Abstract
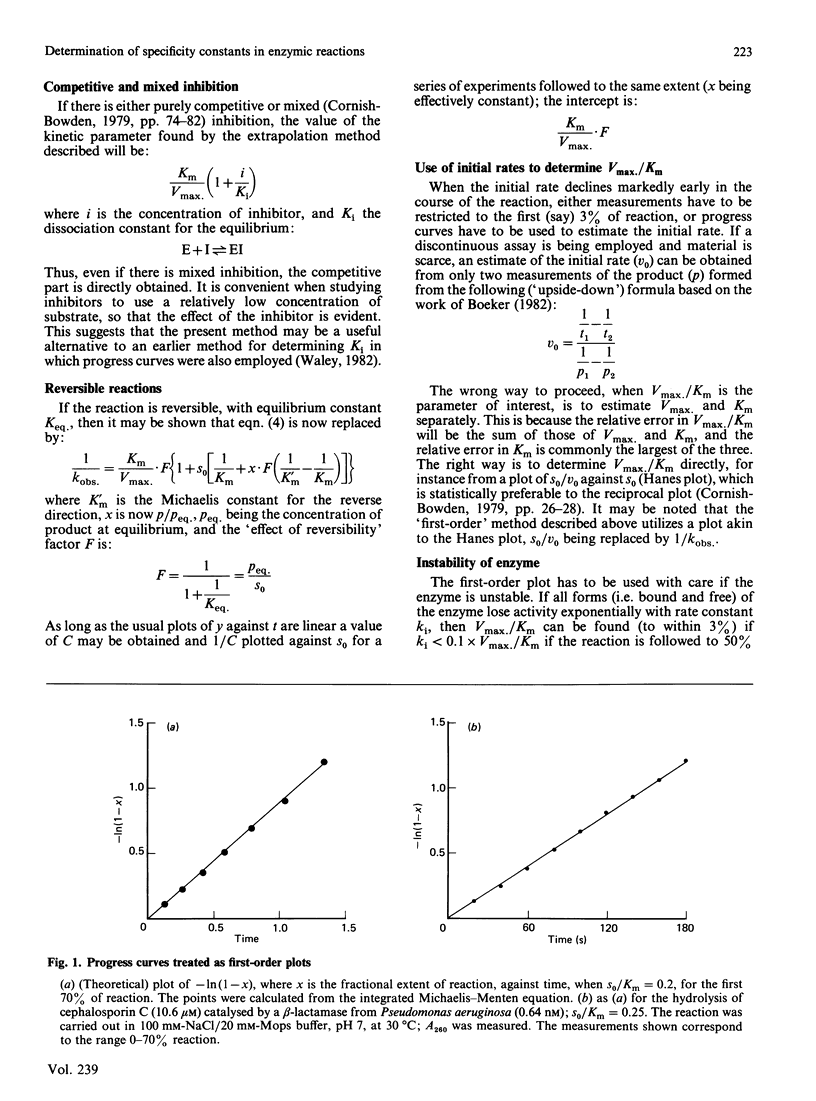
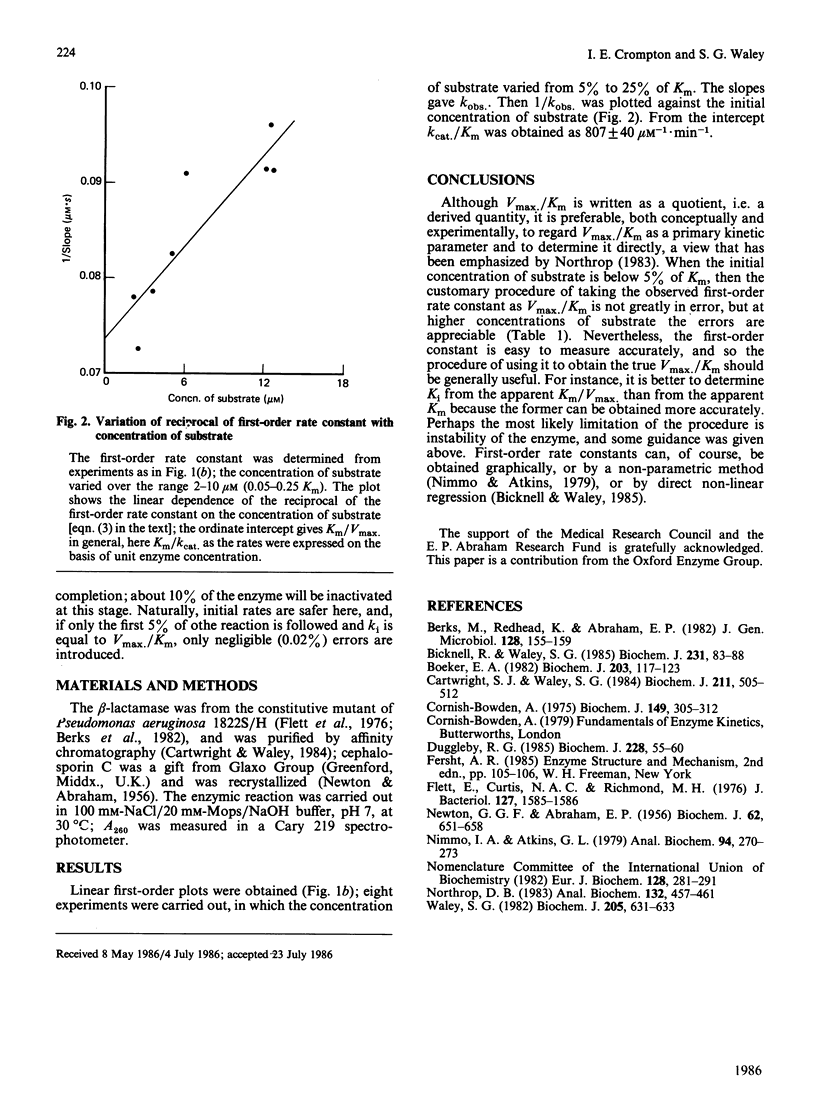
A convenient and accurate procedure for determining the kinetic parameter Vmax./Km is described. This avoids the error in the usual method of taking the observed first-order rate constant of an enzymic reaction at low substrate concentration as Vmax./Km. A series of reactions is used in which the initial concentration of substrate is below Km (e.g. from 5% to 50% of Km). Measurements are taken over the same extent of reaction (e.g. 70%) for each member of the series, and treated as if the kinetics were truly first-order. The reciprocal of the observed first-order rate constant is then plotted against the initial concentration of substrate: the reciprocal of the ordinate intercept is Vmax./Km. The procedure, as well as being applicable to simple reactions, is shown to be valid when there is competitive inhibition by the product, or when the reaction is reversible, or when there is competitive or mixed inhibition. The hydrolysis of cephalosporin C by a beta-lactamase from Pseudomonas aeruginosa is used to illustrate the method.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berks M., Redhead K., Abraham E. P. Isolation and properties of an inducible and a constitutive beta-lactamase from Pseudomonas aeruginosa. J Gen Microbiol. 1982 Jan;128(1):155–159. doi: 10.1099/00221287-128-1-155. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Waley S. G. Single-turnover and steady-state kinetics of hydrolysis of cephalosporins by beta-lactamase I from Bacillus cereus. Biochem J. 1985 Oct 1;231(1):83–88. doi: 10.1042/bj2310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeker E. A. Initial rates. A new plot. Biochem J. 1982 Apr 1;203(1):117–123. doi: 10.1042/bj2030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Purification of beta-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984 Jul 15;221(2):505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. The use of the direct linear plot for determining initial velocities. Biochem J. 1975 Aug;149(2):305–312. doi: 10.1042/bj1490305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. Estimation of the initial velocity of enzyme-catalysed reactions by non-linear regression analysis of progress curves. Biochem J. 1985 May 15;228(1):55–60. doi: 10.1042/bj2280055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett F., Curtis N. A., Richmond M. H. Mutant of Pseudomonas aeruginosa 18S that synthesizes type Id beta-lactamase constitutively. J Bacteriol. 1976 Sep;127(3):1585–1586. doi: 10.1128/jb.127.3.1585-1586.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON G. G., ABRAHAM E. P. Isolation of cephalosporin C, a penicillin-like antibiotic containing D-alpha-aminoadipic acid. Biochem J. 1956 Apr;62(4):651–658. doi: 10.1042/bj0620651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo I. A., Atkins G. L. A nonparametric method for fitting a single exponential to biological data. Anal Biochem. 1979 Apr 15;94(2):270–273. doi: 10.1016/0003-2697(79)90359-2. [DOI] [PubMed] [Google Scholar]
- Northrop D. B. Fitting enzyme-kinetic data to V/K. Anal Biochem. 1983 Jul 15;132(2):457–461. doi: 10.1016/0003-2697(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Waley S. G. A quick method for the determination of inhibition constants. Biochem J. 1982 Sep 1;205(3):631–633. doi: 10.1042/bj2050631. [DOI] [PMC free article] [PubMed] [Google Scholar]



