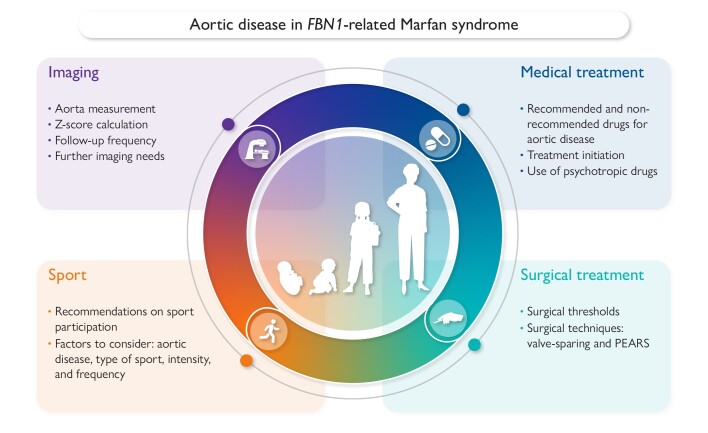
Overview of the four main topics addressed in this joint statement and the most important questions addressed within each topic. This document focuses on follow-up and treatment of aortic disease in children with Marfan syndrome and carrying a pathogenic variant in the fibrillin-1 (FBN1) gene. PEARS, personalized external aortic root support.
Keywords: Marfan syndrome, Aortic disease treatment, Aortic imaging, Exercise recommendation, Children
Abstract
Marfan syndrome (MFS) is a hereditary connective tissue disorder with an estimated prevalence of 1:5000–1:10 000 individuals. It is a pleiotropic disease characterized by specific ocular, cardiovascular, and skeletal features. The most common cardiovascular complication is aortic root dilatation which untreated can lead to life-threatening aortic root dissection, mainly occurring in adult patients. Prompt diagnosis, appropriate follow-up, and timely treatment can prevent aortic events. Currently there are no specific recommendations for treatment of children with MFS, and management is greatly based on adult guidelines. Furthermore, due to the scarcity of studies including children, there is a lack of uniform treatment across different centres. This consensus document aims at bridging these gaps of knowledge. This work is a joint collaboration between the paediatric subgroup of the European Network of Vascular Diseases (VASCERN, Heritable Thoracic Aortic Disease Working Group) and the Association for European Paediatric and Congenital Cardiology (AEPC). A group of experts from 12 different centres and 8 different countries participated in this effort. This document reviews four main subjects, namely, (i) imaging of the aorta at diagnosis and follow-up, (ii) recommendations on medical treatment, (iii) recommendations on surgical treatment, and (iv) recommendations on sport participation.
Preamble
This joint statement document by the paediatric subgroup of the European Reference Network of Vascular Diseases (VASCERN) and the Association for European Paediatric and Congenital Cardiology (AEPC) represents their first collaborative effort. It reviews current knowledge on the follow-up and treatment of children with Marfan syndrome (MFS), providing recommendations based on clinical evidence or on expert opinion. The level of final agreement for each statement has been added to the corresponding table.
Expert panel organization
The lack of standardized care for children with MFS across Europe prompted the development of this document. The expert panel consisted of 14 experts from 12 centres and 8 countries. Members of VASCERN's Heritable Thoracic Aortic Disease (HTAD) Working Group or the AEPC's Genetics, Basic Science and Myocardial Disease Working Group participated.
Methodology
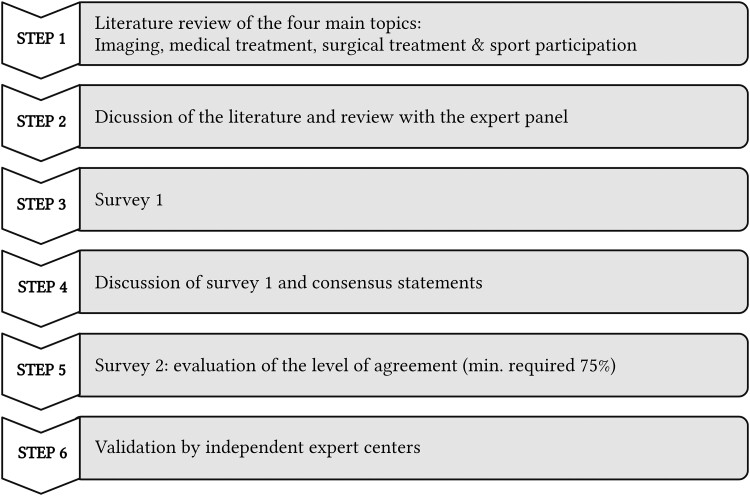
Monthly online discussions, held from November 2021 to May 2023, reviewed the current published literature focused on children with MFS and single-centre practices on four main topics: (i) imaging of the aorta, (ii) medical treatment, (iii) surgical treatment, and (iv) sport participation. Literature search was mainly performed in PubMed and Web of Science. After the online calls, surveys gauged agreement on statements, with rounds repeated until achieving ≥75% agreement. To validate the formulated statements, the consensus document was reviewed by several independent experts who are listed in the Acknowledgements section. The sequential process is shown in Figure 1.
Figure 1.
Stepwise process of the creation of the consensus document. The four main topics treated were (i) imaging of the aorta, (ii) medical treatment, (iii) surgical treatment, and (iv) sport participation. Literature search was mainly performed in PubMed and Web of Science. The online discussions were followed by dedicated surveys to evaluate the level of agreement to each statement. If a statement had <75% agreement, a new round of online questions was performed based on the former feedback of all group members. This process was repeated until statements were formulated with a minimum of 75% agreement. The document was validated in a final step by several independent expert centres as indicated in Acknowledgements
Scope
This document aims to assist paediatric cardiologists in the follow-up and treatment of children with MFS related to pathogenic variants in fibrillin-1 (FBN1), focusing on aortic disease. It excludes mitral valve disease and other cardiovascular problems like cardiomyopathy, as well as other non-FBN1–related HTAD.
Introduction: Marfan syndrome
Marfan syndrome is a connective tissue disorder caused by pathogenic variants in the FBN1 gene encoding the extracellular matrix (ECM) protein fibrillin-1.1 Fibrillin-1 is ubiquitous throughout the organism, thereby accounting for the diverse spectrum of manifestations associated with this condition. The diagnosis is usually clinical and based on the revised Ghent nosology.2 Clinical diagnosis, however, might be challenging because systemic features can be subtle or absent in very young children.3 Some children might also be diagnosed through predictive genetic testing, following the identification of an affected family member.
Clinical spectrum
Marfan syndrome is a highly penetrant condition that demonstrates substantial intra- and interfamilial variability. Part of this variability might be explained by the underlying pathogenic variant.
Early-onset Marfan syndrome
Early-onset MFS (eoMFS) is a severe form of the disease.4 The definition remains however unclear and not recognized internationally. Individuals with eoMFS usually present specific marfanoid characteristics at the time of birth including camptodactyly, arachnodactyly, joint contractures, muscle hypoplasia, and loose skin.4–6 They frequently develop significant cardiorespiratory compromise, including congenital emphysema and atrioventricular valve regurgitation leading to early mortality. These individuals often have de novo pathogenic variants (missense and in-frame deletions) in FBN1 clustered within exons 24–32.7 This document excludes patients with eoMFS, where cardiac failure is the most important cardiovascular feature and determinant for the outcome.
Classic Marfan syndrome
Cardinal manifestations in classic MFS involve the ocular, skeletal, and cardiovascular systems.8 Cardiovascular features in children include dilatation of the aorta at the level of the sinuses of Valsalva, mitral valve prolapse with or without regurgitation, tricuspid valve prolapse, and proximal pulmonary artery enlargement. Aortic root dissection is unusual in children but might occur in adolescents. Severe and prolonged left-sided valve regurgitation can predispose to left ventricular dysfunction and occasionally heart failure.9 Aortic dilatation at distal sites, primary cardiomyopathy, and ventricular arrhythmia leading to sudden cardiac are less common, yet noteworthy cardiovascular complications, reported mainly in adults.10–12
Skeletal manifestations include bone overgrowth and joint laxity; disproportionately long extremities for the size of the trunk (dolichostenomelia); overgrowth of the ribs that can push the sternum in (pectus excavatum) or out (pectus carinatum); and scoliosis that ranges from mild to severe. Ocular findings include myopia (>50% of affected individuals); ectopia lentis (seen in ∼60% of affected individuals); and an increased risk for retinal detachment, glaucoma, and early cataracts.8
Genetics of Marfan syndrome
In the early 1990s, pathogenic variants in FBN1 were identified as the cause of MFS.1,13 Pathogenic variants predisposing to MFS are distributed throughout the gene and are mostly private.8 Missense variants typically disrupting the repetitive calcium-binding epidermal growth factor (cb-EGF)–like domains are the most common type of disease-causing variants.14 Approximately 10% of the disease-causing variants disrupt canonical splice donor or acceptor sites and cause splicing errors, which can lead to in-frame deletion of an entire cb-EGF–like domain. Variants leading to splicing errors can also cause a frameshift in translation and, along with small insertions, deletions, and stop codons, lead to haploinsufficiency. Fibrillin-1 haploinsufficiency is the cause of MFS in 30%–40% of cases.15,16 Up to 7% of MFS pathogenic variants are large or complete deletions of FBN1.15
About 25% of FBN1 pathogenic variants are de novo17 with gonad mosaicism, in which unaffected parents harbour the pathogenic variant in some of their germline cells and somatic mosaicism also being observed.18,19
The most consistent and robust genotype–phenotype association exists with de novo missense pathogenic variants in exons 24–32 in eoMFS.7 Additional correlations include pathogenic variants disrupting cysteine in patients with ectopia lentis7,20 and haploinsufficiency variants associated with more severe skeletal features.16,20 Cardiovascular genotype–phenotype correlations are more debated: pathogenic variants leading to haploinsufficiency and variants disrupting cysteine residues seem to be associated with a more severe phenotype.16 Conversely, missense variants introducing a cysteine residue tend to associate with milder cardiovascular features.16
Diagnosis in paediatric patients
Early diagnosis is crucial for implementing appropriate surveillance and intervention strategies to optimize outcomes in affected individuals. In individuals with a family history of MFS and a known pathogenic variant, genetic testing can be offered. Asymptomatic children diagnosed through cascade screening should be offered comprehensive evaluation every 1–2 years.
When clinical suspicion is high but family history is negative, a comprehensive evaluation including cardiovascular, skeletal, and ocular assessments should be performed to evaluate the clinical features included on the revised Ghent nosology.2 Patients (almost) fulfilling these criteria can be offered genetic testing. Testing may be deferred in very young children if only some skeletal features are present; instead, a follow-up evaluation should be offered.
Aortic disease in Marfan syndrome
The aortic root is the initial segment of the aorta and comprises the aortic valve annulus, the sinuses of Valsalva, and the sinotubular junction (ST-junction). Aortic root dilatation is the hallmark of MFS, present in 75%–80% of affected children.3 The predilection of the aortic root to dilate is determined by a combination of haemodynamic and structural factors. On one hand, the proximal part of the aorta bears the cyclic pressure load from the left ventricular ejection, rendering it more susceptible to dilatation. Conversely, the elastin content at the level of the sinus is higher than in other parts of the arterial tree,21 and therefore, diseases like MFS affecting elastogenesis confer a higher risk of dilatation at this site.
Aortic root dilatation can be typically diagnosed by echocardiography, with computed tomography angiography (CTA) and magnetic resonance imaging (MRI) providing more accurate measurements. In adult patients, prophylactic aortic root replacement is usually indicated when the diameter reaches 50 mm with earlier surgical intervention considered at 45 mm for rapid aortic growth, progressive aortic valve regurgitation, family history of dissection, or pregnancy desire.22,23
If the dilatation advances and surgery is not performed, dissection or rupture may occur. Typically, MFS patients will have a dissection at the level of the proximal aorta (type A dissection), but dissection at distal sites (type B dissection) has also been described in adult cohorts.10,11 While increased aortic diameter correlates with increased risk of dissection,24 some patients experience dissection below surgical thresholds.24 Some factors like dilatation of the ST-junction, rapid aortic growth, or male sex25–27 have been associated with a higher incidence of aortic root replacement but not necessarily with a higher rate of dissection. Aortic stiffness and aortic and vertebral tortuosity have been associated with worse cardiovascular prognosis.28–30
Imaging of the aorta
Accurate and reproducible aortic measurement is crucial for the diagnosis, surveillance, and management. Additionally, surgical planning relies on quantification of absolute diameters. There are no established recommendations on imaging and surveillance in children with MFS except for Canadian guidelines which attempt a time frame for baseline assessment and 6-monthly monitoring with 2D transthoracic echocardiography (2D-TTE), without actually prescribing clear intervals for CTA/MRI.31 It has been common practice to adapt adult guidelines in the absence of a more tailored and children centred approach.22
Echocardiography: technical aspects and calculation of the z-score
2D-TTE is the imaging modality of choice for measuring proximal aortic segments and is commonly used for initial assessment and surveillance of aortic dilatation.
How to measure the aorta
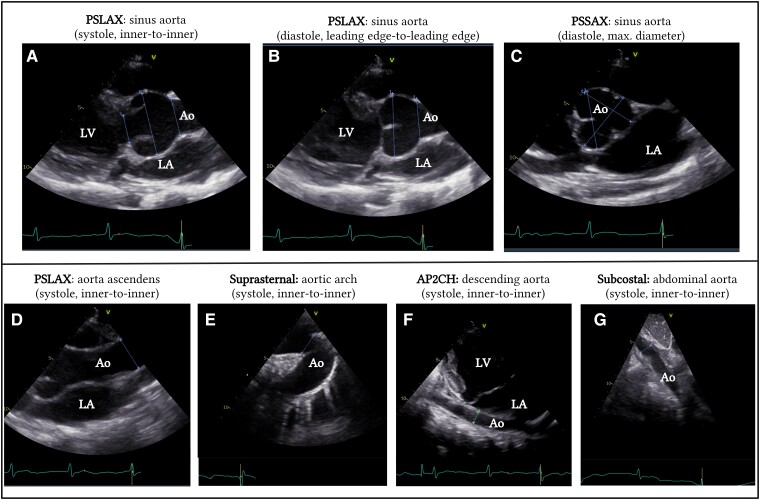
There is no universally accepted way of measuring aortic root diameter. According to the American Society of Echocardiography (ASE) guidelines for paediatric patients, measurements should be taken perpendicular to the long axis of the aorta at specific anatomic landmarks.32 A 2D image of the aorta should be recorded in the parasternal long-axis (PSLAX) view. Aortic diameters at the annulus, the sinuses of Valsalva, ST-junction, and ascending aorta at the level of the right pulmonary artery should be measured (Figure 2A, B, and D). All measurements should be obtained in systole, from inner edge to inner edge, at the broadest diameter. The highest values are used for z-score calculation. Both raw averaged values of aortic diameters and the corresponding z-scores should be reported.
Figure 2.
Echocardiographic imaging of the aorta at different levels. A Parasternal long-axis view of the aortic annulus, sinus of Valsalva, and sinotubular junction measured in systole using the inner-to-inner method. Measurements should be taken perpendicular to blood flow. B Parasternal long-axis view of the sinus of Valsalva and sinotubular junction measured in diastole using the leading edge-to-leading edge method. C Parasternal short-axis view of the aortic valve measured in diastole and using the largest aortic diameter. D Parasternal long-axis of the ascending aorta, measured in systole at the level of the right pulmonary artery, using the inner-to-inner method. E Suprasternal view of the aortic arch measured in systole between the truncus brachiocephalicus and the left carotid artery, using the inner-to-inner diameter. F Modified apical two-chamber view of the descending aorta measured at the level of the left atrium in systole using the inner-to-inner method. G Subcostal view of the abdominal aorta at the level of the liver, measured in systole using the inner-to-inner diameter. Ao, aorta; AP2CH, apical two-chamber view; LA, left atrium; LV, left ventricle; PSLAX, parasternal long-axis view; PSSAX, parastenal short-axis view
In contrast to the paediatric guidelines, the ASE and the European Society of Cardiology (ESC) guidelines for adult patients recommend the use of the leading edge to leading edge method in diastole.33,34 While this working group advocates for using the inner-to-inner diameter in systole, recognized as the most validated method in paediatric patients, some expert centres prefer adult recommendations. This approach ensues consistent follow-up across the lifespan and aligns better with CTA and MRI measurements.35 Studies in children and adults with MFS, as well as healthy controls, demonstrated that standard systolic and diastolic echocardiographic measurements of the aortic root are comparable and show good interobserver agreement.36–39 Maintaining consistency in the chosen method is the most important aspect, regardless of the specific approach.
By using the PSLAX view, the maximum aortic root diameter is determined by measuring the distance between the right and non-coronary cusps. However, due to complex, non-cylindrical geometry of the aortic root, this might not be the largest aortic root diameter. Measurements from the parasternal short axis may help identify significant aortic root asymmetry; however, cross-sectional imaging with CMR or CTA is more reliable (Figure 2C).34
In contrast to adults, visualization of the distal parts of the aorta in children using 2D-TTE is usually feasible. The suprasternal view allows visualization of the aortic arch and proximal descending aorta, while the modified apical two-chamber view and the subcostal view provide views of the descending and abdominal aorta, respectively (Figure 2D–G).34
Applicability of the different z-scores
Z-scores based on body surface area (BSA) play a crucial role in interpreting aortic diameters. Despite numerous published nomograms for aortic measurements, there is no consensus on the preferred one. Nomograms lead to different z-scores due to variations in measuring methods, BSA normalization technique, and diversity of the study population.40 The Haycock formula for BSA is often favoured over the Du Bois and Du Bois formula for its accuracy in younger children.41 An overview of the different nomograms is presented in Table 1.42–49 Among the available options, the Lopez et al. method42 stands out for its inclusion of a large and diverse population (N = 3215), utilizing the inner-to-inner edge method in systole along with the Haycock formula to calculate BSA. However, it is worth noting that this method may yield higher z-score calculation compared with others.40 This needs to be taken into consideration, particularly in diagnostic settings.
Table 1.
Overview of the different methods for calculating z-scores in children
| Reference | TTE method | Population | Year |
|---|---|---|---|
| Roman et al.44 | M-mode and 2D-TTE Leading to leading edge, diastole |
N = 187 total N children: 52 (30 days–15 years) Healthy individuals |
1989 |
| Colan et al.45 (Sluysmans et al.) |
2D-TTE Inner to inner edge, systole BSA method: Haycock |
N = 496 (1 d–20 yr) Healthy individuals |
2005 |
| Warren et al.46 (Halifax method) |
2D-TTE Inner to inner edge, systole BSA method: Boyd |
N = 88 (1 d–18 yr) Only children with BAV |
2006 |
| Pettersen et al.47 (Detroit method) |
M-mode and 2D-2D-TTE Inner to inner edge, systole |
N = 782 Healthy children |
2008 |
| Gautier et al.48 | 2D-TTE Leading to leading edge, diastole BSA method: Du Bois and Du Bois |
N = 353 (2–18 yr) Healthy French children |
2010 |
| Campens et al.43 | 2D-TTE Leading to leading edge, diastole BSA method: Du Bois and Du Bois |
N = 849 total N children: 80 (1–15 yr) |
2014 |
| Lopez et al.42 (Boston method) |
2D-TTE Inner to inner edge, systole BSA method: Haycock |
N = 3215 (1 d–18 yr) Healthy North American children |
2017 |
| Cantinotti et al.49 | 2D-TTE Inner to inner edge, systole BSA method: Haycock |
N = 1151 (1 d–18 yr) Healthy Italian children |
2017 |
2D-TTE, 2D transthoracic echocardiography; BAV, bicuspid aortic valve; BSA, body surface area; d, days; yr, years.
For measurements taken in diastole, the Campens nomograms are preferred43 given its validation across both paediatric and adult populations.
Recommendations for echocardiographic examination (Table 2): specific supporting text
Table 2.
Summary of the different recommendations and level of agreement
| LoA (%) | |
|---|---|
| Imaging | |
| 2D-TTE is the imaging technique of choice for diagnosis and surveillance | 100 |
| The aorta should be measured at the seven different recommended levels | 100 |
| The inner-to-inner edge method in systole is the preferred method to measure the aorta in 2D-TTE | 92 |
| The calculation of the z-scores for 2D-TTE measurements according to Lopez at al. may be considered as a method of choice | 92 |
| Alternatively, the leading edge-to-leading edge in diastole method can be considered. In this case, calculating z-scores according to the Campens nomograms is recommended | 100 |
| 2D-TTE should be performed in each child with MFS at diagnosis; yearly examination thereafter is recommended | 92 |
| In patients with large aortic diameters (≥45 mm) or rapid aortic growth (>5 mm/yr with increase in z-score of >1 SD) biannual surveillance is recommended | 92 |
| A biannual 2D-TTE can be considered during titration of medication or if clinically indicated (e.g. valvular disease) | 92 |
| MRI should be preferred over CTA for surveillance in children to limit radiation exposure | 83 |
| The calculation of the z-score for MRI measurements according to Kaizer et al. is recommended | 92 |
| CTA should be considered as first choice if MRI is not tolerated or would not allow acquisition of diagnostic images, in specific instances for surgical planning (i.e. PEARS), and in the suspicion of an acute aortic event | 100 |
| Cross-sectional imaging (MRI/CTA) can be offered to all children with MFS during adolescence, when no sedation is required, to gather baseline aortic measurements | 83 |
| In children with (almost) normal aortic diameter, appropriate visualization, and no asymmetry of the aortic valve, cross-sectional imaging (MRI/CT) can be deferred to an adult age | 100 |
| Cross-sectional imaging (MRI/CTA) can be considered at regular intervals according to clinical progression | 83 |
| Cross-sectional imaging (MRI/CTA) can be considered at an earlier age or interval (even if sedation or general anaesthesia is needed) in circumstances where 2D-TTE does not provide accurate data, if approaching surgical threshold, or in presence of accelerated aortic growth (>5 mm/year with increase of z-score of >1 SD) | 83 |
| Cross-sectional imaging (MRI/CTA) should be considered at an earlier age or interval if indicated to assess or monitor other lesions (i.e. valvular and/or cardiac function and shunts) | 83 |
| Neck-to-pelvis MRI may be considered in all patients at first MRI examination | 83 |
| MRI of cerebral arteries is not required in children with MFS | 100 |
| Medical treatment | |
| In children with MFS and aortic dilatation, medical treatment with a BB or ARB at maximally tolerated doses is recommended | 83 |
| Treatment can be considered in children with MFS and no aortic dilatation especially in the presence of additional risk factors | 100 |
| Combined therapy with BBs and ARBs should be considered if fast progression is observed during surveillance (5 mm/yr) or if aortic diameter > 40 mm or z-score > 5. | 83 |
| ACE-I can be considered if BBs and ARBs contraindicated | 92 |
| Calcium channel blockers should be avoided as they might increase risk of acute aortic events | 100 |
| Surgical treatment | |
| Surgery is recommended in children when the aortic root diameter reaches 50 mm. | 100 |
| Surgery can be considered at 45 mm when some additional risk factors are associated such as family history of aortic dissection, rapid annual growth rate > 5 mm/yr, and when concomitant valve surgery is indicated | 83 |
| Aortic valve–sparing surgery is preferred to the aortic valve replacement technique | 92 |
| Sport participation | |
| The following aspect should be taken into consideration when recommending sport participation: type of sport, the frequency and the intensity of the sport, and the severity of the aortic disease | 100 |
| Sports at competitive level are not recommended except for skills, low-intensity sports | 83 |
| In children ≤10 yrs, any recreational sport can be considered without restriction, but the possibility of restricting a sport with age should be discussed | 100 |
| In children >10 yrs, power sports are not recommended | 92 |
| In children >10 yrs and an aortic root z-score < 3: skill, mixed, or endurance sports can be considered at a recreative level, at any intensity | 92 |
| In children >10 yrs and an aortic root z-score ≥ 3 or ≥ 40 mm: skill, mixed, or endurance sports can be considered at a moderate level (defined as being able to hold a conversation with a friend during exercise) | 92 |
2D-TTE, 2D transthoracic echocardiography; ACE-I, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BBs, beta-blockers; CTA, computed tomography angiography; LoA, level of agreement; MFS, Marfan syndrome; MRI, magnetic resonance imaging; PEARS, personalized external aortic root support.
The aorta should be measured at seven different levels (annulus, sinus, ST-junction, ascending, arch, and descending and abdominal aorta). The preferred method is using the inner-to-inner edge in systole, according to the ASE recommendation for paediatric patients.
The Lopez et al. method for calculating z-scores is recommended.
When measuring the aortic sinus and ascending aorta using the leading edge to leading edge in diastole, the Campens nomograms are recommended for z-score calculation.
Given the variability of the z-scores depending on the method used, it is recommended to consistently use the same method for follow-up comparison.
Yearly 2D-TTE surveillance is recommended in children with MFS. Children with large aortas, showing accelerated aortic growth (>5 mm/year), having additional cardiac involvement or on the starting phase of medical treatment, might benefit from a more frequent assessment.
Magnetic resonance imaging
Magnetic resonance imaging: technical and practical aspects
While 2D-TTE serves as the standard modality for aortic measurement, cross-sectional imaging through MRI offers superior accuracy, especially in cases of chest wall deformity or with asymmetric aortic roots.50 Magnetic resonance imaging is preferred over CTA, because it does not use ionizing radiation and can often be performed without intravenous contrast. This is particularly important for lifelong surveillance in children to prevent cumulative radiation exposure. However, MRI spatial resolution is inferior to CTA and acquisition time much longer; for these reasons, CTA remains the gold standard in situations requiring rapid assessment of the aortic wall integrity. Metallic implants (even when MRI compatible) can significantly degrade the image quality. Single-institution MRI protocols may vary, and paediatric recommendations are lacking.
For the isolated aortic root assessment, non-contrast MRI sequences are recommended. Electrocardiogram (ECG)-gated imaging decreases motion artefacts, while free-breathing black-blood sequences, 3D steady-state free precession (SSFP), and cine imaging usually allow to obtain a 3D data set for precise and repeatable measurements.51,52
Although less common in MFS than in other connective tissue diseases, adolescents and young adults may present vascular problems distal to the ascending aorta.53,54 Tortuosity of the neck vessels is well recognized, and an established predictor of outcome in children with MFS,34 and therefore a neck-to-pelvis angiography assessment, may be warranted. This usually requires non-contrast sequences like time-of-flight for neck vessels and axial and coronal true fast imaging with steady-state precession (TruFISP) together with contrast-enhanced (single bolus) magnetic resonance angiography (MRA).
When assessing aortic measurements on MRI and CTA, nomogram issues persist, with adult recommendations applied to older children and adolescents.22 Kaiser and colleagues55 published a data set to calculate z-scores for thoracic aorta diameters derived from MRI in children. Despite limitations such as reliance on contrast-enhanced angiography and lack of ECG gating imaging, these are the only nomograms offering a method for z-score calculation in paediatric patients.
Recommendations for magnetic resonance imaging (Table 2): specific supporting text
Cross-sectional imaging using MRI is preferred over CT to limit the cumulative radiation exposure. Computed tomography angiography remains the gold standard for surgical planning, in the acute setting, and when MRI is not tolerated or would yield inconclusive results.
Magnetic resonance imaging screening can be considered when children can tolerate an awake scan, usually around age 10. In children without clear aortic root dilatation and symmetric aortic valve on 2D-TTE, deferring MRI scan until adulthood can be justified.
Regular surveillance with cross-sectional scans can be considered every 5 years especially in patients with large diameters and/or asymmetric aortic valves. Magnetic resonance imaging surveillance should align with clinical needs.
Early or more frequent screening is recommended when 2D-TTE fails to provide adequate images for safe surveillance (poor acoustic window, chest wall deformity, asymmetric aortic root, etc.), when approaching surgical threshold, or in the presence of accelerated aortic growth (>5 mm/year with a significant increase in z-score of at least 1 SD).
Neck-to-pelvis MRI can be considered at baseline to assess tortuosity of neck vessels, a well-recognized and an established predictor of outcome.
Magnetic resonance angiography of cerebral vessels is not required in children with MFS due to the likely low prevalence of cerebral aneurysm and lack of established management protocols.56
Cardiac computed tomography angiography
Computed tomography angiography technique
Computed tomography angiography can be useful in imaging the aorta in patients with MFS, especially in those patients in whom cross-sectional imaging is indicated and who are unable to tolerate an MRI scan without prolonged sedation or general anaesthesia.22 Using third-generation CT scanners with dual sources of radiation/ultrawide detectors with a high temporal resolution (66–75 ms), scans can be obtained in paediatric patients within 1–3 s, thereby enhancing successful acquisition rates.57 While oral sedation may be required for children under 5 years old to facilitate a free-breathing scan, those over 6 years often tolerate awake scans well. To minimize radiation exposure, scans should be acquired using prospective ECG gating, employing techniques like the step-and-shoot, high-pitch spiral acquisition with prospective ECG triggering on dual-source scanners, or target ECG-gated method on scanners with ultrawide detectors.58 The scan should be acquired in end-systole, using a relatively narrow window of acquisition to minimize radiation exposure. Using these low-radiation dose techniques, the field of view can be increased to cover the entire length of the thoracic aorta if required.
Computed tomography angiography serves as a valuable tool for procedure planning in paediatric patients nearing the surgical threshold and is particularly relevant for personalized external aortic root support (PEARS) planning where the spatial data from the high-resolution CT images are used to create a computer-aided design model from which a replica of the individual aorta is made by rapid prototyping.
Recommendations for computed tomography angiography (Table 2): specific supporting text
Computed tomography angiography should be considered over MRI in children who cannot tolerate MRI without prolonged sedation or anaesthesia.
Computed tomography angiography should be considered as a first imaging option in case of suspicion of an acute aortic event.
Computed tomography angiography should be considered before surgical planning, especially in those children candidate for PEARS procedure.
Medical treatment
Different drugs used in treatment
If untreated, individuals with MFS may present earlier progressive aortic root dilatation.59 Improved survival in MFS over recent decades is attributed to better familial screening, regular surveillance, prophylactic medical treatment, and timely surgery.60,61 While it is clear that the arterial wall composition and biomechanical characteristics are altered and responsible for increased fragility, there are no reliable biomarkers to predict aortic events. The best predictor remains aortic root dilatation, and therefore, treatment has aimed at slowing aortic root growth. High blood pressure needs to be aggressively addressed, but it is uncommon in these young patients. Beta-blockers (BBs) are commonly used to slow aortic root growth by lessening strain on the arterial wall, yet their impact on clinical endpoints such as aortic dissection and mortality lacks robust evidence. Several observational studies and only one clinical trial have evaluated the effectiveness of the chronic use of BBs in patients with MFS,62 and the results have been conflicting. Nevertheless, the use of BBs is considered standard of care in patients with MFS. The most widely studied BBs are atenolol and propranolol,62–71 but bisoprolol and metoprolol have also been used in several studies.72,73 Research has more recently focused on agents which could affect the natural history of the disorder by manipulating the signalling pathway of the diseased aorta itself. In particular, the use of angiotensin receptor blockers (ARBs), especially losartan, has been adopted by many.69,70,72,74–77 Irbesartan and valsartan have also been used in several studies.73,78,79 ARBs attenuate the transforming growth factor beta (TGFβ) activity, possibly leading to a reduction in ECM degeneration in the vessel wall, in addition to having a blood pressure–lowering effect. While losartan's efficacy was significant in animal models,80 its effect in human is less pronounced.81 Several groups have investigated the effects of ARBs in humans compared with or in addition to BBs, obtaining variable results.69,70,72,73,76,77 A recent meta-analysis including 1442 patients (children and adults) showed a positive effect of ARB therapy in reducing aortic root z-score, similar to that of BBs.82 Accordingly, it appears both classes of medicines are effective in slowing down the aortic root growth, and combination therapy appears more effective than monotherapy in children with MFS. Current ACC/AHA guidelines recommend use of either BBs or ARBs or a combination of both at maximally tolerated doses (Table 3).22 European guidelines recommend use of BBs and consider ARBs as an alternative treatment.23 Evidence to substantiate the effectiveness of angiotensin-converting enzyme inhibitors (ACE-I) in attenuating aortic dilatation is currently lacking.68 They can be considered in patients who have contraindications to BBs and ARBs. Use of calcium channel blockers has been linked to an increase in acute aortic events, and therefore, they should not be used in MFS.83
Table 3.
Recommended dosage of oral therapy in children with Marfan syndrome
| Drug name | Dosage/kg/day | Max. dosage |
|---|---|---|
| Beta-blocker | ||
| Atenolol | 1–4 mg/kg/d | 250 mg |
| Propranolol | 0.75–3 mg/kg/d | 160 mg |
| Metoprolol | 1–2 mg/kg/d | 200 mg |
| Bisoprolol | 0.05 mg/kg/d | 10 mg |
| Angiotensin receptor blocker | ||
| Losartan | 0.7–1.4 mg/kg/d | 50 mg ≤50 kg 100 mg >50 kg |
| Irbesartan | 1–2 mg/kg/d | 150 mg ≤50 kg 300 mg >50 kg |
| Valsartan | 1–4 mg/kg/d | 80 mg ≤35 kg 160 mg between 35–80 kg 320 mg ≥80 kg |
| ACE-I | ||
| Enalapril | 0.1–1 mg/kg/d | 20 mg |
| Captopril | 0.3–6 mg/kg/d | 100 mg |
The optimal timing for initiating treatment is debated with certain studies indicating more favourable outcomes with early or prolonged therapy, particularly in children with existing aortic root dilatation.67,69 Overall, current available evidence supports initiation of prophylactic medical therapy in children with MFS if (mild) aortic root dilatation is present and the benefit of starting treatment before dilatation occurs; it needs to be individually considered and discussed with the child's parents or guardians. Given that aortic root dilatation in MFS has incomplete penetrance, treating all patients would lead to unnecessary lifelong treatment of about 20%.
Recommendations for medical treatment (Table 2): specific supporting text
The expert group recommends starting medical therapy when the aorta z-score reaches 2 or higher.
Medical treatment can be considered in patients with a confirmed diagnosis and normal aortic size with specific indicators of a more aggressive vascular phenotype: arterial tortuosity, carriers of a truncating variant, or a variant causing loss-of a cysteine residue in a cb-EGF–like domain and family history of early dissection.16
Particular attention should be given to relative contraindication to treatment: for BBs, asthma, hyperinsulinism, or challenging glucose levels and for ARBs, teenage girls without contraception or infants below the age of 1 year. Adequate monitoring of potential side effects should be established.
Combination therapy is suggested to further reduce aortic growth rate and is commonly prescribed when there is at least moderate dilatation (aortic root diameter ≥ 40 mm or z-score ≥ 5) or fast aortic growth rate progression (>5 mm/year with a significant increase in z-score of +1 SD/year).
Medications should be titrated to the highest tolerated dose to achieve the desired effect of slowing dilatation. Effects can be titrated on haemodynamic responses like reduction of baseline heart rate as proposed by some authors.
Psychotropic drugs used for the treatment of attention-deficit/hyperactivity disorder and related conditions
While patients with MFS typically have normal intellectual and gross motor development, up to 50% present with neuropsychological deficits, including attention-deficit/hyperactivity disorder (ADHD).84 Data from the Pediatric Heart Network Marfan trial revealed that ADHD significantly impacts health-related quality of life in children and young adults with MFS.84 The US Food and Drug Administration–approved drugs for treatment of ADHD include stimulant drugs (amphetamine and methylphenidate) and non-stimulant drugs (atomoxetine, clonidine, and guanfacine).85 The European Medicines Agency has not approved the use of clonidine but has a waiver for the use of prolonged clonidine release for paediatric patients.86 Stimulant drugs and atomoxetine may produce a mild increase in heart rate and blood pressure and could delay ventricular repolarization. Conversely, clonidine and guanfacine may reduce heart rate and blood pressure.85 Treatment decisions for ADHD in children with MFS should be made case-by-case. Non-stimulant drugs are considered safer but sometimes less-effective. When stimulant drugs are needed, usual caution and control of the heart rate and blood pressure as per standard guidance is warranted with cessation of the drug if hypertension is detected.
Surgical management
When to consider surgical replacement
Even though medical treatment can decrease the rate at which the aorta dilates and postpone the timing of surgery, aortic root intervention is the only effective way to prevent dissection. In contrast to surgery in urgent settings when a dissection has occurred, prophylactic surgery has a low mortality rate.87,88 Therefore, preventive aortic root intervention has become the standard of care.
The decision for surgical intervention is based on weighing the operative risk against the risk of aortic dissection, influenced by centre expertise, surgical technique, and the need for concurrent valve surgery.89 In-hospital mortality for elective surgery in experienced centres is estimated to be lower than 1%.89–92 Young children, however, have a higher rate of re-intervention, related to increase in size and disease progression.92–94
Scattered case reports exist of aortic dissection or rupture under the age of 10.93,95,96 Data from large national databases point at most of dissections starting to occur during adolescence. Approximately 0.5% of all dissections in MFS patients occur in this age category.27,88,89,97
There are no specific thresholds for preventive aortic root surgery in children with MFS. Current indications are mostly based on absolute aortic root diameters, annual growth rate, and the indication for concomitant valve surgery. The majority of the centres follow the existing adult AHA/ESC guidelines.
Surgical techniques
A paramount consideration is the diameter of the native aortic valve which should be able to accommodate a prosthesis adequate for an adult body size. An annulus diameter of ≥22 mm, measured on echocardiography in PSLAX inner-to-inner diameter, is considered adequate.98
Several techniques to replace the aortic root have been used throughout the years. The dilated aortic root might be replaced alone (aortic-sparing surgery) or combined with the aortic valve (aortic valve replacement surgery). As in adults, the most widely accepted option in children is the valve-sparing root replacement technique, a durable repair achievable with low operative mortality.99–101 Within valve-sparing techniques, three modalities can be distinguished: the reimplantation technique (David V procedure), the remodelling technique (Yacoub procedure), and, more recently, the PEARS procedure. The reimplantation technique is preferred in adults due to the higher rate of re-intervention secondary to aortic regurgitation, observed in the remodelling technique.92 While the PEARS procedure shows promise with low mortality (<1% in adults), its adoption as standard care in children requires further long-term data validation.102
Recommendations for surgical treatment (Table 2): specific supportive text
Surgery is recommended in children when the aortic root diameter reaches 50 mm. It can be considered at 45 mm when some additional risk factors are present such as family history of aortic dissection at low diameter, rapid annual growth rate (>5 mm/year and z-score increase of +1 SD), and concomitant valve surgery indication.
Aortic valve–sparing surgery is preferred to the aortic valve replacement because it avoids the need of lifelong anticoagulation.
Within the aortic valve–sparing surgical modalities, the reimplantation technique is preferred.
Exercise, recreational, and competitive sport
Impact of exercise on the cardiovascular system
In individuals with MFS, strenuous exercise could exacerbate aortic dilatation and increase the risk of aortic dissection over time; therefore, historically, this concern led to recommending avoidance of intense physical activity.
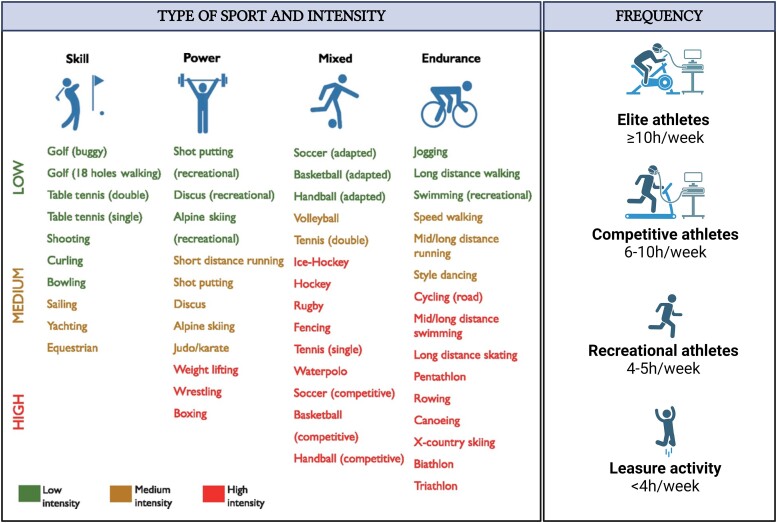
The impact of exercise on the aortic wall varies depending on the type and intensity of the activity. Isotonic activities associated with an increase in cardiac output typically have a moderate effect on systemic blood pressure. In contrast, isometric activities cause a significant increase in diastolic and systolic blood pressure. Most sports combine isotonic and isometric components, which led to a new categorization into skill, power, mixed, and endurance sports103 (Figure 3). The frequency and intensity are also important and can be divided into elite, competitive, or recreational activity based on intensity and amount of training. As a rule, elite athletes train ≥10 h/week, competitive athletes 6–10 h/week, and recreational athletes 4–5 h/week, and <4 h/week is considered leisure sports.
Figure 3.
Differentiation of sports in relation to the predominant isotonic and isometric component, intensity, and frequency (adapted from the 2020 ESC guidelines on sport cardiology and exercise in patients with cardiovascular disease103)
Healthy children typically experience varying blood pressure levels during treadmill, with systolic blood pressure increases >160 mmHg in boys after age 14 while usually remains below 160 mmHg in girls regardless of age.104,105 No data are available on exercise-associated aortic growth. Athletes with bicuspid aortic valve (BAV) generally exhibit similar increase in aortic root and ascending aortic size to non-athletes with BAV.106 Furthermore, exercise-related aortic dissection is rare in healthy individuals,107 with the majority of cases involving adults during heavy lifting.108
Limited literature addresses the effect of exercise on aortic dilatation and dissection in patients with MFS. A recent prospective study suggests that a personalized home training programme using endurance and resistance exercise may be safe for adult patients with MFS with a maximum aortic diameter of 45 mm.109 Additionally, in mouse models of MFS, mild aerobic exercise appears to decrease elastin fibre fragmentation and slow the rate of aortic root dilatation, whereas no training at all or high-intensity training led to a worse aortic phenotype.110,111
Recommendations for sport participation (Table 2): supporting text
Studies addressing sport participation are sparse, and no updated recommendations for children exist. Recommendations were therefore based on adult guidelines adjusted to the physiology of children. It is crucial to adopt a shared decision approach tailored to each child's circumstance when advising on exercise. Notably, additional restrictions related to ocular and skeletal problems are not addressed in our recommendation.
Besides the severity of the aortic disease, the type, frequency, and intensity of the sport should be carefully evaluated with aortic imaging adapted accordingly.
Competitive sport participation is not recommended except for skill, low-intensity sports as bowling, curling, and golf.
In children ≤10 years, any recreational sport can be considered without restriction although the possibility age-related restriction should be discussed with the patient and the parents or guardians.
In children >10 years, power sports are not recommended.
In children >10 years and an aortic root z-score < 3, skill, mixed, or endurance sports can be considered at a recreational level, at any intensity.
In children >10 years and an aortic root z-score ≥ 3 or diameter ≥40 mm, skill, mixed, or endurance sports can be considered at a recreational level, at a moderate intensity (defined as being able to hold a conversation with a friend during exercise).
Similar considerations should be applied when advising patients on future job selection, as certain professions may entail significant physical demands.
Summary
Aortic disease in MFS remains the main cause of morbidity and mortality in this group of patients. Although the incidence of aortic complications in children and adolescents with MFS remains low, early diagnosis and treatment can prevent severe problems in adulthood. The aim of this document is to provide guidance for the follow-up and treatment of children with MFS. Each patient should however be addressed through individualized counselling and treatment.
Acknowledgements
We would like to thank the following reviewers who contributed in improving the results of this consensus statement: Laurence Bal (Fr), Yves Dulac (Fr), Sophie Dupuis-Girot (Fr), Alexandre Guilhem (Fr), Ruth Heying (Be), Lars Idorn (Dk), Stephane Moniotte (Be), Elena Montañés-Delmas (Sp), Sylvie Odent (Fr), Katherine von Klemperer (UK), Yskert von Kodolitsch (De), and Stephane Zuily (Fr).
Contributor Information
Laura Muiño-Mosquera, Department of Paediatrics, division of Paediatric Cardiology, Ghent University Hospital, C. Heymanslaan 10, Ghent 9000, Belgium; Center for Medical Genetics, Ghent University Hospital, Ghent, Belgium.
Elena Cervi, Inherited Cardiovascular Diseases Centre, Cardiology, Great Ormond Street Hospital, London, United Kingdom.
Katya De Groote, Department of Paediatrics, division of Paediatric Cardiology, Ghent University Hospital, C. Heymanslaan 10, Ghent 9000, Belgium.
Wendy Dewals, Department of Paediatrics, division of Paediatric Cardiology, Antwerp University Hospital, Antwerp, Belgium.
Zina Fejzic, Department of Paediatrics, division of Paediatric Cardiology, Radboud University Medical Centre, Nijmegen, The Netherlands.
Kalliopi Kazamia, Department of Paediatric Cardiology, Stockholm-Uppsala, Karolinska University Hospital, Stockholm, Sweden; Department of Women’s and Children’s Health, Karolinska University Hospital, Stockholm, Sweden.
Sujeev Mathur, Department of Cardiovascular Imaging, Guy’s and St Thomas Hospital, London, United Kingdom.
Olivier Milleron, Centre de réference pour le syndrome de Marfan et apparentés, Department of Cardiology, Bichat Claude Bernard Hospital, Université Paris Cité, INSERM U1148, Paris, France.
Thomas S Mir, Childrens Heart Centre, Paediatric Cardiology, University Clinics Hamburg, Hamburg, Germany.
Dorte G Nielsen, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Michal Odermarsky, Children Heart Centre, Skane University Hospital, Lund, Sweden.
Anna Sabate-Rotes, Department of Paediatric Cardiology, Hospital Vall D’Hebron, Barcelona, Spain.
Annelies van der Hulst, Department of Paediatrics, Division of Paediatric Cardiology, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Irene Valenzuela, Department of Clinical and Molecular Genetics, Hospital Vall d’Hebron, Barcelona, Spain.
Guillaume Jondeau, Centre de réference pour le syndrome de Marfan et apparentés, Department of Cardiology, Bichat Claude Bernard Hospital, Université Paris Cité, INSERM U1148, Paris, France.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Declarations
Disclosure of Interest
Nothing to declare.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
L.M.-M. is supported by the Fund of Innovative Search of the Ghent University Hospital (Grant number: KW2296 PED 002 001).
References
- 1. Magenis RE, Maslen CL, Smith L, Allen L, Sakai LY. Localization of the fibrillin (FBN) gene to chromosome 15, band q21.1. Genomics 1991;11:346–51. 10.1016/0888-7543(91)90142-2 [DOI] [PubMed] [Google Scholar]
- 2. Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476–85. 10.1136/jmg.2009.072785 [DOI] [PubMed] [Google Scholar]
- 3. Stheneur C, Tubach F, Jouneaux M, Roy C, Benoist G, Chevallier B et al. Study of phenotype evolution during childhood in Marfan syndrome to improve clinical recognition. Genet Med 2014;16:246–50. 10.1038/gim.2013.123 [DOI] [PubMed] [Google Scholar]
- 4. Zarate YA, Morris SA, Blackshare A, Algaze CA, Connor BS, Kim AJ et al. A clinical scoring system for early onset (neonatal) Marfan syndrome. Genet Med 2022;24:1503–11. 10.1016/j.gim.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 5. Hennekam RCM. Severe infantile Marfan syndrome versus neonatal Marfan syndrome. Am J Med Genet A 2005;139A:1. 10.1002/ajmg.a.30979 [DOI] [PubMed] [Google Scholar]
- 6. Stheneur C, Faivre L, Collod-Béroud G, Gautier E, Binquet C, Bonithon-Kopp C et al. Prognosis factors in probands with an FBN1 mutation diagnosed before the age of 1 year. Pediatr Res 2011;69:265–70. 10.1203/PDR.0b013e3182097219 [DOI] [PubMed] [Google Scholar]
- 7. Faivre L, Collod-Beroud G, Loeys BL, Child A, Binquet C, Gautier E et al. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet 2007;81:454–66. 10.1086/520125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Milewicz DM, Braverman AC, De Backer J, Morris SA, Boileau C, Maumenee IH et al. Marfan syndrome. Nat Rev Dis Primer 2021;7:64. 10.1038/s41572-021-00298-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demolder A, Bianco L, Caruana M, Cervi E, Evangelista A, Jondeau G et al. Arrhythmia and impaired myocardial function in heritable thoracic aortic disease: an international retrospective cohort study. Eur J Med Genet 2022;65:104503. 10.1016/j.ejmg.2022.104503 [DOI] [PubMed] [Google Scholar]
- 10. Mimoun L, Detaint D, Hamroun D, Arnoult F, Delorme G, Gautier M et al. Dissection in Marfan syndrome: the importance of the descending aorta. Eur Heart J 2011;32:443–9. 10.1093/eurheartj/ehq434 [DOI] [PubMed] [Google Scholar]
- 11. den Hartog AW, Franken R, Zwinderman AH, Timmermans J, Scholte AJ, van den Berg MP et al. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol 2015;65:246–54. 10.1016/j.jacc.2014.10.050 [DOI] [PubMed] [Google Scholar]
- 12. Muiño-Mosquera L, De Wilde H, Devos D, Babin D, Jordaens L, Demolder A et al. Myocardial disease and ventricular arrhythmia in Marfan syndrome: a prospective study. Orphanet J Rare Dis 2020;15:300. 10.1186/s13023-020-01581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dietz HC, Cutting CR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM. Marfan syndrome caused by a recurrent missense mutation in the fibrilline 1 gene. Nature 1991;352:337–9. 10.1038/352337a0 [DOI] [PubMed] [Google Scholar]
- 14. Muiño-Mosquera L, Steijns F, Audenaert T, Meerschaut I, De Paepe A, Steyaert W et al. Tailoring the American College of Medical Genetics and Genomics and the Association for Molecular Pathology guidelines for the interpretation of sequenced variants in the FBN1 gene for Marfan syndrome: proposal for a disease- and gene-specific guideline. Circ Genomic Precis Med 2018;11:e002039. 10.1161/CIRCGEN.117.002039 [DOI] [PubMed] [Google Scholar]
- 15. Lerner-Ellis JP, Aldubayan SH, Hernandez AL, Kelly MA, Stuenkel AJ, Walsh J et al. The spectrum of FBN1, TGFβR1, TGFβR2 and ACTA2 variants in 594 individuals with suspected Marfan syndrome, Loeys–Dietz syndrome or thoracic aortic aneurysms and dissections (TAAD). Mol Genet Metab. 2014;112:171–6. 10.1016/j.ymgme.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 16. Arnaud P, Milleron O, Hanna N, Ropers J, Ould Ouali N, Affoune A et al. Clinical relevance of genotype–phenotype correlations beyond vascular events in a cohort study of 1500 Marfan syndrome patients with FBN1 pathogenic variants. Genet Med 2021;23:1296–304. 10.1038/s41436-021-01132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu HH, Wu MH, Chen HC, Kao FY, Huang SK. Epidemiological profile of Marfan syndrome in a general population: a national database study. Mayo Clin Proc 2014;89:34–42. 10.1016/j.mayocp.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 18. Arnaud P, Morel H, Milleron O, Gouya L, Francannet C, Da Costa A et al. Unsuspected somatic mosaicism for FBN1 gene contributes to Marfan syndrome. Genet Med 2021;23:865–71. 10.1038/s41436-020-01078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernández-Álvarez P, Codina-Sola M, Valenzuela I, Teixidó-Turá G, Cueto-González A, Paramonov I et al. A systematic study and literature review of parental somatic mosaicism of FBN1 pathogenic variants in Marfan syndrome. J Med Genet 2022;59:605. 10.1136/jmedgenet-2020-107604 [DOI] [PubMed] [Google Scholar]
- 20. De Backer J, Campens L, Muiño Mosquera L. Looking for the missing links. Circ Genomic Precis Med 2018;11:e002185. 10.1161/CIRCGEN.118.002185 [DOI] [PubMed] [Google Scholar]
- 21. Grant J. Content and distribution of aortic collagen, elastin and carbohydrate in different species. J Athero Res 1967;7:463–72. 10.1016/S0368-1319(67)80024-3 [DOI] [PubMed] [Google Scholar]
- 22. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW, Bolen MA et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334–482. 10.1161/CIR.0000000000001106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Authors/Task Force members; Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult * The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 24. de Beaufort HWL, Trimarchi S, Korach A, Di Eusanio M, Gilon D, Montgomery DG et al. Aortic dissection in patients with Marfan syndrome based on the IRAD data. Ann Cardiothorac Surg. 2017;6:633–41. 10.21037/acs.2017.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roman MJ, Devereux RB, Preiss LR, Asch FM, Eagle KA, Holmes KW et al. Associations of age and sex with Marfan phenotype clinical perspective: the National Heart, Lung, and Blood Institute GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) registry. Circ Cardiovasc Genet. 2017;10:e001647. 10.1161/CIRCGENETICS.116.001647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoskoppal A, Menon S, Trachtenberg F, Burns KM, De Backer J, Gelb BD et al. Predictors of rapid aortic root dilation and referral for aortic surgery in Marfan syndrome. Pediatr Cardiol 2018;39:1453–61. 10.1007/s00246-018-1916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hascoet S, Edouard T, Plaisancie J, Arnoult F, Milleron O, Stheneur C et al. Incidence of cardiovascular events and risk markers in a prospective study of children diagnosed with Marfan syndrome. Arch Cardiovasc Dis 2020;113:40–9. 10.1016/j.acvd.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 28. Selamet Tierney ES, Levine JC, Sleeper LA, Roman MJ, Bradley TJ, Colan SD et al. Influence of aortic stiffness on aortic-root growth rate and outcome in patients with the Marfan syndrome. Am J Cardiol 2018;121:1094–101. 10.1016/j.amjcard.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franken R, el Morabit A, de Waard V, Timmermans J, Scholte AJ, van den Berg MP et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. Int J Cardiol. 2015;194:7–12. 10.1016/j.ijcard.2015.05.072 [DOI] [PubMed] [Google Scholar]
- 30. Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation 2011;124:388–96. 10.1161/CIRCULATIONAHA.110.990549 [DOI] [PubMed] [Google Scholar]
- 31. Bradley TJ, Alvarez NAM, Horne SG. A practical guide to clinical management of thoracic aortic disease. Can J Cardiol 2016;32:124–30. 10.1016/j.cjca.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 32. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465–95. 10.1016/j.echo.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 33. Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:119–82. 10.1016/j.echo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 34. Evangelista A, Sitges M, Jondeau G, Nijveldt R, Pepi M, Cuellar H et al. Multimodality imaging in thoracic aortic diseases: a clinical consensus statement from the European Association of Cardiovascular Imaging and the European Society of Cardiology working group on aorta and peripheral vascular diseases. Eur Heart J—Cardiovasc Imaging 2023;24:e65–85. 10.1093/ehjci/jead024 [DOI] [PubMed] [Google Scholar]
- 35. Rodríguez-Palomares JF, Teixidó-Tura G, Galuppo V, Cuéllar H, Laynez A, Gutiérrez L et al. Multimodality assessment of ascending aortic diameters: comparison of different measurement methods. J Am Soc Echocardiogr 2016;29:819–826.e4. 10.1016/j.echo.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 36. Muraru D, Maffessanti F, Kocabay G, Peluso D, Bianco LD, Piasentini E et al. Ascending aorta diameters measured by echocardiography using both leading edge-to-leading edge and inner edge-to-inner edge conventions in healthy volunteers. Eur Heart J—Cardiovasc Imaging 2014;15:415–22. 10.1093/ehjci/jet173 [DOI] [PubMed] [Google Scholar]
- 37. Son MK, Chang SA, Kwak JH, Lim HJ, Park SJ, Choi JO et al. Comparative measurement of aortic root by transthoracic echocardiography in normal Korean population based on two different guidelines. Cardiovasc Ultrasound 2013;11:28. 10.1186/1476-7120-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bossone E, Yuriditsky E, Desale S, Ferrara F, Vriz O, Asch FM. Normal values and differences in ascending aortic diameter in a healthy population of adults as measured by the pediatric versus adult American Society of Echocardiography guidelines. J Am Soc Echocardiogr 2016;29:166–72. 10.1016/j.echo.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 39. Servato ML, Teixidó-Turá G, Sabate-Rotes A, Galian-Gay L, Gutiérrez L, Valente F et al. Are aortic root and ascending aorta diameters measured by the pediatric versus the adult American Society of Echocardiography guidelines interchangeable? J Clin Med 2021;10:5290. 10.3390/jcm10225290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutten DWE, Aarts-Janssen IJH, Kempers MJE, Reimer AG, Udink ten Cate FEA, Loeys BL et al. Comparability of different Z-score equations for aortic root dimensions in children with Marfan syndrome. Cardiol Young 2021;31:1962–8. 10.1017/S1047951121001311 [DOI] [PubMed] [Google Scholar]
- 41. Sigurdsson TS, Lindberg L. Six commonly used empirical body surface area formulas disagreed in young children undergoing corrective heart surgery. Acta Paediatr 2020;109:1838–46. 10.1111/apa.15208 [DOI] [PubMed] [Google Scholar]
- 42. Lopez L, Colan S, Stylianou M, Granger S, Trachtenberg F, Frommelt P et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity. Circ Cardiovasc Imaging 2017;10:e006979. 10.1161/CIRCIMAGING.117.006979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campens L, Demulier L, De Groote K, Vandekerckhove K, De Wolf D, Roman MJ et al. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol 2014;114:914–20. 10.1016/j.amjcard.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 44. Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989;64:507–12. 10.1016/0002-9149(89)90430-X [DOI] [PubMed] [Google Scholar]
- 45. Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 2005;99:445–57. 10.1152/japplphysiol.01144.2004 [DOI] [PubMed] [Google Scholar]
- 46. Warren AE, Boyd ML, O’Connell C, Dodds L. Dilatation of the ascending aorta in paediatric patients with bicuspid aortic valve: frequency, rate of progression and risk factors. Heart 2006;92:1496. 10.1136/hrt.2005.081539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of Z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr 2008;21:922–34. 10.1016/j.echo.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 48. Gautier M, Detaint D, Fermanian C, Aegerter P, Delorme G, Arnoult F et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am J Cardiol 2010;105:888–94. 10.1016/j.amjcard.2009.11.040 [DOI] [PubMed] [Google Scholar]
- 49. Cantinotti M, Giordano R, Scalese M, Murzi B, Assanta N, Spadoni I et al. Nomograms for two-dimensional echocardiography derived valvular and arterial dimensions in Caucasian children. J Cardiol 2017;69:208–15. 10.1016/j.jjcc.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 50. Meijboom LJ, Groenink M, van der Wall EE, Romkes H, Stoker J, Mulder BJM. Aortic root asymmetry in Marfan patients; evaluation by magnetic resonance imaging and comparison with standard echocardiography. Int J Card Imaging 2000;16:161–8. 10.1023/A:1006429603062 [DOI] [PubMed] [Google Scholar]
- 51. Veldhoen S, Behzadi C, Derlin T, Rybczinsky M, von Kodolitsch Y, Sheikhzadeh S et al. Exact monitoring of aortic diameters in Marfan patients without gadolinium contrast: intraindividual comparison of 2D SSFP imaging with 3D CE-MRA and echocardiography. Eur Radiol 2015;25:872–82. 10.1007/s00330-014-3457-6 [DOI] [PubMed] [Google Scholar]
- 52. Weinrich JM, Avanesov M, Lenz A, Tahir E, Henes FO, Schoennagel BP et al. Reliability of non-contrast magnetic resonance angiography-derived aortic diameters in Marfan patients: comparison of inner vs. outer vessel wall measurements. Int J Cardiovasc Imaging 2020;36:1533–42. 10.1007/s10554-020-01850-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lopez-Sainz A, Mila L, Rodriguez-Palomares J, Limeres J, Granato C, La Mura L et al. Aortic branch aneurysms and vascular risk in patients with Marfan syndrome. J Am Coll Cardiol 2021;77:3005–12. 10.1016/j.jacc.2021.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mariucci EM, Lovato L, Rosati M, Palena LM, Bonvicini M, Fattori R. Dilation of peripheral vessels in Marfan syndrome: importance of thoracoabdominal MR angiography. Int J Cardiol 2013;167:2928–31. 10.1016/j.ijcard.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 55. Kaiser T, Kellenberger CJ, Albisetti M, Bergsträsser E, Valsangiacomo Buechel ER. Normal values for aortic diameters in children and adolescents—assessment in vivo by contrast-enhanced CMR-angiography. J Cardiovasc Magn Reson 2008;10:56. 10.1186/1532-429X-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim JH, Kim JW, Song SW, Ahn SJ, Park M, Park SK et al. Intracranial aneurysms are associated with Marfan syndrome. Stroke 2021;52:331–4. 10.1161/STROKEAHA.120.032107 [DOI] [PubMed] [Google Scholar]
- 57. Long CM, Long SS, Johnson PT, Mahesh M, Fishman EK, Zimmerman SL. Utility of low-dose high-pitch scanning for pediatric cardiac computed tomographic imaging. J Thorac Imaging 2015;30:W36–40. 10.1097/RTI.0000000000000131 [DOI] [PubMed] [Google Scholar]
- 58. Han BK, Rigsby CK, Hlavacek A, Leipsic J, Nicol ED, Siegel MJ et al. Computed tomography imaging in patients with congenital heart disease part I: rationale and utility. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT): endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr 2015;9:475–92. 10.1016/j.jcct.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 59. Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med 1972;286:804–8. 10.1056/NEJM197204132861502 [DOI] [PubMed] [Google Scholar]
- 60. Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ et al. Life expectancy in the Marfan syndrome. Am J Cardiol 1995;75:157–60. 10.1016/S0002-9149(00)80066-1 [DOI] [PubMed] [Google Scholar]
- 61. Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM. Marfan syndrome. Circulation 1995;91:728–33. 10.1161/01.CIR.91.3.728 [DOI] [PubMed] [Google Scholar]
- 62. Shores J, Berger K, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. NEJM 1994;330:1335–41. 10.1056/NEJM199405123301902 [DOI] [PubMed] [Google Scholar]
- 63. Tahernia A. Cardiovascular anomalies in Marfan’s syndrome: the role of echocardiography and beta-blockers. South Med J 1993;86:305–10. 10.1097/00007611-199303000-00012 [DOI] [PubMed] [Google Scholar]
- 64. Salim MA, Alpert BS, Ward JC, Pyeritz RE. Effect of beta-adrenergic blockade on aortic root rate of dilation in the Marfan syndrome. Am J Cardiol 1994;74:629–33. 10.1016/0002-9149(94)90762-5 [DOI] [PubMed] [Google Scholar]
- 65. Rossi-Foulkes R, Roman MJ, Rosen SE, Kramer-Fox R, Ehlers KH, O’Loughlin JE et al. Phenotypic features and impact of beta blocker or calcium antagonist therapy on aortic lumen size in the Marfan syndrome. Am J Cardiol 1999;83:1364–8. 10.1016/S0002-9149(99)00101-0 [DOI] [PubMed] [Google Scholar]
- 66. Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS et al. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr 2007;150:77–82. 10.1016/j.jpeds.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 67. Ladouceur M, Fermanian C, Lupoglazoff JM, Edouard T, Dulac Y, Acar P et al. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol 2007;99:406–9. 10.1016/j.amjcard.2006.08.048 [DOI] [PubMed] [Google Scholar]
- 68. Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol 2005;95:1125–7. 10.1016/j.amjcard.2005.01.032 [DOI] [PubMed] [Google Scholar]
- 69. Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD et al. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014;371:2061–71. 10.1056/NEJMoa1404731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Forteza A, Evangelista A, Sánchez V, Teixidó-Turà G, Sanz P, Gutiérrez L et al. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur Heart J 2016;37:978–85. 10.1093/eurheartj/ehv575 [DOI] [PubMed] [Google Scholar]
- 71. Muiño-Mosquera L, De Nobele S, Devos D, Campens L, De Paepe A, De Backer J. Efficacy of losartan as add-on therapy to prevent aortic growth and ventricular dysfunction in patients with Marfan syndrome: a randomized, double-blind clinical trial. Acta Cardiol 2017;72:616–24. 10.1080/00015385.2017.1314134 [DOI] [PubMed] [Google Scholar]
- 72. Milleron O, Arnoult F, Ropers J, Aegerter P, Detaint D, Delorme G et al. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur Heart J 2015;36:2160–6. 10.1093/eurheartj/ehv151 [DOI] [PubMed] [Google Scholar]
- 73. Mueller GC, Stierle L, Stark V, Steiner K, von Kodolitsch Y, Weil J et al. Retrospective analysis of the effect of angiotensin II receptor blocker versus β-blocker on aortic root growth in paediatric patients with Marfan syndrome. Heart 2014;100:214. 10.1136/heartjnl-2013-304946 [DOI] [PubMed] [Google Scholar]
- 74. Pees C, Laccone F, Haql M, De Brauwer V, Moser E, Michel-Behnke I. Usefulness of losartan on the size of the ascending aorta in an unselected cohort of children, adolescent and young adults with Marfan syndrome. Am J Cardiol 2013;112:1477–83. 10.1016/j.amjcard.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 75. Pees C, Heno J, Michel-Behnke I. Initial angiotensin receptor blocker response in young Marfan patients decreases after 3 years of treatment. Pediatr Cardiol 2022;43:586–95. 10.1007/s00246-021-02761-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008;358:2787–95. 10.1056/NEJMoa0706585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chiu HH, Wu MH, Wang JK, Lu CW, Chiu SN, Chen CA et al. Losartan added to β-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013;88:271–6. 10.1016/j.mayocp.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 78. Mullen M, Jin XY, Child A, Stuart AG, Dodd M, Aragon-Martin JA et al. Irbesartan in Marfan syndrome (AIMS): a double-blind, placebo-controlled randomised trial. Lancet 2019;394:2263–70. 10.1016/S0140-6736(19)32518-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Olfe J, Kanitz JJ, Stark VC, Stute F, von Kodolitsch Y, Biermann D et al. Prophylactic effect of angiotensin receptor blockers in children with genetic aortopathies: the early bird catches the worm. Clin Res Cardiol 2023;112:1610–9. 10.1007/s00392-023-02221-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK et al. Losartan, a AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312:117–21. 10.1126/science.1124287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Isselbacher EM. Losartan for the treatment of Marfan syndrome: hope fades∗. J Am Coll Cardiol 2018;72:1619–21. 10.1016/j.jacc.2018.07.051 [DOI] [PubMed] [Google Scholar]
- 82. Pitcher A, Spata E, Emberson J, Davies K, Halls H, Holland L et al. Angiotensin receptor blockers and β blockers in Marfan syndrome: an individual patient data meta-analysis of randomised trials. Lancet 2022;400:822–31. 10.1016/S0140-6736(22)01534-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Doyle JJ, Doyle AJ, Wilson NK, Habashi JP, Bedja D, Whitworth RE et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. eLife 2015;4:e08648. 10.7554/eLife.08648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Handisides JC, Hollenbeck-Pringle D, Uzark K, Trachtenberg FL, Pemberton VL, Atz TW et al. Health-related quality of life in children and young adults with Marfan syndrome. J Pediatr 2019;204:250–255.e1. 10.1016/j.jpeds.2018.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2019;144:e20192528. 10.1542/peds.2019-2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. European Medicines agency decision of 10 June 2022 on the refusal of a paediatric investigation plan and on the granting of a waiver for clonidine (EMEA-003198-PIP01-22) in accordance with Regulation (EC) No 1901/20006 of the European Parliament and of the Council.
- 87. Groth KA, Stochholm K, Hove H, Kyhl K, Gregersen PA, Vejlstrup N et al. Aortic events in a nationwide Marfan syndrome cohort. Clin Res Cardiol 2017;106:105–12. 10.1007/s00392-016-1028-3 [DOI] [PubMed] [Google Scholar]
- 88. Gott VL, Greene PS, Alejo DE, Cameron DE, Naftel DC, Miller DC et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med 1999;340:1307–13. 10.1056/NEJM199904293401702 [DOI] [PubMed] [Google Scholar]
- 89. Knadler JJ, LeMaire S, McKenzie ED, Moffett B, Morris SA. Thoracic aortic, aortic valve, and mitral valve surgery in pediatric and young adult patients with Marfan syndrome: characteristics and outcomes. Semin Thorac Cardiovasc Surg 2019;31:818–25. 10.1053/j.semtcvs.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Javier Delmo EM, Javier MdM, Hetzer R. Consecutive surgical sequelae in children and adolescents with Marfan syndrome after primary cardiovascular surgical interventions. Eur J Cardiothorac Surg 2020;57:54–62. 10.1093/ejcts/ezz143 [DOI] [PubMed] [Google Scholar]
- 91. Martín C, Evangelista A, Serrano-Fiz S, Villar S, Ospina V, Martínez D et al. Aortic complications in Marfan syndrome: should we anticipate preventive aortic root surgery? Ann Thorac Surg 2020;109:1850–7. 10.1016/j.athoracsur.2019.08.096 [DOI] [PubMed] [Google Scholar]
- 92. Fraser CD III, Liu RH, Zhou X, Patel ND, Lui C, Pierre AS et al. Valve-sparing aortic root replacement in children: outcomes from 100 consecutive cases. J Thorac Cardiovasc Surg 2019;157:1100–9. 10.1016/j.jtcvs.2018.09.148 [DOI] [PubMed] [Google Scholar]
- 93. Gillinov AM, Zehr KJ, Redmond JM, Gott VL, Deitz HC, Reitz BA et al. Cardiac operations in children with Marfan’s syndrome: indications and results. Ann Thorac Surg 1997;64:1140–5. 10.1016/S0003-4975(97)00849-7 [DOI] [PubMed] [Google Scholar]
- 94. Everitt MD, Pinto N, Hawkins JA, Mitchell MB, Kouretas PC, Yetman AT. Cardiovascular surgery in children with Marfan syndrome or Loeys-Dietz syndrome. J Thorac Cardiovasc Surg 2009;137:1327–33. 10.1016/j.jtcvs.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 95. Knirsch W, Hillebrand D, Horke A, Lewin MAG, Rein J, Uhlemann F. Aortic aneurysm rupture in infantile Marfan’s syndrome. Pediatr Cardiol 2001;22:156–9. 10.1007/s002460010185 [DOI] [PubMed] [Google Scholar]
- 96. Kato Y, Ohashi H, Tsutsumi Y, Kawai T. Emergent david-V operation for a ruptured aortic root aneurysm in a 9-year-old child. Eur J Cardiothorac Surg 2007;31:744–6. 10.1016/j.ejcts.2007.01.022 [DOI] [PubMed] [Google Scholar]
- 97. El Habbal MH. Cardiovascular manifestations of Marfan’s syndrome in the young. Am Heart J 1992;123:752–7. 10.1016/0002-8703(92)90516-X [DOI] [PubMed] [Google Scholar]
- 98. Lange R, Badiu CC, Vogt M, Voss B, Hörer J, Prodan Z et al. Valve-sparing root replacement in children with aortic root aneurysm: mid-term results†. Eur J Cardiothorac Surg 2013;43:958–64. 10.1093/ejcts/ezs598 [DOI] [PubMed] [Google Scholar]
- 99. David TE. Current readings: aortic valve–sparing operations. Semin Thorac Cardiovasc Surg 2014;26:231–8. 10.1053/j.semtcvs.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 100. David TE. Aortic valve repair and aortic valve–sparing operations. J Thorac Cardiovasc Surg 2015;149:9–11. 10.1016/j.jtcvs.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 101. Escobar Kvitting JP, Kari FA, Fischbein MP, Liang DH, Beraud AS, Stephens EH et al. David valve-sparing aortic root replacement: equivalent mid-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg 2013;145:117–127.e5. 10.1016/j.jtcvs.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Van Hoof L, Rega F, Golesworthy T, Verbrugghe P, Austin C, Takkenberg JJM et al. Personalised external aortic root support for elective treatment of aortic root dilation in 200 patients. Heart 2021;107:1790. 10.1136/heartjnl-2021-319300 [DOI] [PubMed] [Google Scholar]
- 103. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S et al. 2020 eSC guidelines on sports cardiology and exercise in patients with cardiovascular disease: the task force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 2021;42:17–96. 10.1093/eurheartj/ehaa605 [DOI] [PubMed] [Google Scholar]
- 104. Maffulli N, Greco R, Greco L, D’Alterio D. Treadmill exercise in neapolitan children and adolescents. Acta Paediatr 1994;83:106–12. 10.1111/j.1651-2227.1994.tb12964.x [DOI] [PubMed] [Google Scholar]
- 105. Becker M, Silva O, Moreira IEG, Victor EG. Pressão arterial em adolescentes durante teste ergométrico. Arq Bras Cardiol 2007;88:297–300. 10.1590/S0066-782X2007000300012. [DOI] [PubMed] [Google Scholar]
- 106. Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317–25. 10.1001/jama.300.11.1317 [DOI] [PubMed] [Google Scholar]
- 107. Gati S, Malhotra A, Sedgwick C, Papamichael N, Dhutia H, Sharma R et al. Prevalence and progression of aortic root dilatation in highly trained young athletes. Heart 2019;105:920. 10.1136/heartjnl-2018-314288 [DOI] [PubMed] [Google Scholar]
- 108. Thijssen CGE, Bons LR, Gökalp AL, Van Kimmenade RRJ, Mokhles MM, Pelliccia A et al. Exercise and sports participation in patients with thoracic aortic disease: a review. Expert Rev Cardiovasc Ther 2019;17:251–66. 10.1080/14779072.2019.1585807 [DOI] [PubMed] [Google Scholar]
- 109. Jouini S, Milleron O, Eliahou L, Jondeau G, Vitiello D. Effects of a personalized home-based training program among patients suffering from Marfan syndrome: a pilot randomized and controlled study. Intractable Rare Dis Res 2021;10:263–8. 10.5582/irdr.2021.01080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gibson C, Nielsen C, Alex R, Cooper K, Farney M, Gaufin D et al. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J Appl Physiol 2017;123:147–60. 10.1152/japplphysiol.00132.2017 [DOI] [PubMed] [Google Scholar]
- 111. Mas-Stachurska A, Siegert A, Batlle M, Gorbenko del Blanco D, Meirelles T, Rubies C et al. Cardiovascular benefits of moderate exercise training in Marfan syndrome: insights from an animal model. J Am Heart Assoc. 2017;6:e006438. 10.1161/JAHA.117.006438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this paper.