Abstract
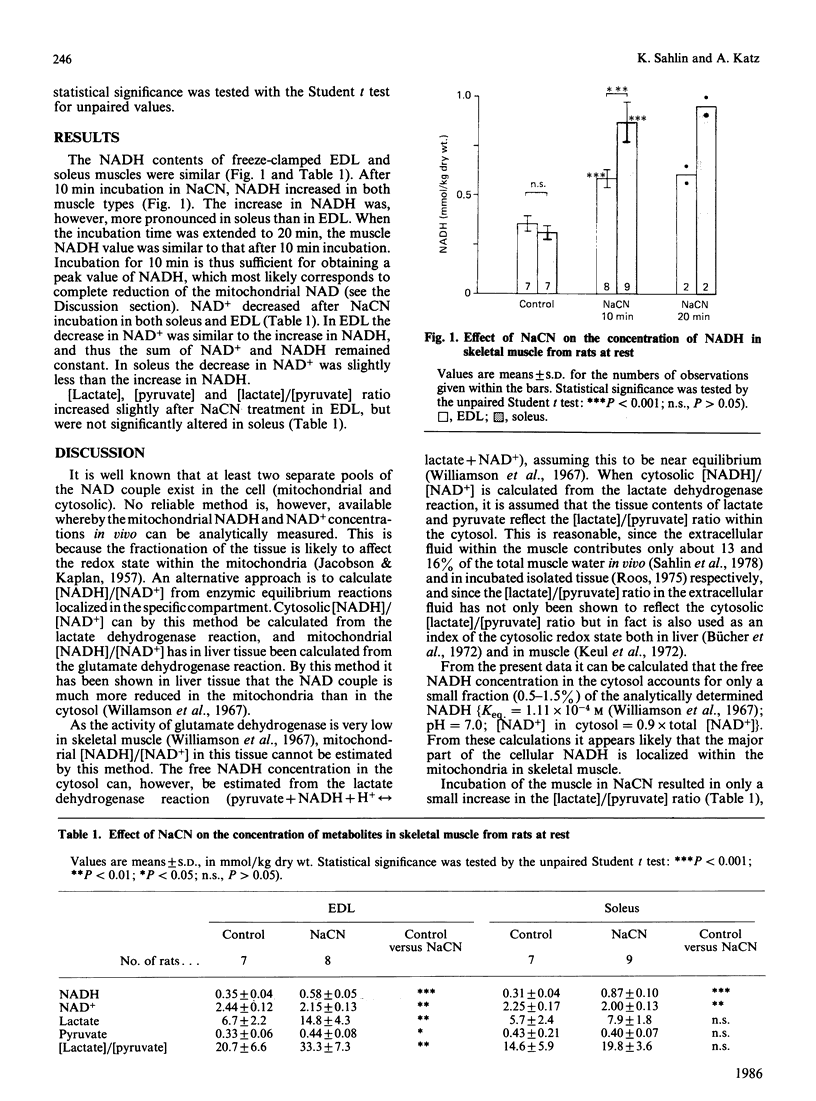
The concentration of NADH was determined a high-oxidative muscle (soleus) and a high-glycolytic muscle (extensor digitorum longus, EDL) from resting rats. The NADH content of freeze-clamped control muscles was 0.35 +/- 0.04 (mean +/- S.D.) and 0.31 +/- 0.04 mmol/kg dry wt. in EDL and soleus respectively, and increased to peak values of 0.58 +/- 0.05 (EDL) and 0.87 +/- 0.10 (soleus) after 10 min of NaCN treatment. The [lactate]/[pyruvate] ratio, which was not significantly changed in soleus and increased only slightly in EDL after NaCN incubation, shows that only minor changes occurred in the cytosolic NADH concentration. Provided that the major part of muscle NADH is located in the mitochondria it can be calculated that the mitochondrial NADH content in skeletal muscle at rest is about 36 (soleus) and 60% (EDL) of the anoxic value, respectively. These results are in contrast with previous studies with the surface-fluorescence technique, where mitochondrial NAD appeared to be almost completely reduced in resting skeletal muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bücher T., Brauser B., Conze A., Klein F., Langguth O., Sies H. State of oxidation-reduction and state of binding in the cytosolic NADH-system as disclosed by equilibration with extracellular lactate-pyruvate in hemoglobin-free perfused rat liver. Eur J Biochem. 1972 May 23;27(2):301–317. doi: 10.1111/j.1432-1033.1972.tb01840.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem. 1961 May;236:1534–1543. [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. IV. The pathway of electron transfer. J Biol Chem. 1961 May;236:1562–1568. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Edström L., Hultman E., Sahlin K., Sjöholm H. The contents of high-energy phosphates in different fibre types in skeletal muscles from rat, guinea-pig and man. J Physiol. 1982 Nov;332:47–58. doi: 10.1113/jphysiol.1982.sp014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- JACOBSON K. B., KAPLAN N. O. Pyridine coenzymes of subcellular tissue fractions. J Biol Chem. 1957 Jun;226(2):603–613. [PubMed] [Google Scholar]
- Jöbsis F. F., Duffield J. C. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–1047. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Stainsby W. N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol. 1968 May;4(3):292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Bryla J., Williamson J. R. Feedback interactions in the control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1972 Feb 10;247(3):667–679. [PubMed] [Google Scholar]
- Meno H., Kanaide H., Okada M., Nakamura M. Total adenine nucleotide stores and sarcoplasmic reticular Ca transport in ischemic rat heart. Am J Physiol. 1984 Sep;247(3 Pt 2):H380–H386. doi: 10.1152/ajpheart.1984.247.3.H380. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Paddle B. M. A cytoplasmic component of pyridine nucleotide fluorescence in rat diaphragm: evidence from comparisons with flavoprotein fluorescence. Pflugers Arch. 1985 Aug;404(4):326–331. doi: 10.1007/BF00585343. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K., Alvestrand A., Brandt R., Hultman E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Sep;45(3):474–480. doi: 10.1152/jappl.1978.45.3.474. [DOI] [PubMed] [Google Scholar]
- Sahlin K. NADH and NADPH in human skeletal muscle at rest and during ischaemia. Clin Physiol. 1983 Oct;3(5):477–485. doi: 10.1111/j.1475-097x.1983.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Sahlin K. NADH in human skeletal muscle during short-term intense exercise. Pflugers Arch. 1985 Feb;403(2):193–196. doi: 10.1007/BF00584099. [DOI] [PubMed] [Google Scholar]
- Sugano T., Oshino N., Chance B. Mitochondrial functions under hypoxic conditions. The steady states of cytochrome c reduction and of energy metabolism. Biochim Biophys Acta. 1974 Jun 28;347(3):340–358. doi: 10.1016/0005-2728(74)90074-7. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


