Abstract
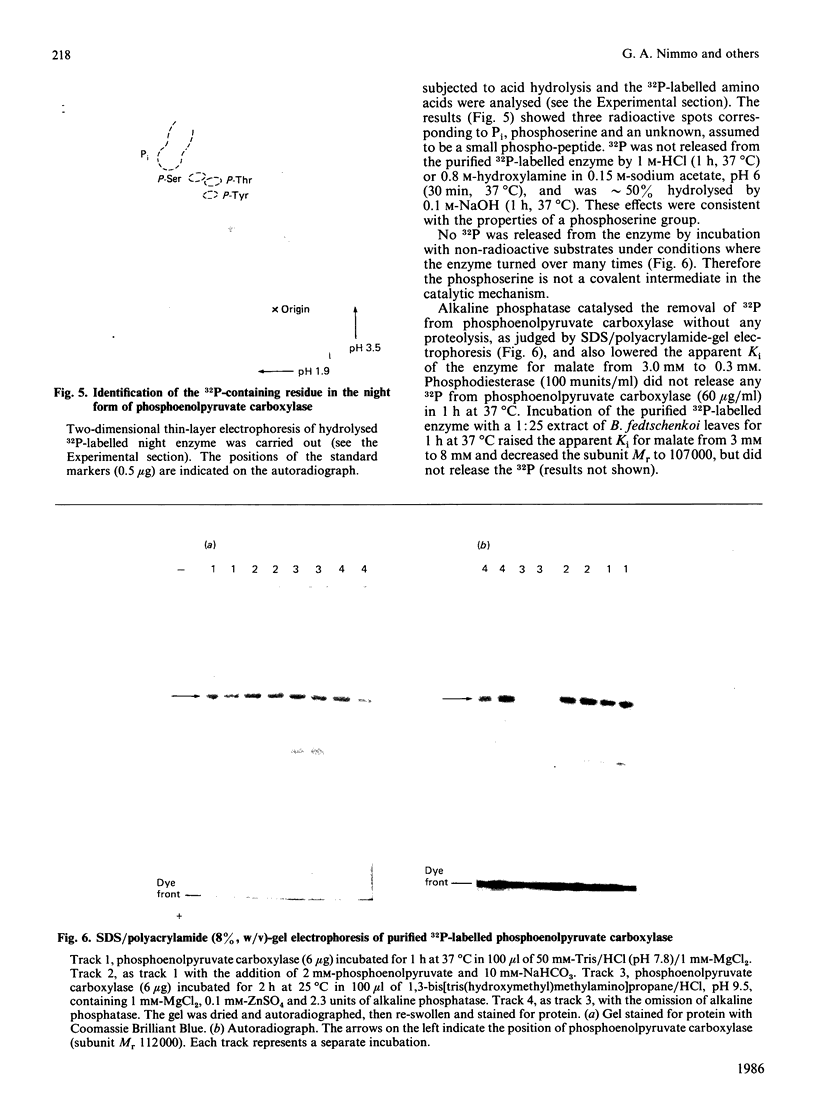
Phosphoenolpyruvate carboxylase of Bryophyllum fedtschenkoi was shown to exist in two forms: a night form, which is phosphorylated and has low sensitivity to inhibition by malate, and a day form, which is dephosphorylated and 10 times more sensitive to malate. The day and night forms of the enzyme were purified retaining their distinct malate sensitivities and phosphorylation states. The purified enzymes contained a major protein (subunit Mr 112,000) and a minor protein (subunit Mr 123,000). The two polypeptides appeared to have closely related amino acid sequences and were present in a similar ratio in extracts that had been prepared rapidly. The phosphate present in the night form of the enzyme was covalently bound to serine. It was not a catalytic intermediate. Alkaline phosphatase removed the phosphate group in vitro and increased the malate sensitivity of the enzyme to that observed for the day form. Both the day and night forms of the enzyme were probably tetramers, and their apparent Mr was lowered by the presence of malate, but was unaffected by Mg2+ ions, EDTA, a rise in pH or a 10-fold change in enzyme concentration. The rapid loss of malate sensitivity, observed in extracts of leaves prepared during the day and at night, was shown to be due to proteolysis of the enzyme. It was slowed in the presence of malate and by phosphorylation of the enzyme.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Jones R., Wilkins M. B., Coggins J. R., Fewson C. A., Malcolm A. D. Phosphoenolpyruvate carboxylase from the crassulacean plant Bryophyllum fedtschenkoi Hamet et Perrier. Purification, molecular and kinetic properties. Biochem J. 1978 Nov 1;175(2):391–406. doi: 10.1042/bj1750391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Nimmo G. A. A general method for the localization of enzymes that produce phosphate, pyrophosphate, or CO2 after polyacrylamide gel electrophoresis. Anal Biochem. 1982 Mar 15;121(1):17–22. doi: 10.1016/0003-2697(82)90551-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]