Abstract
Background
Celiac plexus block has been commonly utilized for the treatment of chronic pancreatitis-associated abdominal pain. Prospective studies suggest efficacy in 30 to 50% of patients, although no randomized sham-controlled trials have been performed. The objective of this study is to assess the effect of endoscopic ultrasound (EUS)-guided celiac plexus block on abdominal pain in patients with documented chronic pancreatitis.
Methods
This is a two-arm randomized sham-controlled trial with blinded evaluators. The study will be conducted at multiple academic sites in the United States who are members of the United States Pancreatic Disease Study Group (USPG). Patients referred for EUS to exclude chronic pancreatitis as a cause of abdominal pain as well as those with established painful chronic pancreatitis undergoing EUS for another indication will be eligible. At the time of EUS with confirmation of chronic pancreatitis by standard EUS diagnostic criteria, patients will be randomized to either celiac plexus block or sham whereby an anesthetic and steroid combination will be injected into the celiac plexus or saline will be injected into the gastric lumen with the same type of needle as used for celiac plexus block, respectively. The main outcome measure will be a 50% reduction in abdominal pain using the Brief Pain Inventory Short Form (BPI-SF) at 1 month post-intervention. A number of secondary outcomes will be measured including visual analog scale (VAS), Comprehensive Pain Assessment Tool Short Form (COMPAT-SF) pain scores, and quality of life using a pancreas-specific validated measure (PANQOLI).
Discussion
In this study, the effect of celiac plexus block on abdominal pain in patients with chronic pancreatitis will be compared to a sham intervention. This randomized trial will offer a definitive assessment of the role of celiac plexus block for the treatment of abdominal pain in this setting.
Trial registration {2}
ClinicalTrials.gov NCT 06178315. Registered on December 21, 2023
Keywords: Chronic pancreatitis, Celiac plexus block, Abdominal pain, Endoscopic ultrasonography
Introduction
Background and rationale {6a}
Chronic pancreatitis is a complex fibroinflammatory disease arising from numerous etiological factors and with a variety of clinical manifestations [1]. Although poorly understood [2], abdominal pain is the most debilitating complication and can result in significant morbidity and impact on quality of life. Studies demonstrate that abdominal pain is the major cause for reduction in quality of life as well as disability [3–5]. When mild, medical therapy may be effective. When the pain is more severe, narcotics can be helpful, although with its inherent significant side effects. For many years, surgery has been the mainstay of therapy but is generally limited to those with a dilated pancreatic duct. Total pancreatectomy may be considered in very selected cases; however, given the significant risks and potential morbidity associated with pancreatic surgery, less invasive therapies for pain should be first attempted [6].
Celiac plexus block (CPB) has been employed for almost four decades for the treatment of debilitating abdominal pain [7–10]. Traditionally, blocks were given percutaneously using fluoroscopy or by computed tomographic (CT) guidance. Given its ability to visualize the celiac axis and use of Doppler to identify the surrounding vasculature, endoscopic ultrasonography (EUS) has been increasingly utilized for CPB. Injection of alcohol (celiac plexus neurolysis) is typically performed for patients with malignancy whereas use of a steroid in combination with a local anesthetic is used for patients with benign disease like chronic pancreatitis.
Prior studies evaluating the efficacy of EUS-CPB, including two prior meta-analyses [11, 12], have shown EUS-CPB to be safe and effective with an overall efficacy of approximately 50%. However, data from adequately powered, prospective, well-designed randomized trials are lacking. In addition, no sham-controlled trials have been performed in this setting which are critically important to perform when any intervention is used to treat pain [13].
Objectives {7}
The primary aim of the study is to evaluate the efficacy of EUS-CPB on relief of abdominal pain in patients with chronic pancreatitis in comparison to a sham group measured by the Brief Pain Inventory Short Form (BPI-SF). This measure contains 11 items which evaluate the severity and interference of pain with daily functioning [14]. It is widely utilized in clinical trials and can be rapidly completed. Secondary outcomes include pain scores using the VAS as well as COMPAT-SF score the latter of which is a validated pain measurement in chronic pancreatitis [15]. Additional secondary outcomes included technical success, procedure-related adverse events, pain medication usage, need for hospitalization or emergency room visits during the period of observation, quality of life [16, 17], and cross-over to EUS-CPB for those randomized to sham.
Trial design {8}
This is a two-arm randomized sham-controlled trial with the patient and evaluator blinded to the group allocation. The study is reported in accordance with established clinical trial reporting standards (CONSORT). The study has been approved by the institutional review board at Orlando Health (protocol number 2103069-1; 12.7.2023) and by the local review boards at other participating sites.
Methods: participants, interventions, and outcomes
Study setting {9}
The study will be performed at the Medical Centers as listed. Each center received approval from their institutional review board. The study has been registered with ClinicalTrials.gov NCT 06178315.
Eligibility criteria {10}
All patients with a diagnosis of abdominal pain consistent with chronic pancreatitis and whereby EUS demonstrates characteristic changes of chronic pancreatitis [18] will be eligible.
The inclusion criteria are as follows:
Age ≥ 18 years
Abdominal pain of at least 3 months duration and a visual analog score (VAS) greater than or equal to 3, with or without the use of narcotic analgesics
Diagnosis of chronic pancreatitis on EUS examination, with ≥5 features on EUS [18]
No other cause of abdominal pain clinically or by EUS
The exclusion criteria are as follows:
Age < 18 years
Use of anticoagulants that cannot be discontinued for the procedure
Clinically significant allergy to bupivacaine or triamcinolone
Unable to obtain consent for the procedure from either the patient or designate
Intrauterine pregnancy
Prior EUS or other endoscopic procedure to treat abdominal pain
Who will take informed consent? {26a}
Informed consent will be performed by a dedicated blinded research coordinator. Informed consent will be signed by the participant after the study design and rationale have been explained and prior to the endoscopic procedure.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
This is not applicable for the study.
Interventions
Explanation for choice of comparators {6b}
The CPB procedure technique (see below) is the current standard used by the majority of endosonographers. No sham trial has been previously performed. Use of the same brand of needle with injection of saline into the stomach will best mimic the actual procedure and further help with blinding. Placing the needle in the celiac axis without any injection of a placebo represents an increased risk and could theoretically exacerbate pain.
Intervention description {11a}
Patients with chronic abdominal pain suspected to be secondary to chronic pancreatitis, who have been referred for diagnostic EUS examination, will be enrolled in the study. Additional patients with established painful chronic pancreatitis by cross sectional imaging undergoing EUS for other indications are also eligible. Prior to the EUS examination, the patients will be seen by a member of the research team to discuss the study, obtain consent, and administer assessment tools for pain and quality of life—VAS score, COMPAT-SF [15], and the PANQOLI [16, 17] as well as obtain additional demographic and pertinent medical history.
Celiac plexus block—intervention group
Day 0: index intervention
All endoscopic interventions will be performed in the endoscopy unit.
All patients will be placed in the left lateral decubitus position and undergo monitored anesthesia care (MAC) using propofol or other anesthetic agents, administered by staff anesthesiologists or nurse anesthetists.
Carbon dioxide will be used in all cases for insufflation.
Linear echoendoscope will be used (Olympus America Inc., Center Valley, PA, USA, Pentax Medical, NJ, USA, or Fujifilm Inc., Valhalla, NY) in all cases.
Pre-procedure antibiotics will not be administered.
In all patients, the pancreas will be examined using EUS to identify features of chronic pancreatitis. Diagnosis of chronic pancreatitis is defined as the presence of ≥5 (of 9) features on EUS based on standard (classic) criteria [18]. The criteria comprise the following 9 features: hyperechoic foci, hyperechoic strands, lobularity, calcifications/stones, cysts, main pancreatic duct dilation, main pancreatic duct irregularity, hyperechoic main pancreatic duct walls, and visible side branches.
If the patient does not meet EUS criteria for chronic pancreatitis, then they will be considered a screen failure and will be removed from the study.
Once the diagnosis of chronic pancreatitis is established by EUS, the randomization envelope will be opened by the endoscopist intra-procedurally.
- Depending on the randomization assignment, patient will undergo either:
-
A.EUS-CPB:
- The area of the celiac plexus is identified as the site of celiac artery take-off from the aorta on EUS.
- A 22-gauge FNA needle (Expect; Boston Scientific, Marlborough, MA, USA) is inserted into the area of celiac plexus.
- Using both color Doppler and aspiration to confirm that the needle tip is not intravascular, 10 mL of 0.25% bupivacaine, followed by 80 mg of triamcinolone, is injected into the celiac plexus. One injection will be made into the celiac plexus.
-
B.Sham:
- A 22-gauge FNA needle (Expect; Boston Scientific, Marlborough, MA, USA) is inserted into the echoendoscope.
- Ten milliliters of normal saline solution will be injected into the lumen of the gastric body.
- After completion of the EUS procedure, the echoendoscope will be withdrawn from the patient and the patient will be taken to the recovery area for routine post-procedure monitoring and discharge as clinically indicated by the treating physician.
- Given the differences in procedural techniques, endoscopists will not be blinded to the treatment allocation. However, study participants and research coordinators who conduct the outcome assessments will be blinded to the type of intervention performed.
-
A.
Following the procedure, we will use the following script for staff and patients.
We will document in the procedure note as follows: “Patient was recruited for participation in the randomized trial comparing celiac plexus block versus sham therapy and was assigned to a treatment arm.” We will bill/code as we would for any EUS—43259.
After 1 month, we will make an addendum to the procedure report and add CPT code 43253 if in fact they received a block treatment.
For the patient, we will tell beforehand that “they may or may not get a block based on randomization” and that only at 1 month, we will inform them of the treatment delivered.
For the staff, we will tell them that the patient participated in a clinical trial evaluating treatment options for chronic pancreatitis.
24 h post-index procedure (±7 days)
The following information will be obtained from all patients at 24 h post-procedure:
Adverse events related to the procedure or chronic pancreatitis
Hospitalization or emergency room visit since the index procedure
2 weeks post-index procedure (±7 days)
Telephone calls are made by the research personnel to all patients at 2 weeks post-index procedure (±7 days) to collect the following information:
Adverse events related to the procedure or underlying disease since last follow-up
Information on any repeat radiological imaging since last follow-up
Interim hospitalization or emergency department visit since last follow-up
Any additional endoscopic, radiological, or surgical interventions performed for abdominal pain since last follow-up
Pain scores measured using the BPI-SF
Pain scores measured using the VAS
Pain scores measured using the COMPAT-SF
Quality of life measured using the PANQOLI
Pain medications regime
1 month post-index procedure (±7 days)
- Telephone calls are made by the research personnel to all patients at 1 month post-index procedure (±7 days) to collect the following information:
- Adverse events related to the procedure or underlying disease since last follow-up.
- Information on any repeat radiological imaging since last follow-up.
- Interim hospitalization or emergency department visit since last follow-up.
- Any additional endoscopic, radiological, or surgical interventions performed for abdominal pain since last follow-up.
- Pain scores measured using the BPI-SF.
- Pain scores measured using the VAS.
- Pain scores measured using the COMPAT-SF.
- Quality of life measured using the PANQOLI.
- Pain medication regime—morphine equivalent intake over 24 h.
- At 1 month, all patients will be asked which group they feel they were assigned to as a guide to the strength of the blinding.
Patients originally assigned to the sham group and without adequate pain relief at 1-month post-index intervention (i.e., without a 50% reduction in pain from the baseline VAS pain score) at 1-month follow-up can be crossed over to the CPB group. Those crossed over will have unblinded assessments to 1 month as before.
3 months post-index procedure (±7 days)
- Telephone calls will be made by the research personnel to all patients at 3 months post-index procedure (±7 days) to collect the following information:
- Adverse events related to the procedure or underlying disease since last follow-up
- Information on any repeat radiological imaging since last follow-up
- Interim hospitalization or emergency department visit since last follow-up
- Any additional endoscopic, radiological, or surgical interventions performed for abdominal pain since last follow-up
- Pain scores measured using the BPI-SF
- Pain scores measured using the VAS
- Pain scores measured using the COMPAT-SF
- Quality of life measured using the PANQOLI
- Pain medications regime
Criteria for discontinuing or modifying allocated interventions {11b}
There will be no change in assignments. Following the study completion, if a patient who is randomized to sham wishes to have the intervention, they will be scheduled accordingly. If a patient is lost to follow-up, the analysis will be by intention to treat.
Strategies to improve adherence to interventions {11c}
Patients will be given a copy of the original baseline forms to be used for comparison on follow-up. Before discharge from the unit, patients will be given appointment cards for the dates of the subsequent phone calls and the assessment documents will again be reviewed.
Relevant concomitant care permitted or prohibited during the trial {11d}
All patients will be managed as is standard of care. Pain medications can be adjusted by the primary care physician and any additional abdominal imaging is permitted. Any admission to the hospital or emergency room visit will be recorded. Patients will be urged to not undergo any additional interventions for pain unless they have had no response, and the pain becomes unbearable.
Provisions for post-trial care {30}
The participants will be given the phone numbers of the research coordinator and the principal investigator at each site if questions should arise. In addition, if for some reason the blind needs to be broken, the principal investigator can be contacted. These numbers will also be in the blinded procedure note. These procedures are generally safe, but patients who experience any complication will be referred to the appropriate emergency department.
Outcomes {12}
The main outcome measure will be comparison of BPI-SF and with a 50% reduction in BPI-SF. Additional outcome measures will be pain scores using the VAS, COMPAT-SF scores, and quality of life using the PANQOLI. In addition, any adverse events, hospitalizations, or need for any other intervention will be assessed.
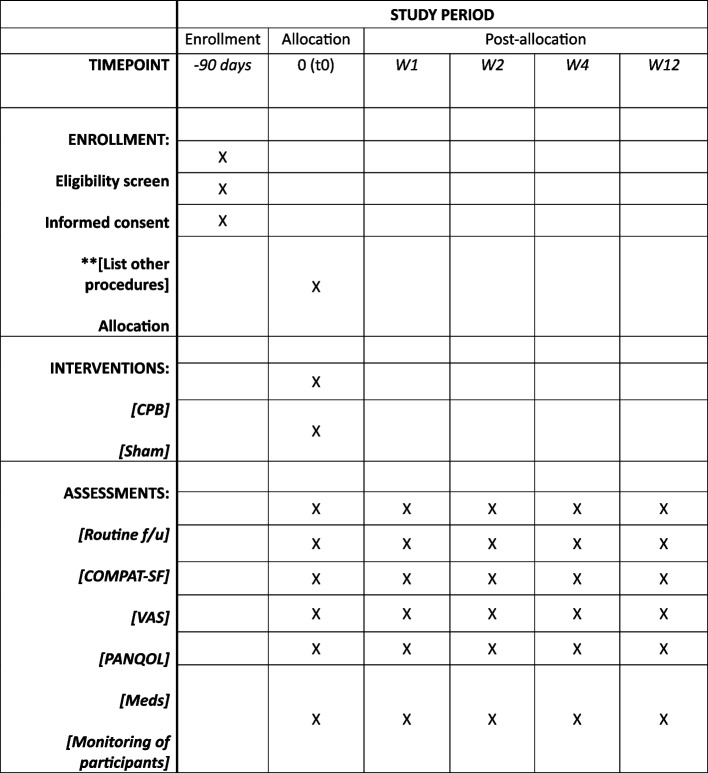
Participant timeline {13}
Figure 1 displays the participant timeline and assessments.
Fig. 1.
Schedule of enrollment, interventions, and assessments. Abbreviations: COMPAT-SF, Comprehensive Pain Assessment Tool Short Form; CPB, celiac plexus block; PANQOL, pancreatitis quality of life instrument; VAS, visual analog scale; W, week; Meds, medications
Sample size determination {14}
A two-sided sample size calculation was performed to detect a 30% difference in the primary outcome measure, based on the primary outcome of the proportion of patients with at least a 50% reduction in the composite pain scores on BPI at 1-month follow-up. To detect a difference of 30% between the CPB group and the placebo group (1-10) (assuming 60% for CPB and 30% for placebo and no differences across sites), with 80% power, two-sided 0.05 alpha level likelihood ratio test, the estimated sample size was 84 patients (42 per treatment group) and hence was set at 94 patients (47 patients per treatment group) to account for a 10% dropout rate (PASS 15 Power Analysis and Sample Size Software, NCSS, LLC., Kaysville, UT, USA).
Recruitment {15}
Patients will be recruited from those seen in the participating centers’ endoscopy unit undergoing EUS. Patients referred for EUS because of abdominal pain to exclude chronic pancreatitis will be eligible. There will be participants with established chronic pancreatitis and abdominal pain undergoing EUS for some other indication at which time if they meet the inclusion criteria can be enrolled.
Assignment of interventions: allocation
Sequence generation {16a}
The selected participants will be allocated into 2 groups, group 1 will be the active intervention and group 2 the sham group using a simple randomization process. Each participant will have an equal probability of being randomly allocated to either group.
Concealment mechanism {16b}
Each site will have concealed allocation cards. They will be numbered consecutively in opaque envelopes. The envelopes will be sealed and then will be stored in a secure cabinet. Randomization and concealed allocation will be carried out by the principal researcher. The researcher in charge of administering the treatments will open the envelopes just prior to the intervention. Following the procedure, the envelope will be resealed and given to the research coordinator.
Implementation {16c}
Two independent research coordinators blinded to the treatment allocation will be collecting interval data at 2 weeks, 4 weeks, and 12 weeks. This data will be collected by phone and all participants will be given a copy of the original data forms to be reviewed at each phone call.
Assignment of interventions: blinding
Who will be blinded {17a}
The research nurses evaluating the patient at 2 days, 2 weeks, 4 weeks, and 12 weeks will be blinded to the randomization and intervention. They will only be responsible for collecting the evaluation documents and will receive no information regarding the group randomized. The principal researcher and endoscopist responsible for the treatment will not be blinded due to the nature of the interventions.
Procedure for unblinding if needed {17b}
The evaluators would not be allowed to unlock the blinding. If for some reason the blind needs to be broken, for example, a significant adverse event, then the principal investigator at each site will be contacted as they will have the information regarding the group.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Following completion of the study, the principal investigator will receive all data forms. The data will then be uploaded to an Excel spreadsheet and stored on a password-protected institutional computer.
Plans to promote participant retention and complete follow-up {18b}
Given the short duration of the study, we hope there is minimal dropout. We will communicate weekly with the patient by text and/or email to encourage continued participation. No specific monetary compensation will be provided.
Data management {19}
The principal investigator will be responsible for data management.
Confidentiality {27}
Human subjects’ names will be kept on a password-protected file and will be linked only with a study identification number for this research. The study identification number will be kept separate from the data collected and will be destroyed according to Orlando Health policy once all data is collected and before data analysis. All data will be entered using Orlando Health computers according to Orlando Health policies for data storage. De-identified data from other centers will be sent electronically to the principal investigator at Orlando in a password-protected file and stored in password-protected Orlando Health computers according to Orlando Health policies for data storage. All data will be stored in a locked office of the investigators and maintained for a minimum of 3 years after the completion of the study.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
This is not applicable to this study.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Baseline characteristics of the recruited patients, clinical details, technical, and procedure outcomes will be summarized by study arm as means (with standard deviation) and medians (with interquartile range and range) for continuous data and as frequencies and proportions for categorical data. For comparison of categorical data between arms, chi-square or Fisher’s exact test will be used as indicated, whereas the two-sample t-test or the Wilcoxon rank-sum test will be used as appropriate for comparison of continuous data. Logistic regression analysis will be performed with the primary outcome measure as the response variable. Time-to-event analysis will be performed using Cox regression model. Poisson regression model (or negative binomial model) will be performed for count data and analysis of quality-of-life data will be performed.
An intention to treat analysis will be performed. Statistical significance will be determined as p < 0.05 and two-sided p values will be reported for comparison of all outcome measures. 95% confidence intervals will be reported as indicated. All statistical analyses will be performed using Stata 17 (Stata Corp, College Station, TX, USA).
Interim analyses {21b}
No interim analysis is planned.
Methods for additional analyses {20b}
Subgroup analysis may be compared based upon duration and severity of pain, severity of disease based on EUS criteria, age/gender, and medication usage.
Methods and analysis to handle protocol nonadherence and any statistical methods to handle missing data {20c}
Patients failing to complete the study will be handled as intent to treat.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
Not applicable to this study.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
Orlando Health will be the coordinating center. All data forms and information will be sent to Orlando Health for computer entry.
Composition of the data monitoring committee, its role and reporting structure {21a}
The principal investigator as well as other investigators at Orlando Health will monitor the study. We will review study progress and any unforeseen difficulties with study implementation at 3 monthly intervals.
Adverse events reporting and harms {22}
Any adverse events or complications will be reported to the appropriate institutional review board.
Frequency and plans for auditing trial conduct {23}
Not applicable for this study.
Plans for communicating important protocol amendments to relevant parties {25}
Any changes to the study protocol will be disseminated to all investigators.
Dissemination plans {31a}
The results of the study will be published in a scientific journal.
Discussion
Abdominal pain is a major complication of chronic pancreatitis. In this setting, pain is difficult to treat and is associated with significant debility, disability, and reduction in quality of life [3–5, 19, 20]. When more severe, management of pain is often first initiated with narcotics, but this has many potential downsides. Multimodality medical therapy including narcotics, gabapentin/pregabalin, and antidepressants have been used with variable effectiveness [21, 22]. In patients with longstanding pain, central sensitization may be a mechanism for pain requiring a different approach to management [2]. While technical success is high, endoscopic therapy is generally unsatisfactory [23, 24]. Surgery can be effective but is applicable to very select patients [25]. Therefore, effective therapies are desperately needed.
Blockade of the celiac plexus to treat abdominal pain has been used for many years but with variable results. In patients with chronic pancreatitis, a number of studies have evaluated the role of CPB both percutaneously and with EUS guidance. Response rates vary from ~30 to 50% with EUS-CPB which is superior to a percutaneous approach [11, 12]. Unfortunately, these studies have rarely been randomized and there has only been one published sham-controlled trial to our knowledge of any therapy to treat abdominal pain in the setting of chronic pancreatitis [26]. We all recognize the high placebo response rate for any intervention for pain thus necessitating a sham-controlled trial for definitive assessment of efficacy [27–29].
Given this well-structured protocol using state-of-the-art pancreas-specific outcome measures, this trial will provide a definitive assessment of the role of EUS-CPB in the treatment of chronic pancreatitis-associated abdominal pain. The results will have significant implications for management.
Trial status
Protocol version #1 recruitment to begin 1.1.2024. Recruitment is estimated to be completed by 12.15.2025.
Acknowledgements
Not applicable.
Members of the US Pancreatic Disease Study Group
C. Mel Wilcox, MD1, Ji Young Bang, MD1, Akwi Asombang, MD2, Chloe Bennett, MD3, Yan Bi, MD4, Wojciech Blogowski, MD5,6, James Buxbaum, MD7, Wei-Shen Chin, MD8, Darwin Conwell, MD9, Gregory A. Coté, MD10, Timothy B. Gardner, MD11, Pramod Garg, MD12, Nalini Guda, MD13, Robert Hawes, MD1, Yasmin G. Hernandez-Barco, MD2, Emily Jonica, MD10, Prashant Kedia, MD14, Thomas Kowalski, MD15, Vivek Kumbhari, MD4, Linda Lee, MD16, Jorge Machicado, MD17, Desiree Morgan, MD18, Thiruvengadam Muniraj, MD3, Udayakumar Navaneethan, MD1, Veeral Oza, MD19, Swati Pawa, MD20, Rajesh Puri, MD21, Amit Rastogi, MD22, D. Nageshwar Reddy, MD23, Monica Saumoy, MD24, Mandeep Sawhney, MD25, Santhi Swaroop Vege, MD26, Rupjyoti Talukdar, MD23, Paul Tarnasky, MD14, Felix Tellez-Avila, MD27, Shyam Thakkar, MD28, Nikhil Thiruvengadam, MD29, Elaina Vivian, MPH14, Irving Waxman, MD5, Field F. Willingham, MD30, Shyam Varadarajulu, MD1
1Digestive Health Institute, Orlando Health, Orlando, FL; 2Harvard, Massachusetts General Hospital, Boston, MA; 3Yale School of Medicine, New Haven, CT; 4Mayo Clinic, Jacksonville, FL; 5Rush University Medical Center, Chicago, IL; 6University of Zielona Gora, Poland; 7Keck Medicine of USC, Los Angeles, CA; 8Department of Radiology, Orlando Health, Orlando, FL; 9University of Kentucky College of Medicine, Lexington, KY; 10Oregon Health & Science University, Portland, OR; 11Dartmouth Geisel School of Medicine, Hanover, NH; 12Department of Gastroenterology at All India Institute of Medical Sciences, New Delhi, India; 13Aurora Health Care, Milwaukee, WI; 14Methodist Health, Dallas, TX; 15Jefferson Health, Philadelphia, PA; 16Harvard, Brigham and Women’s Hospital, Boston, MA; 17University of Michigan, Ann Arbor, MI; 18Department of Radiology, University of Alabama, Birmingham, AL; 19Prizma Health, Greenville, SC; 20Wake Forest University School of Medicine, NC; 21Institute of Digestive & Hepatobiliary Sciences, Medanta, Gurgaon, Haryana, India; 22University of Kansas Medical Center, Kansas City, KS; 23Asian Institute of Gastroenterology, Hyderabad, India; 24Penn Medicine Princeton Medical Center, Philadelphia, PA; 25Beth Israel Deaconess Hospital, Boston, MA; 26Mayo Clinic, Rochester, MN; 27University of Arkansas Medical System, Little Rock, AR; 28West Virginia University, Morgantown, WV; 29Loma Linda University Health, Loma Linda, CA; 30Emory University Department of Medicine, Atlanta, GA.
Abbreviations
- EUS
Endoscopic ultrasound
- CPB
Celiac plexus block
Authors’ contributions
CW, JB, and SV initially conceptualized and designed the study. The manuscript was written by CW. All authors read and approved the final manuscript.
Funding {4}
There is no specific funding for the study.
Availability of data and materials {29}
All authors will have complete access to the data.
Declarations
Ethics approval and consent to participate {24}
The institutional reviews boards for each center have approved the study.
Consent for publication {32}
No personal-identifying details will be published. All authors have consented to publication of the study when completed.
Competing interests {28}
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. Mel Wilcox, Email: charles.wilcox@orlandohealth.com.
on behalf of the US Pancreatic Disease Study Group:
C. Mel Wilcox, Ji Young Bang, James Buxbaum, Timothy B. Gardner, Robert Hawes, Prashant Kedia, Thiruvengadam Muniraj, Udayakumar Navaneethan, Paul Tarnasky, Shyam Thakkar, Irving Waxman, Shyam Varadarajulu, Akwi Asombang, Chloe Bennett, Yan Bi, Wojciech Blogowski, Wei-Shen Chin, Darwin Conwell, Gregory A. Coté, Pramod Garg, Nalini Guda, Yasmin G. Hernandez-Barco, Emily Jonica, Thomas Kowalski, Vivek Kumbhari, Linda Lee, Jorge Machicado, Desiree Morgan, Veeral Oza, Swati Pawa, Rajesh Puri, Amit Rastogi, D. Nageshwar Reddy, Monica Saumoy, Mandeep Sawhney, Santhi Swaroop Vege, Rupjyoti Talukdar, Felix Tellez-Avila, Nikhil Thiruvengadam, Elaina Vivian, and Field F. Willingham
References
- 1.Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: an international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drews AM, Bouwense SA, Campbell CM, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720–31. [DOI] [PubMed] [Google Scholar]
- 3.de Rijk F, van Veldhuisen C, Kempeneers M, et al. Quality of life in patients with definite chronic pancreatitis: a nationwide longitudinal cohort study. Am J Gastroenterol. 2023;118:1428–38. [DOI] [PubMed] [Google Scholar]
- 4.Machicado JD, Amann ST, Anderson MA, et al. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking, and associated co-morbidities. Am J Gastroenterol. 2017;112:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blachnio K, Szymocha A, Kovalsky M, et al. Quality of life and pain in patients with chronic pancreatitis. Pancreas. 2023;52:321–7. [DOI] [PubMed] [Google Scholar]
- 6.Abu-El-Haija M, Anazawa T, Beilman GJ, et al. The role of total pancreatectomy with islet autotransplantation in the treatment of chronic pancreatitis: a report from the international consensus guidelines in chronic pancreatitis. Pancreatology. 2020;20:762–71. [DOI] [PubMed] [Google Scholar]
- 7.Bell S, Cole R, Roberts-Thomson I. Coeliac plexus block for control of pain in chronic pancreatitis. Br Med J. 1980;281:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900–5. [DOI] [PubMed] [Google Scholar]
- 9.Stevens T, Costanzo A, Lopez R, Kapural L, Parsi MA, Vargo JJ. Adding triamcinolone to endoscopic ultrasound-guided celiac plexus blockade does not reduce pain in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10:186–91. [DOI] [PubMed] [Google Scholar]
- 10.ASGE Standards of Practice Committee, Sheth SG, Machicado, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of chronic pancreatitis: summary and recommendations. Gastrointest Endosc. 2024:S0016–5107. In press. [DOI] [PubMed]
- 11.Kaufman M, Singh G, Das S, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–34. [DOI] [PubMed] [Google Scholar]
- 12.Puli SR, Reddy JBK, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pan: a meta-analysis and systemic review. Dig Dis Sci. 2009;54:2330–7. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox CM. Tinkering with a tarnished technique: isn’t it time to abandon celiac plexus blockade for the treatment of abdominal pain in chronic pancreatitis? Clin Gastroenterol Hepatol. 2012;10:106–8. [DOI] [PubMed] [Google Scholar]
- 14.Hohenschurz-Schmidt D, Cherkin D, Rice ASC, et al. Methods for pragmatic randomized clinical trials of pain therapies: IMMPACT statement. Pain. 2024;00:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhlmann L, Teo K, Olesen SS, et al. Development of the comprehensive pain assessment tool short form for chronic pancreatitis: validity and reliability testing. Clin Gastroenterol Hepatol. 2022;20:e770–83. [DOI] [PubMed] [Google Scholar]
- 16.Wassef W, DeWitt J, McGreevy K, et al. Pancreatitis quality of life instruments: a psychometric evaluation. Am J Gastroenterol. 2016;111:1177–86. [DOI] [PubMed] [Google Scholar]
- 17.Hart P, Anderson D, Lyons E, et al. Clinical trials in pancreatitis: opportunities and challenges in the design and conduct of patient-focused clinical trials in recurrent acute and chronic pancreatitis: summary of a national institute of diabetes and digestive and kidney diseases workshop. Pancreas. 2022;51:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Pozo D, Loves E, Tabernero S, et al. Conventional versus Rosemont endoscopic ultrasound criteria for chronic pancreatitis: interobserver agreement in same day back-to-back procedures. Pancreatology. 2012;12:284–7. [DOI] [PubMed] [Google Scholar]
- 19.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartelle AL, Shah I, Bocchino R, et al. Long-term follow-up of disabled patients with chronic pancreatitis: evaluation of clinical characteristics, outcomes, and predictors. J Clin Gastroenterol. 2024;58:98–102. [DOI] [PubMed] [Google Scholar]
- 21.Bouwense SA, Olesen SS, Drewes AM, et al. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized controlled trial. PLoS One. 2012;7:e42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouwense SA, de Vries M, Schreuder LT, et al. Systematic mechanism-oriented approach to chronic pancreatitis pain. World J Gastroenterol. 2015;21:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parhiala M, Nojgaard C, Bartholdy A, et al. Quality of life after endoscopic procedures for chronic pancreatitis: a multicentre study. United European Gastroenterol J. 2023;11:884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassar N, Cromwell P, Duggan S, et al. Surgery versus endoscopy for the management of painful chronic pancreatitis: a systematic review and meta-analysis of randomized trials. Dig Surg. 2024;41:1–11 (in press). [DOI] [PubMed] [Google Scholar]
- 25.Issa Y, Kempeneers MA, Bruno MJ, et al. Effect of early surgery vs endoscopy-first approach on pain in patients with chronic pancreatitis. The ESCAPE randomized clinical trial. JAMA. 2020;323:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talukdar R, Olesen SS, Unnisa M, et al. Extracorporeal shock-wave lithotripsy and endoscopy for the treatment of pain in chronic pancreatitis: a sham-controlled, randomized trial. Ann Intern Med. 2024;177:749–58. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox CM. Exploring the use of the sham design for interventional trials: implications for endoscopic research. Gastrointest Endosc. 2008;67:123–7. [DOI] [PubMed] [Google Scholar]
- 28.Cotton PB, Durkalski V, Romagnuolo J, et al. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy: the EPISOD randomized clinical trial. JAMA. 2014;311:2101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palma C, David C, Fernandes RM, et al. The sham effect of invasive interventions in chronic coronary syndromes: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2022;22:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors will have complete access to the data.