Abstract
Background
Though the survival of breast cancer (BC) patients with malignant pleural effusion (MPE) has been studied, this has not been specifically studied in the luminal B subtype. Therefore, this study investigated the characteristics and survival of luminal B-BC patients presenting with MPE.
Methods
We retrospectively analyzed 141 patients diagnosed with postoperative advanced Luminal B breast cancer, including 54 cases with MPE and 87 cases without MPE at the Tianjin Cancer Hospital from January 2012 to January 2015. We assessed the clinical characteristics between the groups.
Results
The mean age of all patients was 47 years, with no significant difference between the two groups. Altogether, 29 (33%), 24 (28%), 28 (32%), 45 (52%), and 10 (11%) patients had lung, liver, bone, lymph node, and chest wall metastases, respectively. In addition. The difference in overall survival between the two groups was not significant (P>0.05). However, cox regression analysis showed that only the tumor clinical stage at initial diagnosis was related to short overall survival. Further, we conducted a subgroup analysis and found that the higher the clinical stage at initial diagnosis in age < 50 years patients, the shorter the overall survival, while age > 50 years patients was not. (P < 0.05).
Conclusions
There was no difference in the overall survival between luminal B-BC patients with MPE and those without. Clinical stages at initial diagnosis were an independent prognostic factor for age < 50 years luminal B BC with MPE overall survival. Our results may help clinicians make positive decisions regarding personalized treatment of luminal B-BC with MPE.
Keywords: Breast cancer, Malignant pleural effusion, Clinical features, Survival, Luminal B
Introduction
Malignant pleural effusion (MPE) is a medical condition often secondary to a variety of diseases, especially cancers and tuberculosis [1]. Cancers that cause MPE in order of frequency are lung cancer, breast cancer, and lymphoma. Breast cancer (BC) is one of the most common cancers and the second leading cause of cancer-associated deaths in women. Some studies have shown that advanced breast carcinoma is the second most common cause of malignant effusions ranging from 7–23% depending on increases in advanced stages, and is often associated with poor prognosis [2, 3]. Although systemic therapy options (surgery, radiotherapy, and chemotherapeutic agents) have been tested, those are largely palliative, including symptomatic control and chemical pleurodesis, which promotes symphysis of the parietal and visceral pleura to prevent exudate accumulation. Furthermore, as previously emphasized, a diverse array of management strategies is now accessible for the management of MPE. Nevertheless, each of these interventions carries its unique implications, encompassing both the patient’s burden and the financial strain imposed on the healthcare system [4, 5]. Thus, the recently updated pleural guidelines from the British Thoracic Society (BTS) in 2023 present a comprehensive framework for addressing dyspnea associated with malignant pleural effusions (MPEs). These guidelines meticulously outline various procedural and strategic interventions, emphasizing not only the prognostic implications and potential for lung re-expansion, but also the paramount importance of aligning treatment with patient priorities and preferences. Recognizing the absence of a universally ‘correct’ definitive management strategy, the BTS guidelines underscore the need for a highly individualized approach that integrates patient-centered considerations, including the choice between inpatient and ambulatory care, to optimize outcomes and ensure patient satisfaction [6].
Luminal B is one of the most frequently occurring in five clinically and biologically relevant molecular subtypes of breast cancer in the population. Compared to patients with the other subtype, those with luminal B tumors have worse prognoses [7]. Moreover, several studies have suggested that the increased approximately 18–45% relapse risk associated with the luminal B phenotype appears to have a predilection for pleural metastases and occur MPE within the first 5 years after surgery [8–10]. Despite the establishment of endocrine therapy and anti-HER2 therapy as cornerstone treatments for luminal B breast cancer, this subtype of malignancy continues to pose a significant therapeutic challenge. Regrettably, despite remarkable advances in patient survival outcomes over recent years, attributed to the employment of diverse therapeutic strategies, luminal B breast cancer remains an incurable condition [11, 12]. Furthermore, prior investigative endeavors have unveiled that individual diagnosed with luminal B subtype of breast cancer, in whom the presence of MPE, irrespective of its extent, may portend a less favorable prognostic outlook. [13, 14]. Therefore, it is essential to judge the prognosis of luminal B breast cancer patients with MPE to better stratify patients and guide their therapy. However, as far as we know, the survival in luminal B BC patients presenting with MPE has been seldom reported.
This study aimed to investigate previously unreported observations on outcomes in luminal B BC patients presenting with MPE.
Methods
Study design
A retrospective review of all consecutive primary BC patients who developed MPE and aged between 18 and 80 years, were eligible for the study. The study site was the Tianjin Cancer Hospital and lasted from January 2012 to January 2015. The inclusion criteria were as follows: (a). diagnosis of luminal B breast cancer verified by biopsy or surgical resection; The exclusion criteria were as follows: (a). patients with double primary malignant tumors; (b). patients without a complete clinical history; (c). patients who developed MPE within one month after surgery. A total of 141 consecutive patients diagnosed with advanced luminal B BC were included in the study. The detailed pathological and histologic type, and Clinical stages at initial diagnosis of the primary tumor were recorded in a questionnaire. The study protocol was approved by the Tianjin Cancer Hospital ethics committee and The Affiliated General Hospital Binhai Hospital of Tianjin Medical University, and informed consent was obtained from all patients. An immunohistochemical (IHC) analysis was performed to classify the patients into molecular subtypes (IHC-based subtypes) [15].
Clinicopathological evaluation
We retrospectively evaluated clinicopathological factors such as estrogen receptor (ER) and progesterone receptor (PR) status, HER2 status (0–3 + score as recommended by the Dako HercepTest kit scoring guidelines with > 3 + defined as positive), and Ki67 status (positivity cut-off 14%). Cases with IHC scores of 2 + with gene amplification by fluorescence in situ hybridization (FISH) were considered positive for HER2. A cut-off value of 1% for both receptors was used to classify the expression of ER and PR according to the criteria proposed by the American Society of Clinical Oncology (ASCO) [16]. When immunostaining was observed in more than 1% of the tumor nuclei, the tumor was considered ER- or PR- positive. The definition of luminal B was as follows: either HER2-negative and ER-positive, or HER2-negative and either Ki-67 high or PR low; luminal B-like was either HER2- and ER-positive, or HER2 overexpressed or amplified, and low or high Ki-67 and PR. The immunostaining results were evaluated independently by two pathologists.
Diagnosis and therapy for pleural effusions
A posteroanterior chest X-ray (CXR-PA), chest computed tomography (CT) scan or ultrasonography was carried out for the determination of the volume of the effusion and the affected area. MPE was defined by positive pleural fluid cytology, tissue histology or multidisciplinary meeting consensus. The various treatments (chemotherapy, radiotherapy, and hormone therapy) and therapeutic options (thoracenteses and bedside insertion of an indwelling pleural catheter) were extracted from the electronic medical charts.
Statistical analyses
The primary endpoint of this study was overall survival (OS). OS was calculated from the date of primary BC first postoperative diagnosis to the date of death or last follow-up to December 2015. The Student’s t-test and the chi-squared test were used to compare the clinical characteristics between groups. Continuous and categorical variables are expressed as percentages. P < 0.05 was considered statistically significant. For categorical variables, missing data were included as a “Unknown” category. The OS rates were calculated using the Kaplan-Meier method, and the groups were compared using the log-rank test. For the multivariate analysis, Cox regression analysis was used. All analyses were conducted with SPSS version 24.0 statistical software (Chicago, IL, USA).
Results
Demographics
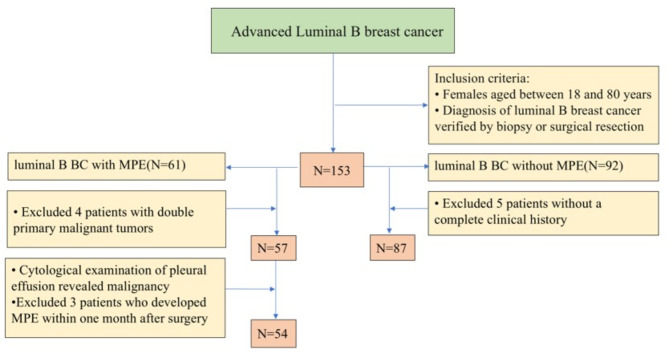
The cohort comprised 141 luminal B BC patients among whom 54 (38.3%) had MPE. Amongst the MPE cases, 2 had MPE due to congestive cardiac failure, and they responded to heart disease medical treatment. We analyzed all the 54 cases with MPE [Fig. 1]. All cases were female. The histological distribution included 50 (92.6%) invasive ductal carcinomas, 1 (1.9%) invasive lobular carcinoma, and 3 (5.6%) other types of carcinomas (1 low-grade adenocarcinoma, 1 papillary carcinoma, and 1 neuroendocrine carcinoma). Eight (14.9%) patients were HER2-positive while 46 (85.2%) were negative. The clinical-stage data was available for only 54 patients. The number of patients with tumors in stage I, II, III, and unknown stage was 5 (9.3%), 15 (27.8%), 24 (44.4%), and 10 (18.5%), respectively. The percentage of patients with lung metastatic, liver metastatic, bone metastatic, and others sites metastatic was 22 (40.7%),12 (22.2%), 27 (50.0%), and 42 (77.8%), respectively. Treatments instituted included chemotherapy (45 patients, 83.3%), radiotherapy (24 patients, 44.4%), endocrine therapy (34 patients, 63.0%), and neoadjuvant chemotherapy (11 patients, 20.3%) [Table 1]. Most of the patients were between 30 and 70 years of age (youngest was 23 years and oldest was 70 years) with mean 47.0 ± 10.3. In this study, 4 (25.9%), 18 (33.3%), and 22 (40.7%) patients presented with left-sided, right-sided, and bilateral MPE, respectively.
Fig. 1.
Flow diagram of enrolled patients
Table 1.
Characteristic of luminal B breast cancer patients with and without malignant pleural effusions
| Variables | With MPE (n = 54) | Without MPE (n = 87) | P |
|---|---|---|---|
| Age at surgery (years) | 0.096 | ||
| < 50 | 32 (59.3%) | 39(44.8%) | |
| ≥ 50 | 22(40.7%) | 48 (55.2%) | |
| Primary tumor location | |||
| Left | 25 (46.3%) | 50 (57.5%) | 0.186 |
| Right | 26 (48.1%) | 36 (41.4%) | |
| Bilateral | 3 (5.6%) | 1 (1.1%) | |
| Clinical stages at initial diagnosis | |||
| Stage I | 5 (9.3%) | 11 (12.6%) | 0.088 |
| Stage II | 15 (27.8%) | 26 (29.9%) | |
| Stage III | 22 (44.4%) | 36 (41.4%) | |
| Unknown | 10 (18.5%) | 4 (4.6%) | |
| Histological type | |||
| Invasive ductal carcinoma | 50 (92.6%) | 83 (95.4%) | 0.787 |
| Invasive lobular carcinoma | 1(1.9%) | 1(1.1%) | |
| Others | 3 (5.6%) | 3 (3.4%) | |
| HER-2 | |||
| Positive | 8 (14.9%) | 12 (13.8%) | 0.866 |
| Negative | 46 (85.2%) | 75 (86.2%) | |
| Metastatic site | 0.502 | ||
| Lung | 22(40.7%) | 29(33.3%) | |
| Liver | 12(22.2%) | 24(27.6%) | |
| Bone | 27(50.0%) | 28(32.2%) | |
| Others | 42(77.8%) | 50(57.5%) | |
| Neoadjuvant therapy | |||
| Yes | 11 (20.3%) | 13 (14.9%) | 0.404 |
| No | 43 (79.6%) | 74 (85.0%) | |
| Adjuvant Therapy | |||
| Chemotherapy | 45 (83.3%) | 68 (78.2%) | 0.316 |
| Endocrine therapy | 34 (63.0%) | 51 (58.6%) | |
| Radiotherapy | 24 (44.4%) | 22 (25.3%) | |
| Surgical approach | |||
| Radical surgery | 49 (83.3%) | 84 (96.6%) | 0.260 |
| Conservative surgery | 5 (9.3%) | 3 (3.4%) |
MPE, Malignant pleural effusion
Survival analysis
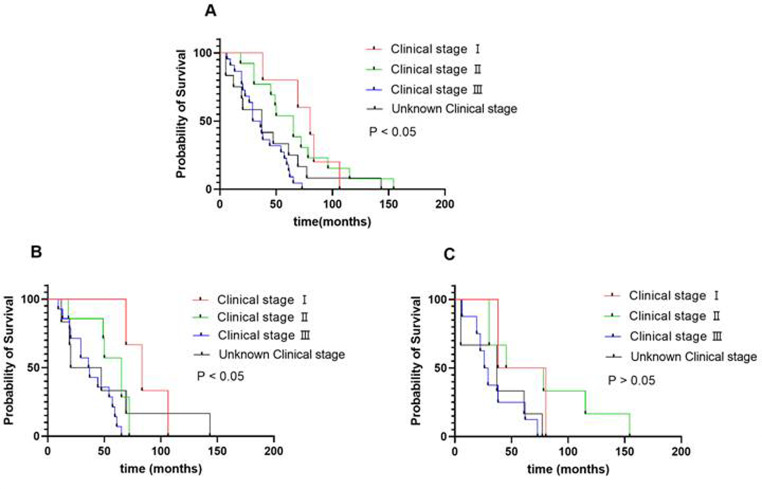
In this study, the median OS for patients with MPE was 44 months. The effect on survival was not significantly different between with MPE and without MPE group (P > 0.05) [Fig. 2]. We conducted a univariate analysis and found that clinical stages at initial diagnosis and histological types for OS were significant, and then into the multivariate Cox regression for analysis [Table 2]. Further, we conducted a subgroup analysis and found that the higher the clinical stage at initial diagnosis in age < 50 years patients, the shorter the overall survival, while age > 50 years patients was not. (P < 0.05) [Fig. 3; Table 3].
Fig. 2.
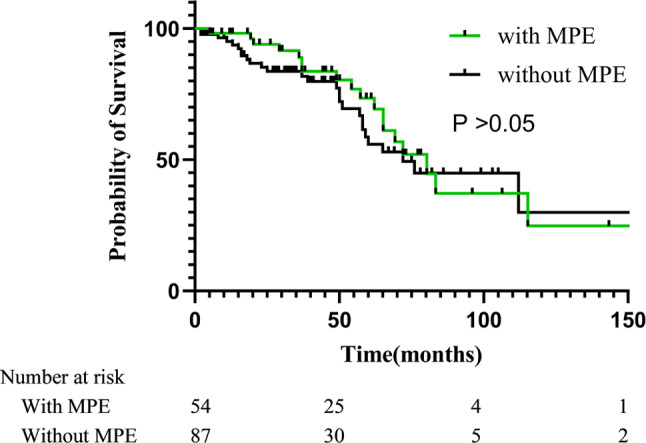
Survival of patients with malignant pleural effusions or without
Table 2.
Univariable and multivariable analyses for overall survival
| Variables | Patients, No |
BC with MPE | P | |
|---|---|---|---|---|
| HR | CI (95%) | |||
| Univariable analyses | ||||
| Age at surgery | ||||
| <50 | 22(40.7%) | REF a | REF | 0.888 |
| ≥50 | 32(59.2%) | 1.041 | 0.597–1.815 | |
| Primary tumor location | 0.963 | 0.551–1.685 | 0.672 | |
| Left | 25(46.3%) | 2.065 | 0.271–15.755 | |
| Right | 26(48.1%) | 2.391 | 0.315–18.141 | |
| Bilateral | 3(5.6%) | REF | REF | |
| Clinical stages at initial diagnosis | 0.036 | |||
| Stage I | 5(9.3%) | 2.244 | 0.488–10.316 | |
| Stage II | 15(27.8%) | 5.837 | 1.262–27.002 | |
| Stage III | 22(40.7%) | 3.626 | 0.756–17.394 | |
| Unknown | 10(18.5%) | REF | REF | |
| Histological type | 0.064 | |||
| Invasive ductal carcinoma | 50(92.6%) | 1.400 | 0.430–4.561 | |
| Invasive lobular carcinoma | 1(1.9%) | 17.669 | 1.493–209.4 | |
| Others | 3(5.6%) | REF | REF | |
| Effusion location | 0.281 | |||
| Right side | 18(33.3%) | 0.506 | 0.204–1.257 | |
| Left side | 14(25.9%) | 0.636 | 0.296–1.370 | |
| Bilateral | 22(40.7%) | REF | REF | |
| Her2 expression | 8(14.9%) | 1.442 | 0.593–3.508 | 0.419 |
| Neoadjuvant | 11(20.4%) | 1.081 | 0.466–2.507 | 0.856 |
| Surgical approach | 49(90.7%) | 1.230 | 0.470–3.213 | 0.673 |
| Multivariable analysis | ||||
| Clinical stages at initial diagnosis | 0.021 | |||
| Stage I | 0.158 | 0.028–0.883 | ||
| Stage II | 0.394 | 0.130–1.195 | ||
| Stage III | 1.350 | 0.504–3.617 | ||
| Unknown | REF | REF | ||
HR, hazard ratio; CI, confidence interval
a For calculation of HR, a group of patients was defined as a reference
b The number of patients was not enough for further calculation
Fig. 3.
A. Overall survival of different clinical stages at initial diagnosis patients with MPE; BC. Overall survival of patients with PE of age < 50 and age > 50 years with different clinical stages
Table 3.
Univariable and multivariable analyses luminal B BC with MPE at different age for overall survival
| Variables | age < 50 years | P | age > 50 years | P | ||
|---|---|---|---|---|---|---|
| HR | CI 95%) | HR | CI (95%) | |||
| Univariable analyses | ||||||
| Clinical stages at initial diagnosis | 0.013 | 0.151 | ||||
| Stage I | 0.103 | 0.018–0.602 | 0.395 | 0.064–2.417 | ||
| Stage II | 0.331 | 0.102–1.075 | 0.294 | 0.066–1.321 | ||
| Stage III | 1.139 | 0.391–3.320 | 1.359 | 0.395–4.678 | ||
| Unknown | REF | REF | REF | REF | ||
| Her2 expression | 2.781 | 0.915–8.449 | 0.071 | 0.818 | 0.269–2.487 | 0.723 |
| Neoadjuvant | 0.954 | 0.422–2.156 | 0.909 | 3.755 | 0.724–19.483 | 0.115 |
| Surgical approach | 2.459 | 0.915–6.605 | 0.074 | 0.152 | 0.016–1.466 | 0.103 |
| Adjuvant Chemotherapy | 0.166 | 0.057–0.486 | 0.001 | 3.975 | 0.514–30.740 | 0.186 |
| Adjuvant Endocrine therapy | 1.019 | 0.461–2.252 | 0.963 | 1.145 | 0.462–2.838 | 0.770 |
| Adjuvant Radiotherapy | 0.563 | 0.270–1.174 | 0.126 | 0.972 | 0.384–2.460 | 0.952 |
| Multivariable analysis | ||||||
| Clinical stages at initial diagnosis | 0.026 | |||||
| Stage I | 0.173 | 0.027–1.112 | - | - | ||
| Stage II | 0.574 | 0.153–2.151 | - | - | ||
| Stage III | 1.887 | 0.600-5.935 | - | - | ||
| Unknown | REF | REF | - | - | ||
Discussion
To the best of our knowledge, this is the first research on luminal B BC patients with MPE that has examined survival. Advanced BC patients with MPE were typically considered to have a worse prognosis than those without. However, as shown in the study, we followed up the 141 patients with advanced Luminal B breast cancer, and statistical results revealed that among these BC patients with or without MPE, the survival rate for patients with MPE was not significantly worse than those without MPE. Accordingly, by shedding light on the prognostic value of MPEs in Luminal B BC, our study has not only filled a gap in the existing literature but also provided a fresh perspective that could potentially lead to improved patient stratification, tailored treatment strategies, and more accurate prognosis predictions.
Luminal B BC as one of the most common of the five related molecular subtypes of breast cancer, which has a higher recurrence risk and a distinctive dissemination pattern. Several studies have suggested luminal BC appear to have a predilection for metastasis to bone and pleura. Besides predilection for the pleura, about 2–11% of patients develop MPEs during the disease course [17, 18]. Moreover, approximately 80% of patients with pleural recurrences develop MPEs within the first 5 years after primary surgery, although pleural recurrences more than 10 years after surgery are rare [19, 20]. Although pleura is a common metastatic site of BC [21], MPE has rarely been reported as the first diagnose in patients with luminal BC. The main reasons the rarely reported of these patients may be underestimated because of the delay in follow-up examinations or the lack of reliable and efficient methods of examination. Conversely, in our research, 38% of luminal B BC patients multisite metastatic breast cancer concomitant with MPE, which was higher than the previous studies. The probable reason for this is that we have a complete follow-up team and have various diagnostic methods of MPE in our organization, thus increasing the corresponding diagnostic rate.
Patients with MPE usually have dyspnea, cough, poor exercise tolerance, an impaired quality of life, and their life expectancy is usually short. In an earlier study of 284 patients with MPE, their median survival time was approximately 13.2 months depending on the stage and histological type of BC [22]. However, our study shows that the median survival time was only 9.8 months in patients diagnosed with luminal B BC after surgery, which was far less in the previous studies. This discrepancy can be attributed to several factors. Firstly, the prognosis of luminal BC patients with multiple metastatic sites was significantly worse than that of patients with single-site metastasis, furthermore, concomitant visceral metastases have a significant negative impact on the patient’s prognosis [23, 24]. In our study, 40.7% of patients with advanced Luminal BC had concurrent lung metastases, and 22.2% had liver metastases, potentially contributing to the poorer prognosis. Further analysis revealed that approximately one-third of patients with liver metastases also had simultaneous lymph node, lung, and bone metastases. Previous studies have shown that untreated liver metastases from breast cancer result in a survival time of only 4–8 months [25]. Secondly, the prognosis may be adversely affected by inadequate interdisciplinary therapeutic approaches for treating Luminal B BC with MPE. Specifically, Luminal-HER2-positive tumors are associated with poor outcomes without systemic targeted therapy. The advent of HER2-directed therapies, including monoclonal antibodies, tyrosine kinase inhibitors (TKIs), and more recently, antibody-drug conjugates (ADCs), has improved the prognosis for patients with advanced Luminal-HER2-positive breast cancer. The DESTINY-Breast03 trial, for example, has established ADC T-DM1 as a preferred second-line treatment in recent years [26]. However, in our study, there was a lack of targeted therapies for this group of advanced luminal-HER2-positive breast cancers for economic reasons or the associated complications. Alternatively, Subclinical multiple organ failure syndrome may also be one of the major causes of poor prognosis in advanced luminal B breast cancer. Unfortunately, because our data lacked specific information on monitoring for subclinical multi-organ failure syndrome, we were unable to examine how multi-organ failure syndrome affected survival rates.
Interestingly, multivariate analysis using the Cox regression model showed that the higher the tumor clinical stage at initial diagnosis, the shorter the OS. Further, we conducted a subgroup analysis and found that the higher the clinical stage in age < 50 years patients, the shorter the overall survival, while age > 50 years patients was not. While, the study boasts a robust evidence base, employing a multi-center dataset and sophisticated statistical analyses to comprehensively evaluate the independent prognostic impact of MPEs. In addition, of the comparison between patients with and without MPEs reinforces the clinical relevance and significance of the findings. Further studies with more patients and prospective design are needed to better interpret the situation.
This study had several limitations. First, because of the retrospective design of the study, different chemotherapy schemas were used depending on the oncologist’s decision, and the indication for surgery depended on the surgeon. Second, the sample size of the luminal B BC with MPE group was small, therefore, the prognostic impact of clinical features in luminal B BC with MPE should be investigated in a larger population in subsequent studies. An additional limitation was that the specific cause of death for these patients was not identified. With regard to the missing clinical stages at initial diagnosis in our dataset, we acknowledge that this is an important limitation that merits clarification. This can be attributed to several factors, including the retrospective nature of our data collection, variations in documentation practices across institutions, and the potential for incomplete medical records. And we have conducted sensitivity analyses to assess the impact of excluding patients with unknown clinical stages on our main findings. These analyses help us understand the robustness of our results and the potential bias introduced by the missing data. Despite these limitations, this is the first comprehensive report on the clinical features and survival of patients with luminal B presenting with MPE.
Conclusions
In conclusion, there was no difference in the overall survival between luminal B-BC patients with MPE and those without. Clinical stages at initial diagnosis were an independent prognostic factor for age < 50 years luminal B BC with MPE overall survival. Our results may help clinicians make positive decisions regarding personalized treatment of luminal B-BC with MPE.
Acknowledgements
We are also grateful to Tianjin Cancer Hospital for providing the data essential for this research.
Author contributions
Conception and design: Youming Han and Yan Dong; Administrative support: Youming Han; Provision of study materials or patients: Hailong Wang and Han Youming; Collection and assembly of data: Youming Han, Yan Dong, and Hailong Wang; Data analysis and interpretation: Youming Han and Yan Dong; Manuscript writing: Yongming Han; Manuscript revision: Xiang-Min Li, Xiao-Zhang, Xin-Yu Wei, Feng-Wen Qian, and Zhi-Gang Li. Final approval of manuscript: All authors.
Funding
This work was supported by TianjinBinhai New Area Health Research Project (2023BWKY008).
Data availability
All data supporting the findings of this study are available within the paper.
Declarations
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and has received ethical approval from the Institutional Review Board (IRB) of the Tianjin Cancer Hospital and The Affiliated General Hospital Binhai Hospital of Tianjin Medical University, approval number 20240101. Patients were consented by an informed consent process that was reviewed by the Ethics Committee of the Affiliated General Hospital Binhai Hospital of Tianjin Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
You-ming Han and Yan-Dong contributed equally to this work and considered co-first authors.
References
- 1.Hassan M, M R, Mercer NA, Maskell R, Asciak DJ, McCracken E, et al. Survival in patients with malignant pleural effusion undergoing talc pleurodesis. Lung Cancer. 2019;137:14–8. [DOI] [PubMed]
- 2.Li W, Zheng Y, Wu H, Li X. Breast-conserving therapy versus mastectomy for breast cancer: a ten-year follow-up single-center real-world study. Gland Surg. 2022;11(7):1148–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shameek Gayen. Malignant pleural effusion: presentation, diagnosis, and management. Am J Med. 2022;135(10):1188–92. [DOI] [PubMed] [Google Scholar]
- 4.Peel AM, Mishra EK. The Psychosocial Impact of Indwelling Pleural catheters: a scoping review. Cureus. 2023;15:e41689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina S, Martinez-Zayas G, Sainz PV, et al. Breast and lung effusion survival score models: improving survival prediction in patients with malignant pleural effusion and metastasis. Chest. 2021;160:1075–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark E, Roberts NM, Rahman NA, Maskell AC, Bibby, Kevin G, Blyth JP, Corcoran A, Edey J, McCracken R, Mercer, Eleanor K, Mishra, Andrew G, Nicholson. Farinaz Noorzad, Kirstie Opstad, Maria Parsonage, Andrew E Stanton, Steven Walker. British thoracic Society Guideline for pleural disease. Thorax. 2023;78: 1–42.
- 7.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. [DOI] [PubMed] [Google Scholar]
- 9.Ian H, Kunkler LJ, Williams, Wilma JL, Jack DA, Cameron JM, Dixon. Breast-conserving surgery with or without irradiation in early breast Cancer. J N Engl J Med. 2023;388(7):585–94. [DOI] [PubMed] [Google Scholar]
- 10.Majid Akrami P, Arasteh T, Eghbali HR, Shahraki S, Tahmasebi V, Zangouri A, Rezaianzadeh, Abdolrasoul Talei. Introducing novel and comprehensive models for predicting recurrence in breast cancer using the group LASSO approach: are estimates of early and late recurrence different? World J Surg Oncol. 2018;16(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard Schmiester N, Kristensen A, Frigessi. Aleix Prat, Alvaro Köhn-Luque. Computational Model Predicts Patient Outcomes in Luminal B Breast Cancer Treated with Endocrine Therapy and CDK4/6 Inhibition.Clin Cancer Res. 2024; 30(17):3779–3787. [DOI] [PubMed]
- 12.Vo TH, Abdelaal EE-S, Jordan E. Orla O’Donovan, Edel A McNeela, Jai Prakash Mehta, Sweta Rani. miRNAs as biomarkers of therapeutic response to HER2-targeted treatment in breast cancer: a systematic review. Biochem Biophys Rep. 2023;37:101588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu K, Wang Y, Qi J, et al. Analysis of prognostic factors and clinical characteristics for patients with Limited Stage Small Cell Lung Cancer with Pleural Effusion. Zhongguo Fei Ai Za Zhi. 2018;21(1):16–23. 10.3779/j.issn.1009-3419.2018.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arellano-Orden E, Romero-Romero B, Sánchez-López V, et al. Survivin is a negative prognostic factor in malignant pleural effusion. Eur J Clin Invest. 2018;48(4). 10.1111/eci.12895. [DOI] [PubMed]
- 15.Goldhirsch A, Wood WC, Coates AS, et al. Panel members. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–47. 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Wolff AC, et al. American society of clinical oncology/college of American pathologist’s guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–7. 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofia Molina G, Martinez-Zayas PV, Sainz CH, Leung L, Li HB, Grosu. Roberto Adachi, David E Ost. Breast and lung effusion survival score models: improving survival prediction in patients with malignant pleural effusion and metastasis. Chest. 2021;160(3):1075–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cansu E, Önder, Teresa J, Ziegler R, Becker, Sara Y, Brucker AD, Hartkopf T, Engler. André Koch. Advancing Cancer therapy predictions with patient-derived Organoid models of metastatic breast Cancer. Cancers (Basel). 2023;15(14):3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karampinis I, Dionysopoulou A, Galata C, Almstedt K, Grilli M, Hasenburg A, et al. Hyperthermic intrathoracic chemotherapy for the treatment of malignant pleural effusion caused by breast and ovarian cancer: a systematic literature review and pooled analysis. Thorac Cancer. 2022;13(7):883–8. [DOI] [PMC free article] [PubMed]
- 20.Chen L, Meng Z, Zhou Z, Li X, Zhao L, Jia Z, Chen J, Tian Y. Qingju Meng, Yibing Liu. Immunotherapy Combined with Chemotherapy in Relapse metaplastic breast Cancer. Onco Targets Ther. 2023;16:885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Li C, Shao W, Liu X. Luhao Sun, Zhiyong Yu. Survival analysis and prognosis of patients with breast cancer with pleural metastasis. Front Oncol. 2023;13:1104246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielsa S, Salud A, Martínez M, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med. 2008;19(5):334–9. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura J, Kamigaki S, Fujita J, Osato H, Manabe H, Tanaka Y, et al. New insights into patterns of first metastatic sites influencing survival of patients with hormone receptor-positive, HER2-negative breast cancer: a multicenter study of 271 patients. BMC Cancer. 2021;21(1):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer (Oxford Engl 1990). 2020;129:60–70. [DOI] [PubMed] [Google Scholar]
- 25.Laura A, Huppert Z, Walker M, Li M-O, Kim J, Callan D, Brandman M, Majure ME, Melisko, Hope S, Rugo S, Behr. A Jo Chien. Clinical characteristics and outcomes in patients with metastatic breast cancer and pseudocirrhosis: a single center retrospective cohort study. Breast Cancer Res Treat. 2023;197(1):137–48. [DOI] [PubMed] [Google Scholar]
- 26.Sara A, Hurvitz R, Hegg W-P, Chung S-A, Im W, Jacot V, Ganju JWY, Chiu B, Xu E, Hamilton S, Madhusudan H, Iwata S, Altintas J-W, Henning S-B, Kim V, Petry C-S, Huang W, Li J-S, Frenel. Silvia Antolin, Winnie Yeo, Giampaolo Bianchini, Sherene Loi, Junji Tsurutani, Anton Egorov, Yali Liu, Jillian Cathcart, Shahid Ashfaque, Javier Cortés. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023, 401(10371):105–117. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the paper.