Abstract
Sepsis and septic shock remain global healthcare problems associated with high mortality rates despite best therapy efforts. Circulating biomarkers may identify those patients at risk for poor outcomes, however, current biomarkers, most prominently lactate, are non-specific and have an inconsistent impact on prognosis and/or disease management. Activation of the renin-angiotensin- system (RAS) is an early event in sepsis patients and elevated levels of circulating renin are more predictive of worse outcomes than lactate. The precursor protein Angiotensinogen is another key component of the circulating RAS; it is the only known substrate for renin and the ultimate source of the vasopressor Angiotensin II (Ang II). We postulate that lower Angiotensinogen concentrations may reflect a dysfunctional RAS characterized by high renin concentrations but attenuated Ang II generation, which is disproportionate to the high renin response and may compromise adequate support of blood pressure and tissue perfusion in septic patients. The current study compared the association between serum Angiotensinogen with mortality to that of lactate and renin in the VICTAS cohort of sepsis patients at baseline (day 0) by receiver operating characteristic (ROC) and Kaplan–Meier curve analyses. Serum concentration of Angiotensinogen was more strongly associated with 30-day mortality than either the serum concentrations of renin or lactate in sepsis patients. Moreover, the clinical assessment of Angiotensinogen may have distinct advantages over the typical measures of renin. The assessment of intact Angiotensinogen may potentially facilitate more precise therapeutic approaches (including exogenous angiotensin II) to restore a dysfunctional RAS and improve patient outcomes. Additional prospective validation studies are clearly required for this biomarker in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05120-w.
Keywords: Sepsis, Septic shock, Outcomes, Biomarkers, Renin, Lactate, Angiotensinogen
Introduction
Sepsis and septic shock remain global healthcare problems associated with mortality rates of up to 40% despite best care efforts in the millions of patients affected every year. Circulating markers of sepsis severity may identify those patients at risk for poor outcomes early in the course of the disease; however, currently used disease biomarkers, most prominently lactate, are non-specific and have an inconsistent impact on prognosis and or disease management [1]. Activation of the renin–angiotensin–aldosterone system (RAS) is an early event in sepsis and elevated levels of circulating renin, a protease that activates the RAS cascade, is more predictive of worse outcomes in septic patients than lactate and may be a relevant biomarker in guided therapy [2–5]. Various conditions associated with sepsis and/or septic shock may stimulate the release of renin from the kidney including a reduction in blood pressure, higher adrenergic tone, increased circulating levels of succinate and lower tubular fluid Na + content (Fig. 1) [6–8]. Apart from an increase in renin which, other aspects of an altered RAS in sepsis include lower levels of angiotensin converting enzyme (ACE), reduced expression or responsiveness of the Ang II type 1 receptor (AT1R), and a lower serum ratio of Ang II/Ang I. The precursor protein angiotensinogen that is primarily released by the liver into the blood is another key component of the circulating RAS; it is the only known substrate for renin and the ultimate source for angiotensin peptides including the downstream vasopressor product angiotensin II (Ang II). Although angiotensinogen is typically thought to circulate at saturating concentrations with respect to renin, the concentrations of the precursor in humans approximate the optimal kinetic value for renin (Michaelis–Menten constant or Km of 1000 nM), such that changes in the circulating levels of angiotensinogen may impact the subsequent generation of Ang II [9, 10]. Indeed, siRNA-based approaches that chronically downregulate expression of angiotensinogen are promising antihypertensive therapies by reducing the formation of Ang II and the subsequent activation of downstream signaling event through the AT1 receptor (AT1R) [10].
Fig. 1.
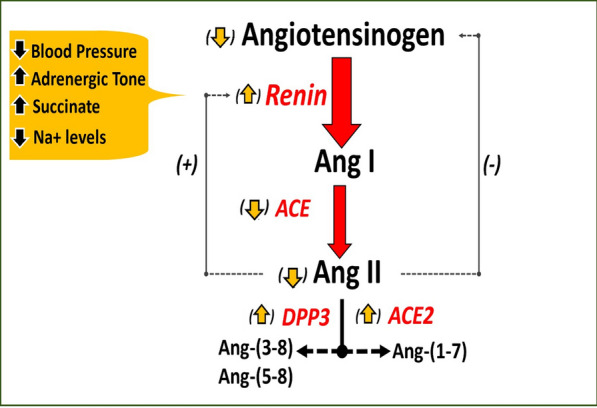
Dysregulation of the RAS in sepsis and septic shock. Circulating angiotensinogen is processed by renin to angiotensin I (Ang I), which is immediately converted to Ang II by ACE. Ang II is metabolized by dipeptidyl peptidase 3 (DPP3) to Ang-(3–8) and Ang-(5–8) while ACE2 converts Ang II to Ang-(1–7). Ang II inhibits the release of renin while Ang II stimulates the release of angiotensinogen. In sepsis and septic shock, lower blood pressure and tubular Na + but higher adrenergic tone and succinate stimulate renin release. Reduced levels of ACE but higher DPP3 and ACE2 contribute to blunted Ang II levels despite increased renin levels. Reduced Ang II and AT1 receptor (AT1R) responsiveness, as well as high renin levels may lead to lower circulating angiotensinogen, which may further depress the generation of Ang II. Lower Ang II and AT1R may also stimulate the release of renin. [6]
Adapted from Schaich et al.
We previously reported the association of elevated renin content to disease severity in the VICTAS cohort (Vitamin C, Thiamine and Steroids in Sepsis trial) [5]. Here we saw that the Ang II response was disproportionate to the high renin levels in those patients who exhibited increased mortality [5, 6]. We now extend our previous work, with the postulation that lower circulating concentrations of angiotensinogen in sepsis and septic shock patients may reflect a dysfunctional RAS characterized by high renin levels, but an attenuated generation of Ang II [2–6]. A blunted Ang II response may be detrimental in critically-ill patients regarding the inability to maintain adequate blood pressure and tissue perfusion [3, 5, 6, 8].
Methods
The current post-hoc study of the VICTAS cohort (Vitamin C, Thiamine and Steroids in Sepsis trial) compared the association between circulating levels of intact angiotensinogen with mortality compared to lactate and active renin in a subset of sepsis and septic shock patients at baseline (day 0, N = 103). Intact human angiotensinogen, which contains the angiotensin I (Ang I) domain released by renin and subsequently converted to Ang II by ACE was determined by a human Enzyme-Linked Immunosorbent Assay (ELISA) (IBL America, Minneapolis MN USA). The thawed samples on ice were pretreated with the specific renin inhibitor aliskerin (1 µM, MedChem, Monmouth NJ, USA) to stabilize the intact protein during the assay. Active renin protein was measured by a human renin ELISA (DRG International Fisher Scientific, Waltham MA, USA) that recognizes the active site of renin as described [5]. Serum lactate was determined by standard methods [3]. Serum biomarker discrimination for all-cause, 30-day mortality was compared graphically via receiver operating characteristic (ROC) curves generated by multivariable logistic regression models adjusted for age, sex, sequential organ failure assessment (SOFA) score, systolic blood pressure, and in-hospital vasopressor administration. Area under the curve (AUC) for angiotensinogen and renin was compared to lactate (DeLong’s test). The association of angiotensinogen, renin, and lactate with mortality was assessed by Kaplan–Meier curves with log rank test and hazard ratios derived from Cox regression. Analyses were completed in R 4.2.3 (2023) with a 2-sided α = 0.05. Additionally, the Youden index was calculated for these biomarkers.
Results
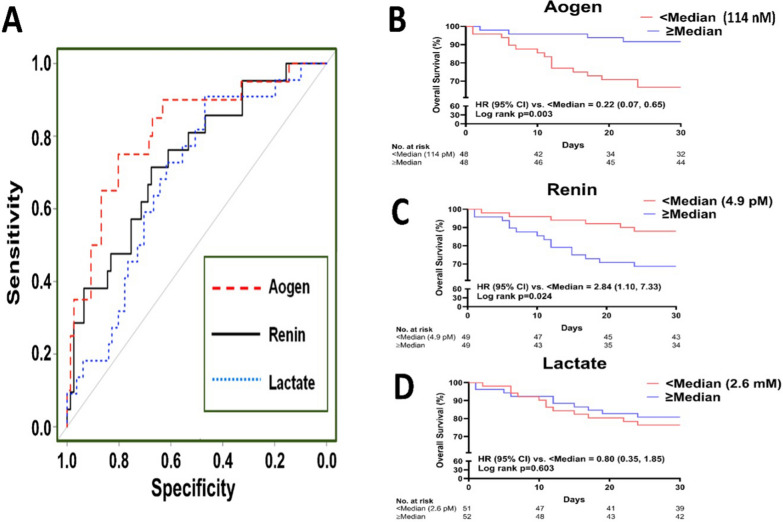
The patient characteristics for this cohort are included in the supplemental table (Table S1). Note the VICTAS study lacks patient data on use or type of RAS blockers. The median [interquartile range] serum concentrations were 114 nM [78.2–205.4] for intact angiotensinogen, 4.9 pM [2.2–13.6] for active renin and 2.6 mM [1.7–3.8] for lactate. The ROC curves revealed better discrimination of angiotensinogen for overall 30-day mortality than either active renin or lactate (Fig. 2A). The AUC of angiotensinogen (0.82, 95% CI: 0.70, 0.93) was significantly greater than that of lactate (AUC = 0.68, 95% CI: 0.57, 0.80; p = 0.022). Kaplan–Meier curves and log rank test also revealed that angiotensinogen concentrations lower than the median value of 114 nM were associated with reduced survival (Fig. 2B). Renin concentrations greater than the median value of 4.9 pM were also associated with higher patient mortality albeit less than angiotensinogen, while serum lactate concentrations (2.6 mM median value) were not associated with mortality in this cohort of patients (Fig. 2C–D). Finally, the Youdin index for angiotensinogen (0.59; optimal threshold = 116.2 nM) was higher than renin (0.36, optimal threshold = 4.1 pM) or lactate (0.41; optimal threshold = 2.3 mM).
Fig. 2.
Panel A: ROC curves reveal better discrimination of circulating angiotensinogen (Aogen; AUC = 0.82 [0.70, 0.93]) for mortality than serum lactate (AUC = 0.68 [0.57, 0.80]) or active renin (AUC = 0.73 [0.61, 0.86]) in the VICTAS cohort of sepsis patients at baseline (day 0, N = 103). Panels B–D: Kaplan–Meier estimates of survival curves reveal a stronger association of serum levels of Aogen (log rank p = 0.003) with mortality than renin (log rank p = 0.024) or lactate (log rank p = 0.603) in the VICTAS cohort at baseline (day 0)
Discussion
The liver is the predominant source of circulating angiotensinogen and perhaps other tissues and receives ~ 25% of cardiac output, as well as constitutes an important site for bacterial clearance and innate immune regulation [11–13]. SOFA scores indicated no overt organ damage in this cohort suggesting other mechanisms may negatively impact angiotensinogen expression rather than liver injury per se [5]. Downregulation of the AT1 receptor (AT1R) is evident in sepsis and reduced ACE activity coupled to enhanced metabolism by ACE2 and DPP3 to generate the angiotensin metabolites angiotensin-(1–7) and angiotensin-(3–8)/(5–8) may further contribute to a blunted Ang II response (Fig. 1) [5–8, 14]. Angiotensin II is a positive regulator of liver angiotensinogen expression via stimulation of the AT1R [15, 16]. In septic patients, a lower Ang II response or reduced AT1R responsiveness may attenuate release of the precursor, particularly in the presence of high circulating renin that would deplete intact angiotensinogen levels. These conditions may further depress Ang II potentially establishing a negative feedback loop for the expression of angiotensinogen (Fig. 1). Additional investigation of the RAS axis is necessary to elucidate the precise regulation of angiotensinogen in sepsis and septic shock patients.
In this well-characterized, multisite cohort of sepsis and septic shock patients, circulating angiotensinogen was more strongly associated with 30-day mortality than either renin or lactate. Lower angiotensinogen content may reflect increased consumption by the high levels of renin, reduced expression of the precursor by liver hepatocytes or both conditions that ultimately attenuate Ang II-AT1R tone to adequately support tissue perfusion and maintain hemodynamics in critically-ill patients, as well as preserve an immune response required for bacterial clearance [3, 5, 6, 17]. The clinical assessment of angiotensinogen may have advantages over renin, particularly as the plasma renin activity (PRA) assay utilizes endogenous angiotensinogen as substrate, and the depletion of intact angiotensinogen may underestimate active renin levels. Prorenin, the inactive precursor that is more abundant in the circulation than renin by up to tenfold, can undergo cryoactivation by improper sample handling that may inflate renin activity or renin content. PRA is also a multi-step assay requiring Ang I measurement and is not suitable as a rapid point-of-care test. The reduced levels of intact angiotensinogen coupled to high active renin content may be indicative of a blunted Ang II response in critically-ill patients. In this regard, the rapid analysis of circulating Ang II in human plasma by direct ELISAs is not presently feasible given evidence of non-specificity of these assays [18]. The relevance of this work is particularly significant knowing the availability of exogenous synthetic Ang II as an approved vasopressor by the United States Food and Drug Agency (FDA) and the European Medicines Agency (EMA). The pivotal Angiotensin II in High Output Shock (ATHOS3) trial demonstrated a significant decrease of elevated renin in patients randomized to receive Ang II compared with placebo which included standard of care vasopressors [19]. When further examining patients with a higher than population median renin level, those who received Ang II had a survival benefit. Our work is limited by the fact that the current data are “hypothesis generating” in that it is a post-hoc sample from a larger cohort of septic patients and that an external validation cohort to confirm the angiotensinogen response is required. Indeed, future studies investigating the prospective assessment of intact angiotensinogen may facilitate more precise therapeutic approaches to restore a dysfunctional ACE-Ang II-AT1R axis of the RAS and improve overall mortality and organ system failure in critically-ill patients [3, 20].
Supplementary Information
Acknowledgements
Supported by grants R01-HL146818 (MCC) and K01AG073581 (CLS) from NIH. Additional funding to Wake Forest investigators from the Groskert Heart Fund, the Wake Forest Venture Fund, the Farley-Hudson Foundation (Jacksonville, NC, USA), and the Cardiovascular Sciences Center Awards (AKK, MCC).
Abbreviations
- ACE
Angiotensin converting enzyme
- Ang II
Angiotensin II
- ELISA
Enzyme-linked immunosorbent assay
- PRA
Plasma renin activity
- RAS
Renin angiotensin system
- SOFA
Sequential organ failure assessment
- VICTAS
Vitamin C, Thiamine and Steroids in Sepsis study
Author contributions
MCC: Conceptualization, writing, reviewing, and editing. CLS: Conceptualization, writing, reviewing, and editing. LWB: Writing, reviewing, and editing. GSM: Writing, reviewing, and editing. JES: Writing, reviewing, and editing. JKH: Writing, reviewing, and editing. AKK: Conceptualization, writing, reviewing, and editing.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The current study was reviewed and approved in April of 2021 with a waiver of consent by the Wake Forest University School of Medicine institutional review board (00073908) and all procedures were followed in accordance with local ethics standards and the Helsinki Declaration of 1975.
Competing interests
AKK has previously received consulting fees from La Jolla Pharma, including grant funding for the ATHOS3 trial. He has also previously received consulting fees from Innoviva Therapeutics and Viatris Pharmaceuticals. The other authors declare that there are no competing financial interests in the work described in the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mark C. Chappell and Christopher L. Schaich have contributed equally to this work.
References
- 1.Weinberger J, Klompas M, Rhee C. What is the utility of measuring lactate levels in patients with sepsis and septic shock? Semin Respir Crit Care Med. 2021;42:650–61. [DOI] [PubMed] [Google Scholar]
- 2.Jeyaraju M, McCurdy MT, Levine AR, Devarajan P, Mazzeffi MA, Mullins KE, et al. Renin kinetics are superior to lactate kinetics for predicting in-hospital mortality in hypotensive critically Ill patients. Crit Care Med. 2022;50:50–60. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Forni LG, Busse LW, McCurdy MT, Ham KR, Boldt DW, et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. A clinical trial. Am J Respir Crit Care Med. 2020;202:1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleeson PJ, Crippa IA, Mongkolpun W, Cavicchi FZ, Van Meerhaeghe T, Brimioulle S, et al. Renin as a marker of tissue-perfusion and prognosis in critically Ill patients. Crit Care Med. 2019;47:152–8. [DOI] [PubMed] [Google Scholar]
- 5.Busse LW, Schaich CL, Chappell MC, McCurdy MT, Staples EM, Ten Lohuis CC, et al. Association of active renin content with mortality in critically Ill patients: a post hoc analysis of the Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial. Crit Care Med. 2024;52:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaich CL, Leisman DE, Goldberg MB, Filbin MR, Khanna AK, Chappell MC. Dysfunction of the renin-angiotensin-aldosterone system in human septic shock. Peptides. 2024;176:171201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310:H137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitker L, Burrell LM. Classic and nonclassic renin-angiotensin systems in the critically Ill. Crit Care Clin. 2019;35:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahlon T, Carlisle S, Otero Mostacero D, Williams N, Trainor P, DeFilippis AP. Angiotensinogen: more than its downstream products: evidence from population studies and novel therapeutics. JACC Heart Fail. 2022;10:699–713. [DOI] [PubMed] [Google Scholar]
- 10.Desai AS, Webb DJ, Taubel J, Casey S, Cheng Y, Robbie GJ, et al. Zilebesiran, an RNA interference therapeutic agent for hypertension. N Engl J Med. 2023;389:228–38. [DOI] [PubMed] [Google Scholar]
- 11.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. [DOI] [PubMed] [Google Scholar]
- 12.Kim TS, Choi DH. Liver Dysfunction in Sepsis. Korean J Gastroenterol. 2020;75:182–7. [DOI] [PubMed] [Google Scholar]
- 13.Kukida M, Cai L, Ye D, Sawada H, Katsumata Y, Franklin MK, et al. Renal angiotensinogen is predominantly liver derived in nonhuman primates. Arterioscler Thromb Vasc Biol. 2021;41:2851–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leisman DE, Fernandes TD, Bijol V, Abraham MN, Lehman JR, Taylor MD, et al. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 2021;99:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the rel A (nuclear factor-kappaB p65) transcription factor: one mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol Endocrinol. 1996;10:252–64. [DOI] [PubMed] [Google Scholar]
- 16.Klett C, Nobiling R, Gierschik P, Hackenthal E. Angiotensin II stimulates the synthesis of angiotensinogen in hepatocytes by inhibiting adenylylcyclase activity and stabilizing angiotensinogen mRNA. J Biol Chem. 1993;268:25095–107. [PubMed] [Google Scholar]
- 17.Leisman DE, Privratsky JR, Lehman JR, Abraham MN, Yaipan OY, Brewer MR, et al. Angiotensin II enhances bacterial clearance via myeloid signaling in a murine sepsis model. Proc Natl Acad Sci. 2022;119:e2211370119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell MC, Pirro NT, South AM, Gwathmey TM. Concerns on the specificity of commercial ELISAs for the measurement of angiotensin (1–7) and angiotensin II in human plasma. Hypertension. 2021;77:e29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419–30. [DOI] [PubMed] [Google Scholar]
- 20.Tumlin JA, Murugan R, Deane AM, Ostermann M, Busse LW, Ham KR, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46:949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.