Abstract
Background and aim:
Only a few series of patients with scar sarcoidosis (SS) have been reported. Our aim was to analyse the clinical features of SS patients and their relationship to the prognosis of sarcoidosis.
Methods:
Patients with systemic sarcoidosis with SS diagnosed between 1980-2017 at Bellvitge University Hospital, were enrolled. Their clinical charts were reviewed to collect the following data: age, sex, ethnicity, number of lesions, location of SS, origin of the scar, association with erythema nodosum or other specific cutaneous lesions, radiological stage at diagnosis, chronic systemic sarcoidosis activity.
Results:
Forty two of 728 patients with systemic sarcoidosis presented SS (31 females and 11 males, mean age 47.71±13.747 years). SS was present at the onset of systemic sarcoidosis in 35/42 cases (83.33%). Twelve patients had simultaneously erythema nodosum. In 14 patients SS was the only specific cutaneous lesion of sarcoidosis. Foreign bodies were observed in 16 of 26 biopsied SS lesions (61.54%). Radiological stage at diagnosis was 0 for 2 patients, I for 22, II for 13, III for 4, and IV for 1. The activity of systemic sarcoidosis persisted for more than 5 years in 16/42 patients with SS (38.1%) vs. 186/686 patients with systemic sarcoidosis (27.11%), However the differences were not significant (p=0.154).
Conclusions:
SS was observed in 5.77% of our patients with systemic sarcoidosis. It is usually present at the onset of the disease and is an useful sign for suspicion of a diagnosis of sarcoidosis, but it carries no prognostic significance.
Keywords: Sarcoidosis, Scar, Scar sarcoidosis, Skin
Introduction
Sarcoidosis is a multisystem disease defined by the formation of non-caseating granulomas in various organs, mainly the lungs, lymph nodes, eyes, and skin (1). Specific cutaneous lesions of sarcoidosis accounts for 9-37% of patients with systemic sarcoidosis (2, 3). Multiple types of specific cutaneous sarcoidosis has been described including maculopapular lesions, plaque type lesions, lupus pernio, subcutaneous sarcoidosis, scar sarcoidosis (SS), angiolupoid, hypopigmented, liquenoid, ulcerative, psoriasiforme, verrucous, ichthyosiform, or erythrodermic sarcoidosis. The most frequently reported specific cutaneous lesions are maculopapular lesions, plaque type lesions, lupus pernio, subcutaneous sarcoidosis, and SS (2, 3). Although SS is a well-known specific manifestation of systemic sarcoidosis, there have been only a few studies focusing on it (4, 5). For this reason, its relative frequency, clinical features, and prognostic significance have not been established.
Our objective was to review our series of patients with systemic sarcoidosis with clinical changes in old scars in order to analyze their frequency, the association with other cutaneous specific lesions, and the possible relationship to clinical course of the systemic disease.
Methods
With ethical committee approval, all patients with systemic sarcoidosis and clinical changes in old scars diagnosed between 1980 and 2017 2017 at Bellvitge University Hospital (an 800-bed university referral centre in Barcelona, Spain) were enrolled. The diagnosis of systemic sarcoidosis was based on the classic criteria (1): a compatible clinical and radiological picture, histological demonstration of non-caseous granulomas in one or more tissues, with stains and cultures negative for mycobacteria and fungi, and exclusion of other granulomatous diseases. SS was defined as a clinical change in one or more than one old scars, including cases with erythema and infiltration of scars, the development of papules or plaques over scars, or infiltration beneath scars. In most patients with other suspected specific lesions skin biopsies were taken from these lesions to avoid possible confusion with foreign body sarcoid granuloma that can be associated with scars.
The patients were followed up in the sarcoidosis clinic of Bellvitge University Hospital. Clinical photographs were taken of all skin lesions suspected of being related to sarcoidosis. Clinical photographs of skin lesions of our patients with histopathologically proven specific cutaneous lesions were reviewed. Scar sarcoidosis was confirmed by biopsy only in patients without other specific skin lesions. Patient clinical charts were reviewed to collect the following data: age, sex, date of diagnosis of SS, number of lesions, location of SS, injury type related to the scar (surgical procedure, accidental trauma, injection-related injury or venepuncture, and tattoo), association with erythema nodosum or other specific cutaneous lesions, radiological stage at diagnosis, duration of systemic sarcoidosis activity. According to clinical pictures of the patients, SS were classified into five clinical types: erythematous infiltrated scar, papules or plaques of granulomatous appearance over a scar, subcutaneous nodular lesions beneath a normal appearing scar, multiple puntiform papules over puncture sites, and tattoo infiltration.
Statistical analysis
Data were analysed with SPSS (v17.0 for Windows; SPSS Inc., Chicago, IL, USA). Categorical variables were compared using the χ2 test or Fisher exact test. Continuous variables were compared using Student t-test when normality of data distribution was confirmed. Otherwise, the Mann–Whitney U-test was performed.
Results
Forty-two of 728 patients with systemic sarcoidosis and SS were included in the study. These were 31 females and 11 males, with a mean age 47.71±13.747 years (range 22-75 years). All patients were Caucasian except for 4 women of North African descent. Eighteen patients had a single lesion of SS and 24 multiple lesions (2 in 12, 3 in 5, and 4 or more that 4 in 7). The origin of the scar was surgery in 8 cases, accidental traumatism in 30, injection-related injury or venepuncture in 5, and tattooing in 3 (46 origins, 4 patients had SS involving scars of different origin). Almost all of our patients had other scars not affected by sarcoidosis. According to the clinical pictures, the clinical features of SS were classified as erythematous infiltrated scars in 33 cases, papules or plaques of granulomatous appearance over a scar in 3, subcutaneous nodular lesions beneath a normal appearing scar in 2, multiple puntiform papules over puncture sites in 5, and infiltration of previous tattoos in 3 (46 types). SS involved 53 locations in 42 patients: head and neck in 4 cases, trunk in 9 (abdomen in 6), upper extremities in 17 (elbow 8), and lower extremities in 23 (knees 17). SS was present at diagnosis of the systemic disease in 35 of 42 cases (83.33%). Only 7 patients developed SS after diagnosis of the systemic disease. Twelve patients had simultaneously erythema nodosum.
In 14 patients SS was the only speficic cutaneous lesion of sarcoidosis. In the remaining 28 patients, other specific cutaneous lesions observed (in some of them more than one type of lesion) were: maculopapules in 18 cases, plaques in 6, lupus pernio in 1, and subcutaneous sarcoidosis in 6.
Specific cutaneous lesions of sarcoidosis were diagnosed in 164 of our 728 systemic sarcoidosis patients, so SS was observed in 25.61% of patients with specific cutaneous sarcoidosis. Excluding patients with only SS as specific cutaneous sarcoidosis, 150 patients had other types of specific skin lesions of sarcoidosis.
Foreign bodies were observed in 16 of 26 biopsied lesions of SS (61.54%).
Radiological stage according to Scadding’s classification at diagnosis of sarcoidosis was 0 for 2 patients, I for 22, II for 13, III for 4, and IV for 1.
The activity of systemic sarcoidosis persisted for more than 5 years in 16 of 42 patients with SS (38.1%), while in the remaining 686 patients with systemic sarcoidosis without SS the disease persisted more than 5 years in 186 patients (27.11%). However these differences were not significant (p=0.154).
Table 1 shows the clinical features of patients with SS according to sex. Males were younger that females (p<0.001) and erythema nodosum was significantly more frequent in females (p=0.018).
Table 1.
Clinical features of patients with SS, according to sex
| females n=31/42 (73.81%) |
males n=11/42 (26.19%) |
||
|---|---|---|---|
| Age (years) n=42 | 52.13±12.055 | 35.27±10.403 | P<0.001 |
| Race Caucasian/African descent n=42 | 27/4 | 11/0 | |
| Scar origin (46 origins) Surgery 8/46 (17.39%) Traumatism 30/46 (65.22%) Puncture 5/46 (10.87%) Tattoo 3/46 (6.52%) |
6/34 (17.65%) 22/34 (64.71%) 5/34 (14.71%) 1/34 (2.94%) |
2/12 (16.67%) 8/12 (66.67%) 0/12 (0.%) 2/12 (16.67%) |
|
| Clinical type of SS (46 types) Erythemat. inf. scar 33/46 (71.74%) Papules over scar 3/46 (6.52%) Nodules beneath scar 2/46 (4.35%) Papules puncture site 5/46 (10.87%) Tattoo infiltration 3/46 (6.52%) |
23/34 (67.65%) 3/34 (8.82%) 2/34 (5.88%) 5/34 (14.71%) 1/34 (2.94%) |
10/12 (83.33%) 0/12 (0.%) 0/12 (0.%) 0/12 (0.%) 2/12 (16.67%) |
|
| Location (53 sites) Head and neck 4/53 (7.55%) Trunk 9/53 (16.98%) (abdomen 6, 4 female/2 male) Upper extremities 17/53 (32.08%) (elbow 8, 8 females) Lower extremities 23/53 (43.40%) (knees 17, 15 female/2 male) |
2/40 (5%) 6/40 (15%) 14/40 (25%) 18/40 (45%) |
2/13 (15.38%) 3/13 (23.08%) 3/13 (23.08%) 5/13 (38.46%) |
|
| Single lesion 18/42 (42.86%) | 13/31 (41.94%) | 5/11 (45.45%) | |
| Erythema nodosum 12/42 (28.57%) | 12/31 (38.71%) | 0/11 (0%) | P=0.018 |
| Other specific lesions Maculopap. 18/42 (42.86%) Plaques 6/42 (14.29%) Lupus pernio 1/42 (2.38%) Subcutaneous s. 6/42 (14.29%) |
13/31 (41.94%) 4/31 (12.90%) 1/31 (3.23%) 5/31 (16.13%) |
5/11 (45.45%) 2/11 (18.18%) 0/11 (0%) 1/22 (9.09%) |
|
| Present at diagnosis 35/42 (83.33%) | 26/31 (83.87%) | 9/11 (81.82%) | |
| Foreign bodies at biopsy 16/26 (61.54%) | 13/21(61.90%) | 3/5 (60%) | |
| Radiological Stage 0 2/42 (4.76%) I 22/42 (52.38%) II 13/42 (30.95%) III 4/42 (9.52%) IV 1/42 (2.38%) |
2/31 (6.45%) 18/31 (58.06%) 6/31 (19.35%) 4/31 (12.90%) 1/31 (3.23%) |
0/11 (0%) 4/11 (36.36%) 7/11 (63.63%) 0/11 (0%) 0/11 (0%) |
Table 2 shows the organs affected by sarcoidosis in our SS patients
Table 2.
Organs affected by sarcoidosis in our SS patients
| IT S | Skin* | Ocular | ET nodes | Liver | Articular | Spleen | Salivary | NS | Bone | ORL |
|---|---|---|---|---|---|---|---|---|---|---|
| 40 | 28 | 6 | 6 | 6 | 5 | 4 | 4 | 3 | 2 | 1 |
IT Intrathoracic sarcoidosis; ET Extrathoracic lymph nodes; NS Neurosarcoidosis.
*Specific cutaneous sarcoidosis in addition to SS.
Figure 1 shows clinical images of typical cases of SS.
Figure 1.
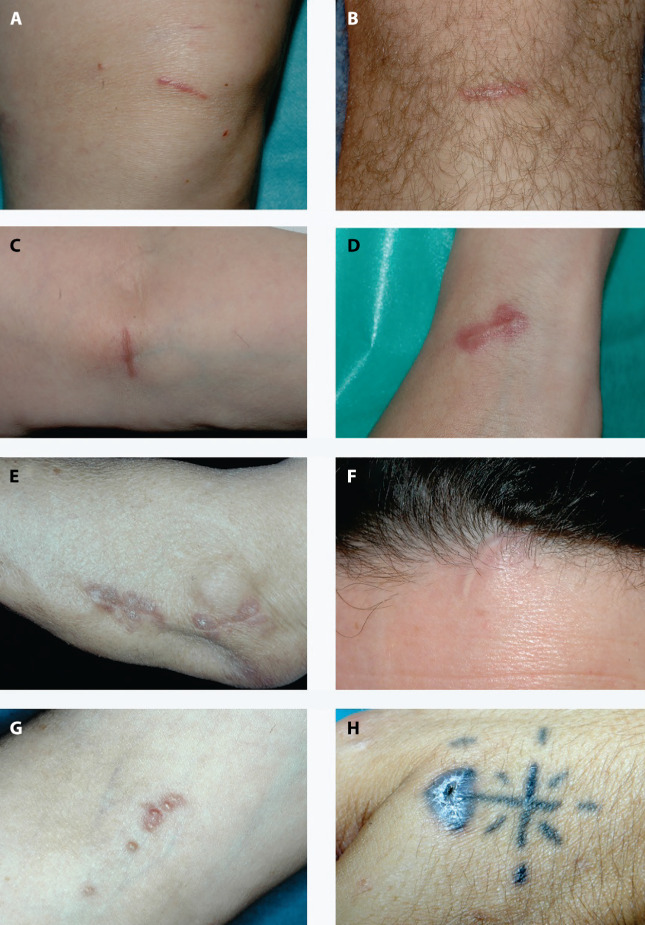
Clinical images of typical cases of SS. (a, d) Infiltrated traumatic erythematous scars. (e) Plaque-type lesions arising over an old surgical scar. (f) Subcutaneous induration beneath a normal appearing scar with sarcoid granulomas at histopathology. (g) Multiple puntiform papules over puncture sites from blood sample extraction. (h) Infiltration of a previous tattoo.
Discussion
SS lesions were present in 5.77% of our 728 patients with systemic sarcoidosis. Two previous large series reported incidences of 2.9% and 5% in systemic sarcoidosis patients (6, 7). Other series only reported the proportion of cases with SS among patients with other specific cutaneous lesions. In these series SS represented 15-36.7% of specific lesions of sarcoidosis (4, 6, 8-10). This disparity may be explained by different diagnostic criteria. Some studies include cases without systemic sarcoidosis while others include only cases of SS with granulomas at biopsy. In our study, we biopsied scars when they were the only possible specific skin lesion in order to avoid confusion with possible foreign body granulomas. The rest of the cases of SS in our series were diagnosed clinically and were photographically documented. With this methodology SS was diagnosed in 25,61% of our patients with specific cutaneous lesions of systemic sarcoidosis.
Most SS cases have been reported to occur in white women in the fifth and sixth decades of life. In our series, SS was also more frequent in females, accounting for 73.81% of cases (2.82:1). The mean age of our patients was 47.71 years, but, curiously, males were significantly younger than females (35.27 vs. 52.13 years, respectively, P<0.001).
Many causes of scars hosting sarcoidosis have been reported including accidental physical trauma, surgery, laser ablation, injection-related injury, venepuncture, vaccination, Mantoux test, tattoos, acne, and herpes zoster (4, 5, 11-16). The most frequent origin of the scars in our patients was accidental traumatisms (30 cases) followed by surgery (8 cases). In patients with sarcoidosis involving multiple scars, these scars may have different aetiologies (4), and patients with SS may also had other scars not affected by sarcoidosis (preserved scars) (4). In our series, 4 patients had SS lesions of more than one origin, and in most of our patients with SS we observed preserved scars.
Only a few small series focusing SS have been published in recent years. In these surveys SS mainly involved the head and neck region (4, 5). However, in our series the most frequent location was the lower extremities, especially the knees (17 cases), which may be explained by a high frequency of traumatic scars in the lower limbs in our population. Clinically, most of our patients developed erythematous-infiltrated scars (33 patients). We also observed papules or plaques of granulomatous appearance over a scar in 3 cases, subcutaneous nodular lesions beneath a normal appearing scar in 2, multiple puntiform papules over puncture sites in 5, and tattoo infiltration in 3 (46 types of lesion).
In 83.33% of our patients, SS appeared at the onset of systemic sarcoidosis and was a useful sign to alert for suspicion of a diagnosis of the systemic disease, mainly in patients without other specific cutaneous lesions. In 14/42 patients (33.33%), SS was the only specific cutaneous lesion of sarcoidosis while in 28/42 patients scar involvement coexisted with other specific cutaneous lesions, predominantly maculopapules and subcutaneous sarcoidosis.
Some studies suggest that SS is associated with chronic forms of sarcoidosis. SS has been reported to be associated with long-lasting pulmonary and mediastinal involvement, uveitis, peripheral lymphadenopathy, bony cysts, and parotid infiltration (7, 10, 17). However, although the small number of cases may have influenced the statistical analysis in our series, SS was not associated with chronic sarcoidosis defined as more than 5 years of persistence of activity. Most of our patients had radiological stage I at diagnosis (22 patients) and only 5 have advanced radiological stages (4 patients with stage III and 1 with stage IV).
The predilection of sarcoidosis to involve old scars is not understood. However some studies suggest that foreign material frequently present in the scars can act as an antigenic stimulus for the induction of granulomas into the scar (18-22). Even the smallest amount of oil used as lubricant in blood sampling needles can induce granulomas in sarcoidosis patients (16). In our study, foreign bodies were observed in 16 of 26 biopsied scar sarcoidosis lesions (61.5%), and this supports the hypothesis that antigens capable of inducing granulomatous responses may act as a nidus for granuloma formation when a patient develops sarcoidosis, thus determining the location of the lesions. Scars or parts thereof without these antigens would not be involved.
Our study is limited by its retrospective design, the small number of cases, and the fact that not all SS lesions were histopathologically confirmed.
In summary, in our series SS lesions were observed in 5.22% of 728 patients with systemic sarcoidosis. In most cases they were present at diagnosis of the systemic disease, and they served as a useful alert to suspicion of the diagnosis. Any patient being studied for possible sarcoidosis should be checked at presentation for the presence of infiltrated old scars as an easy way to confirm the histological diagnosis with a minor invasive procedure. However, the presence of SS carries no prognostic significance. Foreign bodies were observed in 61.5% of biopsied SS lesions, which suggests that this may be the explanation for the predilection of sarcoidosis to involve scars.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
Ethics Approval:
This work was approved by local Ethic Committee (Comitè d’Ètica de la Investigació de Medicaments Hospital de Bellvitge, PR373/23).
Consent to Participate Statement:
Written informed consent was not required according to local Ethic Committee (Comitè d’Ètica de la Investigació de Medicaments Hospital de Bellvitge).
Financial Support for the Research:
None
References
- Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201:e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoval J, Mañá J, Rubio M. Specific cutaneous lesions in patients with systemic sarcoidosis: relationship to severity and chronicity of disease. Clin Exp Dermatol. 2011;36:739–44. doi: 10.1111/j.1365-2230.2011.04128.x. [DOI] [PubMed] [Google Scholar]
- Mañá J, Marcoval J, Graells J, Salazar A, Peyrí J, Pujol R. Cutaneous involvement in sarcoidosis. Relationship to systemic disease. Arch Dermatol. 1997;133:882–8. doi: 10.1001/archderm.1997.03890430098013. [DOI] [PubMed] [Google Scholar]
- Atci T, Baykal C, Kaya Bingöl Z, Polat Ekinci A, Kiliçaslan Z. Scar sarcoidosis: 11 patients with variable clinical features and invariable pulmonary involvement. Clin Exp Dermatol. 2019;44:826–8. doi: 10.1111/ced.13917. [DOI] [PubMed] [Google Scholar]
- Bae KN, Shin K, Kim HS, Ko HC, Kim B, Kim MB. Scar Sarcoidosis: A retrospective investigation into its peculiar clinicopathologic presentation. Ann Dermatol. 2022;34:28–33. doi: 10.5021/ad.2022.34.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525–33. [PubMed] [Google Scholar]
- Yanardağ H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978–82. doi: 10.1016/s0954-6111(03)00127-6. [DOI] [PubMed] [Google Scholar]
- Mangas C, Fernández-Figueras MT, Fité E, Fernández-Chico N, Sàbat M, Ferrándiz C. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772–7. doi: 10.1111/j.1600-0560.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, Pujol RM. The natural history of cutaneous sarcoidosis. Clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178–84. doi: 10.1111/ijd.14218. [DOI] [PubMed] [Google Scholar]
- Veien NK, Stahl D, Brodthagen H. Cutaneous sarcoidosis in Caucasians. J Am Acad Dermatol. 1987;16:534–40. doi: 10.1016/s0190-9622(87)70070-x. [DOI] [PubMed] [Google Scholar]
- Usmani N, Akhtar S, Long E, Phipps A, Walton S. A case of sarcoidosis occurring within an extensive burns scar. J Plast Reconstr Aesthet Surg. 2007;60:1256–9. doi: 10.1016/j.bjps.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kormeili T, Neel V, Moy RL. Cutaneous sarcoidosis at sites of previous laser surgery. Cutis. 2004;73:53–5. [PubMed] [Google Scholar]
- Kwon SH, Jeong KM, Baek YS, Jeon J. Linear scar sarcoidosis on thin blepharoplasty line mimicking a hypertrophic scar: a case report. SAGE Open Med Case Rep. 2018;6:2050313X18803991. doi: 10.1177/2050313X18803991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuja F, Kavoussi SC, Mir MR, Jogi RP, Rosen T. Interferon induced sarcoidosis with cutaneous involvement along lines of venous drainage in a former intravenous drug user. Dermatol Online J. 2009;15:4. [PubMed] [Google Scholar]
- Lee YB, Lee JI, Park HJ, Cho BK, Oh ST. Interferon-alpha induced sarcoidosis with cutaneous involvement along the lines of venous drainage. Ann Dermatol. 2011;23:239–41. doi: 10.5021/ad.2011.23.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoval J, Penín RM, Mañá J. Specific skin lesions of sarcoidosis located at venipuncture points for blood sample collection. Am J Dermatopathol. 2018;40:362–6. doi: 10.1097/DAD.0000000000000928. [DOI] [PubMed] [Google Scholar]
- James DG. Dermatological aspects of sarcoidosis. QJM. 1959;28:109–24. [PubMed] [Google Scholar]
- Marcoval J, Mañá J, Moreno A, Gallego I, Fortuño Y, Peyrí J. Foreign bodies in granulomatous cutaneous lesions of patients with systemic sarcoidosis. Arch Dermatol. 2001;137:485–6. [PubMed] [Google Scholar]
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408–12. doi: 10.1097/00000372-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Callen JP. The presence of foreign bodies does not exclude the diagnosis of sarcoidosis. Arch Dermatol. 2001;137:485–6. [PubMed] [Google Scholar]
- Val-Bernal JF, Sanchez-Quevedo MC, Corral J, Campos A. Cutaneous sarcoidosis and foreign bodies. An electron probe roentgenographic microanalytic study. Arch Pathol Lab Med. 1995;119:471–4. [PubMed] [Google Scholar]
- Walsh NMG, Hanly JG, Tremaine R, Murray S. Cutaneous sarcoidosis and foreign bodies. Am J Dermatopathol. 1993;15:203–7. doi: 10.1097/00000372-199306000-00002. [DOI] [PubMed] [Google Scholar]


