Full text
PDF
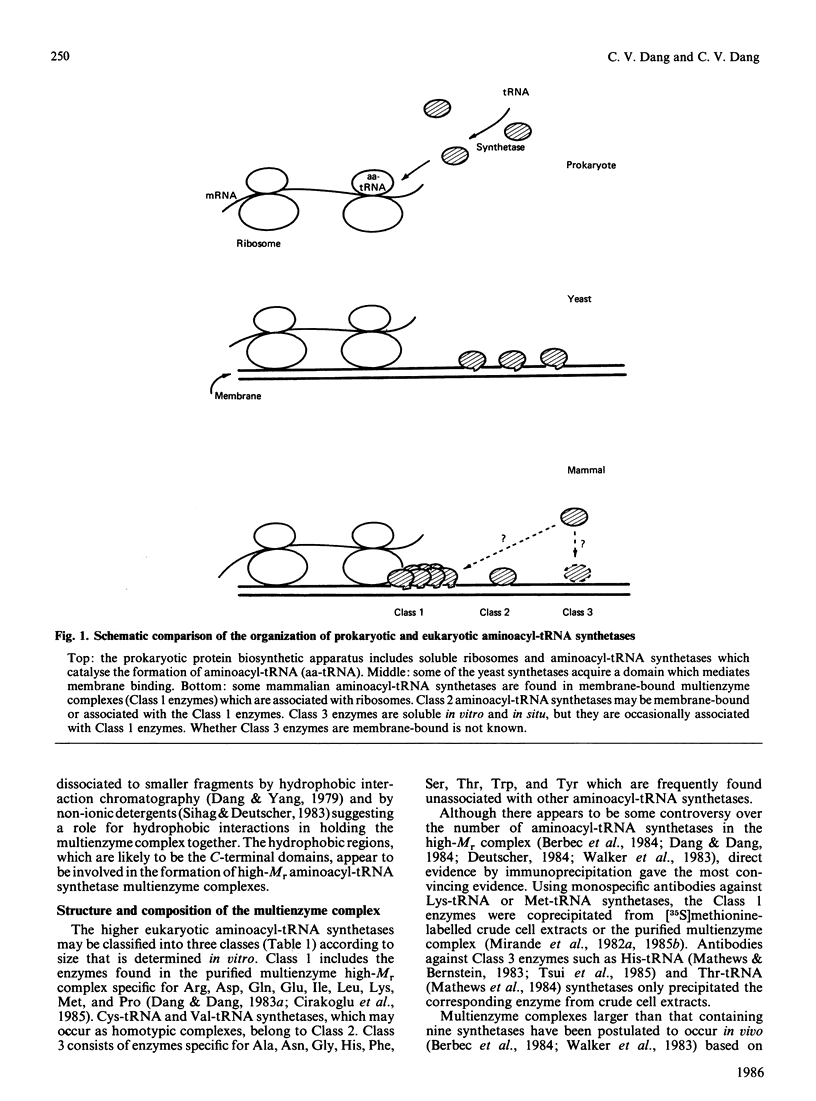
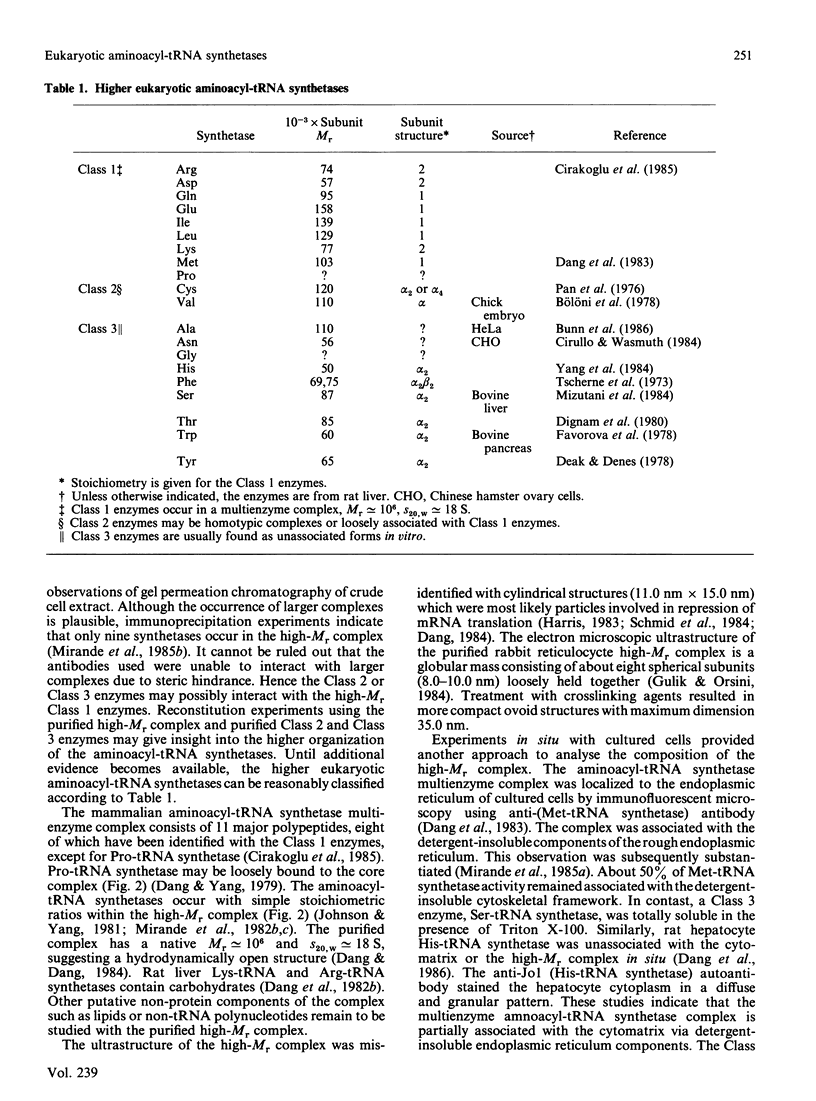
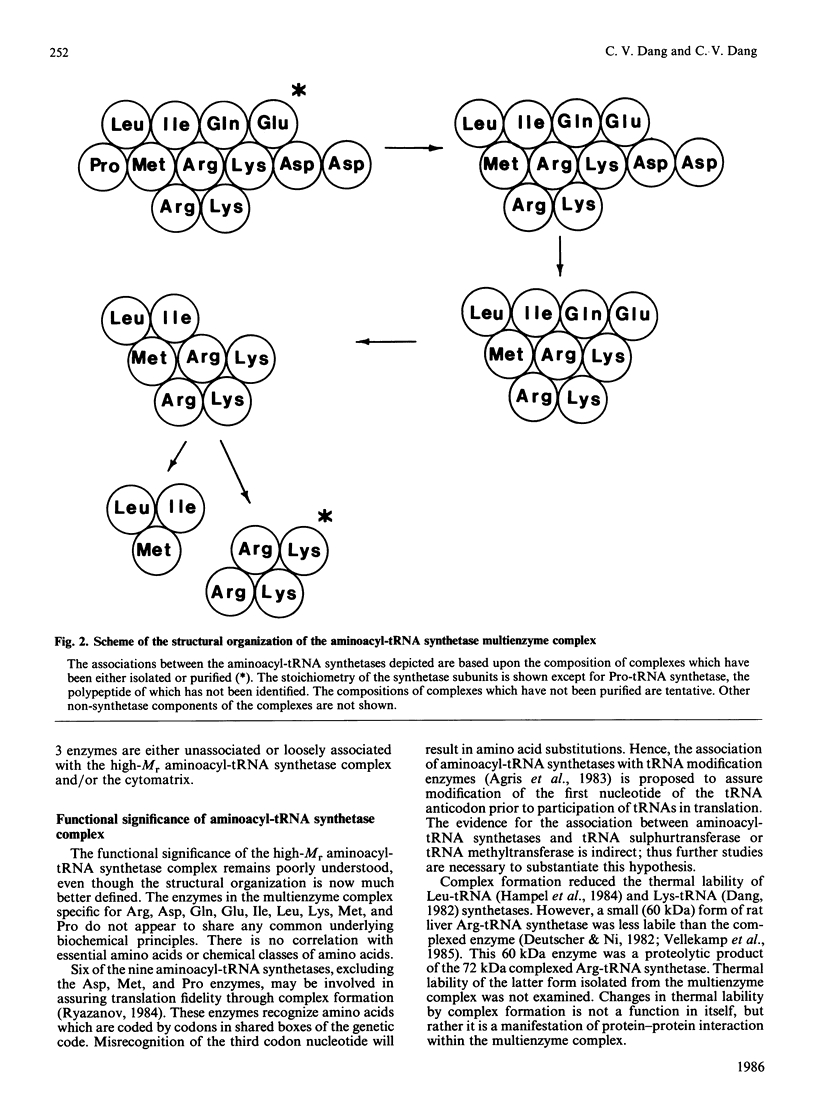



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Playl T., Goldman L., Horton E., Woolverton D., Setzer D., Rodi C. Processing of tRNA is accomplished by a high-molecular-weight enzyme complex. Recent Results Cancer Res. 1983;84:237–254. doi: 10.1007/978-3-642-81947-6_18. [DOI] [PubMed] [Google Scholar]
- Alzhanova A. T., Fedorov A. N., Ovchinnikov L. P., Spirin A. S. Eukaryotic aminoacyl-tRNA synthetases are RNA-binding proteins whereas prokaryotic ones are not. FEBS Lett. 1980 Nov 3;120(2):225–229. doi: 10.1016/0014-5793(80)80303-6. [DOI] [PubMed] [Google Scholar]
- Berbeć H., Paszkowska A., Borkowski T. Heavy and light forms of some aminoacyl-tRNA synthetases in fraction X, microsomes and cytosol of rabbit liver. Mol Cell Biochem. 1984 Jun;62(2):149–155. doi: 10.1007/BF00223305. [DOI] [PubMed] [Google Scholar]
- Bhat T. N., Blow D. M., Brick P., Nyborg J. Tyrosyl-tRNA synthetase forms a mononucleotide-binding fold. J Mol Biol. 1982 Jul 15;158(4):699–709. doi: 10.1016/0022-2836(82)90255-8. [DOI] [PubMed] [Google Scholar]
- Blanquet S., Plateau P., Brevet A. The role of zinc in 5',5'-diadenosine tetraphosphate production by aminoacyl-transfer RNA synthetases. Mol Cell Biochem. 1983;52(1):3–11. doi: 10.1007/BF00230583. [DOI] [PubMed] [Google Scholar]
- Brevet A., Plateau P., Best-Belpomme M., Blanquet S. Variation of Ap4A and other dinucleoside polyphosphates in stressed Drosophila cells. J Biol Chem. 1985 Dec 15;260(29):15566–15570. [PubMed] [Google Scholar]
- Brevet A., Plateau P., Cirakoğlu B., Pailliez J. P., Blanquet S. Zinc-dependent synthesis of 5',5'-diadenosine tetraphosphate by sheep liver lysyl- and phenylalanyl-tRNA synthetases. J Biol Chem. 1982 Dec 25;257(24):14613–14615. [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölöni E., Fónagy A., Holland J., Szabó L. D. Studies on valyl-tRNA synthetase obtained from chick embryo brain. Purification and properties. Acta Biochim Biophys Acad Sci Hung. 1978;13(1-2):35–46. [PubMed] [Google Scholar]
- Cassio D., Waller J. P. Modification of methionyl-tRNA synthetase by proteolytic cleavage and properties of the trypsin-modified enzyme. Eur J Biochem. 1971 May 28;20(2):283–300. doi: 10.1111/j.1432-1033.1971.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Cirakoglu B., Mirande M., Waller J. P. A model for the structural organization of aminoacyl-tRNA synthetases in mammalian cells. FEBS Lett. 1985 Apr 22;183(2):185–190. [PubMed] [Google Scholar]
- Cirakoglu B., Waller J. P. Do yeast aminoacyl-tRNA synthetases exist as soluble enzymes within the cytoplasm? Eur J Biochem. 1985 Jun 3;149(2):353–361. doi: 10.1111/j.1432-1033.1985.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Cirakoglu B., Waller J. P. Leucyl-tRNA and lysyl-tRNA synthetases, derived from the high-Mr complex of sheep liver, are hydrophobic proteins. Eur J Biochem. 1985 Aug 15;151(1):101–110. doi: 10.1111/j.1432-1033.1985.tb09074.x. [DOI] [PubMed] [Google Scholar]
- Cirakoğlu B., Waller J. P. Multiple forms of arginyl- and lysyl-tRNA synthetases in rat liver: a re-evaluation. Biochim Biophys Acta. 1985 Jun 10;829(2):173–179. doi: 10.1016/0167-4838(85)90186-4. [DOI] [PubMed] [Google Scholar]
- Cirullo R. E., Wasmuth J. J. Isolation of Chinese hamster ovary cells that overproduce asparaginyl-tRNA synthetase. Mol Cell Biol. 1984 Sep;4(9):1939–1941. doi: 10.1128/mcb.4.9.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Caudwell F. B., Cohen P. Regulation of the aminoacyl-tRNA synthetase complex of rat liver by phosphorylation/dephosphorylation in vitro and in vivo. Eur J Biochem. 1982 Dec;129(1):57–65. doi: 10.1111/j.1432-1033.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Dang C. V. Hydrodynamic properties and structure of the rat liver 12 S arginyl- and lysyl-tRNA synthetase complex. Biochem Biophys Res Commun. 1983 Dec 16;117(2):464–469. doi: 10.1016/0006-291x(83)91223-8. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Dang C. V. Multienzyme complexes of eukaryotic aminoacyl-tRNA synthetases. Biosci Rep. 1983 Jun;3(6):527–538. doi: 10.1007/BF01120696. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Dang C. V. Structural organization of high-Mr mammalian aminoacyl-tRNA synthetases. Comparison of multi-enzyme complexes from different sources. Mol Cell Biochem. 1984 Sep;63(2):131–136. doi: 10.1007/BF00285220. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Ferguson B., Burke D. J., Garcia V., Yang D. C. Interactions of aminoacyl-tRNA synthetases in high-molecular-weight multienzyme complexes from rat liver. Biochim Biophys Acta. 1985 Jul 1;829(3):319–326. doi: 10.1016/0167-4838(85)90239-0. [DOI] [PubMed] [Google Scholar]
- Dang C. V. High molecular weight complex formation of rat liver lysyl-tRNA synthetase reduces enzyme lability to thermal inactivation. Biochem Biophys Res Commun. 1982 May 14;106(1):44–47. doi: 10.1016/0006-291x(82)92055-1. [DOI] [PubMed] [Google Scholar]
- Dang C. V. Identity of the ubiquitous eukaryotic ring-shaped miniparticle. Cell Biol Int Rep. 1984 Apr;8(4):323–327. doi: 10.1016/0309-1651(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Johnson D. L., Yang D. C. High molecular mass amino acyl-tRNA synthetase complexes in eukaryotes. FEBS Lett. 1982 Jun 1;142(1):1–6. doi: 10.1016/0014-5793(82)80206-8. [DOI] [PubMed] [Google Scholar]
- Dang C. V., LaDuca F. M., Bell W. R. Histidyl-tRNA synthetase, the myositis Jo-1 antigen, is cytoplasmic and unassociated with the cytoskeletal framework. Exp Cell Res. 1986 May;164(1):261–266. doi: 10.1016/0014-4827(86)90474-x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Yang D. C. High molecular weight complexes of eukaryotic aminoacyl-tRNA synthetases. Int J Biochem. 1982;14(7):539–543. doi: 10.1016/0020-711x(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Yang D. C., Pollard T. D. Association of methionyl-tRNA synthetase with detergent-insoluble components of the rough endoplasmic reticulum. J Cell Biol. 1983 Apr;96(4):1138–1147. doi: 10.1083/jcb.96.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Monte U., Capaccioli S., Neri Cini G., Perego R., Caldini R., Chevanne M. Effects of liver regeneration on tRNA contents and aminoacyl-tRNA synthetase activities and sedimentation patterns. Biochem J. 1986 May 15;236(1):163–169. doi: 10.1042/bj2360163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Ni R. C. Purification of a low molecular weight form of rat liver arginyl-tRNA synthetase. J Biol Chem. 1982 Jun 10;257(11):6003–6006. [PubMed] [Google Scholar]
- Deutscher M. P. The eucaryotic aminoacyl-tRNA synthetase complex: suggestions for its structure and function. J Cell Biol. 1984 Aug;99(2):373–377. doi: 10.1083/jcb.99.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F., Dénes G. Purification and some properties of rat liver tyrosyl-tRNA synthetase. Biochim Biophys Acta. 1978 Oct 12;526(2):626–634. doi: 10.1016/0005-2744(78)90153-5. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Rhodes D. G., Deutscher M. P. Purification and structural characterization of rat liver threonyl transfer ribonucleic acid synthetase. Biochemistry. 1980 Oct 28;19(22):4978–4984. doi: 10.1021/bi00563a007. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic L., Godefroy-Colburn T. Interaction entre tARN-ligases et lipides. FEBS Lett. 1974 Sep 1;45(1):194–201. doi: 10.1016/0014-5793(74)80844-6. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Gibson B. W., Walter P., Chatton B., Biemann K., Boulanger Y. Cytoplasmic methionyl-tRNA synthetase from Bakers' yeast. A monomer with a post-translationally modified N terminus. J Biol Chem. 1985 Dec 15;260(29):15571–15576. [PubMed] [Google Scholar]
- Favorova O. O., Madoyan I. A., Kisselev L. L. Evidence for essential histidine residues in tryptophanyl-tRNA synthetase. Eur J Biochem. 1978 May;86(1):193–202. doi: 10.1111/j.1432-1033.1978.tb12299.x. [DOI] [PubMed] [Google Scholar]
- Gerken S. C., Andrulis I. L., Arfin S. M. Histidyl-tRNA synthetase of Chinese hamster ovary cells contains phosphoserine. Biochim Biophys Acta. 1986 Jan 30;869(2):215–217. doi: 10.1016/0167-4838(86)90296-7. [DOI] [PubMed] [Google Scholar]
- Gerken S. C., Arfin S. M. Chinese hamster ovary cells resistant to borrelidin overproduce threonyl-tRNA synthetase. J Biol Chem. 1984 Jul 25;259(14):9202–9206. [PubMed] [Google Scholar]
- Gerken S. C., Arfin S. M. Threonyl-tRNA synthetase from Chinese hamster ovary cells is phosphorylated on serine. J Biol Chem. 1984 Sep 25;259(18):11160–11161. [PubMed] [Google Scholar]
- Grummt F., Weinmann-Dorsch C., Schneider-Schaulies J., Lux A. Zinc as a second messenger of mitogenic induction. Effects on diadenosine tetraphosphate (Ap4A) and DNA synthesis. Exp Cell Res. 1986 Mar;163(1):191–200. doi: 10.1016/0014-4827(86)90572-0. [DOI] [PubMed] [Google Scholar]
- Guedon G., Sovia D., Ebel J. P., Befort N., Remy P. Effect of diadenosine tetraphosphate microinjection on heat shock protein synthesis in Xenopus laevis oocytes. EMBO J. 1985 Dec 30;4(13B):3743–3749. doi: 10.1002/j.1460-2075.1985.tb04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik A., Orsini G. Electron microscopy study of the aminoacyl-tRNA synthetase multienzymatic complex purified from rabbit reticulocytes. Mol Biol Rep. 1984 Jul;10(1):23–30. doi: 10.1007/BF00775150. [DOI] [PubMed] [Google Scholar]
- Hampel A. E., Ritter P. O., Enger M. D. A physically altered leucyl-tRNA synthetase complex in a CHO cell mutant. Nature. 1978 Dec 21;276(5690):844–845. doi: 10.1038/276844a0. [DOI] [PubMed] [Google Scholar]
- Hampel A., Mansukhani A., Condon T. Cell culture mutants as aminoacyl-tRNA synthetase complex probes. Fed Proc. 1984 Dec;43(15):2991–2993. [PubMed] [Google Scholar]
- Hilderman R. H. Characterization of a homogeneous complex of arginyl- and lysyl-tRNA synthetase: zinc and adenosine 5'-phosphate dependent synthesis of diadenosine 5',5'''-P1,P4-tetraphosphate. Biochemistry. 1983 Sep 13;22(19):4353–4357. doi: 10.1021/bi00288a001. [DOI] [PubMed] [Google Scholar]
- Hradec J., Dusek Z. Particulate aminoacyl-tRNA synthetases are retained on heparin bound to Sepharose. Mol Biol Rep. 1980 Dec 31;6(4):245–248. doi: 10.1007/BF00777532. [DOI] [PubMed] [Google Scholar]
- Jasin M., Regan L., Schimmel P. Dispensable pieces of an aminoacyl tRNA synthetase which activate the catalytic site. Cell. 1984 Apr;36(4):1089–1095. doi: 10.1016/0092-8674(84)90059-x. [DOI] [PubMed] [Google Scholar]
- Jasin M., Regan L., Schimmel P. Modular arrangement of functional domains along the sequence of an aminoacyl tRNA synthetase. Nature. 1983 Dec 1;306(5942):441–447. doi: 10.1038/306441a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. L., Van Dang C., Yang D. C. Purification and characterization of lysyl-tRNA synthetase after dissociation of the particulate aminoacyl-tRNA synthetases from rat liver. J Biol Chem. 1980 May 10;255(9):4362–4366. [PubMed] [Google Scholar]
- Johnson D. L., Yang D. C. Stoichiometry and composition of an aminoacyl-tRNA synthetase complex from rat liver. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4059–4062. doi: 10.1073/pnas.78.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. H., McMillan A. J., Fersht A. R., Winter G. Reversible dissociation of dimeric tyrosyl-tRNA synthetase by mutagenesis at the subunit interface. Biochemistry. 1985 Oct 8;24(21):5852–5857. doi: 10.1021/bi00342a024. [DOI] [PubMed] [Google Scholar]
- Kellermann O., Tonetti H., Brevet A., Mirande M., Pailliez J. P., Waller J. P. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. I. Species specificity of the polypeptide composition. J Biol Chem. 1982 Sep 25;257(18):11041–11048. [PubMed] [Google Scholar]
- Lapointe J. Study of the evolution of the genetic code by comparing the structural and catalytic properties of the aminoacyl-tRNA synthetases. Can J Biochem. 1982 Apr;60(4):471–474. doi: 10.1139/o82-055. [DOI] [PubMed] [Google Scholar]
- Lazard M., Mirande M., Waller J. P. Purification and characterization of the isoleucyl-tRNA synthetase component from the high molecular weight complex of sheep liver: a hydrophobic metalloprotein. Biochemistry. 1985 Sep 10;24(19):5099–5106. doi: 10.1021/bi00340a021. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Reichlin M., Hughes G. R., Bernstein R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med. 1984 Aug 1;160(2):420–434. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirande M., Cirakoğlu B., Waller J. P. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. III. Assignment of aminoacyl-tRNA synthetase activities to the polypeptide components of the complexes. J Biol Chem. 1982 Sep 25;257(18):11056–11063. [PubMed] [Google Scholar]
- Mirande M., Cirakoğlu B., Waller J. P. Seven mammalian aminoacyl-tRNA synthetases associated within the same complex are functionally independent. Eur J Biochem. 1983 Mar 1;131(1):163–170. doi: 10.1111/j.1432-1033.1983.tb07244.x. [DOI] [PubMed] [Google Scholar]
- Mirande M., Gache Y., Le Corre D., Waller J. P. Seven mammalian aminoacyl-tRNA synthetases co-purified as high molecular weight entities are associated within the same complex. EMBO J. 1982;1(6):733–736. doi: 10.1002/j.1460-2075.1982.tb01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirande M., Kellermann O., Waller J. P. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. II. Structural characterization of the polypeptide components and immunological identification of the methionyl-tRNA synthetase subunit. J Biol Chem. 1982 Sep 25;257(18):11049–11055. [PubMed] [Google Scholar]
- Mirande M., Le Corre D., Louvard D., Reggio H., Pailliez J. P., Waller J. P. Association of an aminoacyl-tRNA synthetase complex and of phenylalanyl-tRNA synthetase with the cytoskeletal framework fraction from mammalian cells. Exp Cell Res. 1985 Jan;156(1):91–102. doi: 10.1016/0014-4827(85)90264-2. [DOI] [PubMed] [Google Scholar]
- Mirande M., Le Corre D., Waller J. P. A complex from cultured Chinese hamster ovary cells containing nine aminoacyl-tRNA synthetases. Thermolabile leucyl-tRNA synthetase from the tsH1 mutant cell line is an integral component of this complex. Eur J Biochem. 1985 Mar 1;147(2):281–289. doi: 10.1111/j.1432-1033.1985.tb08748.x. [DOI] [PubMed] [Google Scholar]
- Mirande M., Pailliez J. P., Schwencke J., Waller J. P. Sedimentation behaviour of aminoacyl-tRNA synthetases from mixed lysates of yeast and rabbit liver. Biochim Biophys Acta. 1983 Sep 14;747(1-2):71–77. doi: 10.1016/0167-4838(83)90123-1. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Narihara T., Hashimoto A. Purification and properties of bovine liver seryl-tRNA synthetase. Eur J Biochem. 1984 Aug 15;143(1):9–13. doi: 10.1111/j.1432-1033.1984.tb08331.x. [DOI] [PubMed] [Google Scholar]
- Pahuski E., Klekamp M., Condon T., Hampel A. E. Altered aminoacyl-tRNA synthetase complexes in CHO cell mutants. J Cell Physiol. 1983 Jan;114(1):82–87. doi: 10.1002/jcp.1041140114. [DOI] [PubMed] [Google Scholar]
- Pailliez J. P., Waller J. P. Phenylalanyl-tRNA synthetases from sheep liver and yeast. Correlation between net charge and binding to ribosomes. J Biol Chem. 1984 Dec 25;259(24):15491–15496. [PubMed] [Google Scholar]
- Pan F., Lee H. H., Pai S. H., Yu T. C., Guoo J. Y., Duh G. M. Multiple molecular forms of cysteinyl-tRNA synthetase from rat liver: purification and subunit structure. Biochim Biophys Acta. 1976 Nov 8;452(1):271–283. doi: 10.1016/0005-2744(76)90080-2. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Traugh J. A. Alteration of aminoacyl-tRNA synthetase activities by phosphorylation with casein kinase I. J Biol Chem. 1985 Sep 25;260(21):11769–11774. [PubMed] [Google Scholar]
- Putney S. D., Schimmel P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature. 1981 Jun 25;291(5817):632–635. doi: 10.1038/291632a0. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G. Does the complex of aminoacyl-tRNA synthetases and tRNA-modifying enzymes prevent miscoding? FEBS Lett. 1984 Dec 3;178(1):6–9. doi: 10.1016/0014-5793(84)81228-4. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schimmel P., Jasin M., Regan L. Size polymorphism and the structure of aminoacyl-tRNA synthetases. Fed Proc. 1984 Dec;43(15):2987–2990. [PubMed] [Google Scholar]
- Schmid H. P., Akhayat O., Martins De Sa C., Puvion F., Koehler K., Scherrer K. The prosome: an ubiquitous morphologically distinct RNP particle associated with repressed mRNPs and containing specific ScRNA and a characteristic set of proteins. EMBO J. 1984 Jan;3(1):29–34. doi: 10.1002/j.1460-2075.1984.tb01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui F. A., Yang D. C. Generation of multiple forms of methionyl-tRNA synthetase from the multi-enzyme complex of mammalian aminoacyl-tRNA synthetases by endogenous proteolysis. Biochim Biophys Acta. 1985 Apr 5;828(2):177–187. doi: 10.1016/0167-4838(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Sihag R. K., Deutscher M. P. Perturbation of the aminoacyl-tRNA synthetase complex by salts and detergents. Importance of hydrophobic interactions and possible involvement of lipids. J Biol Chem. 1983 Oct 10;258(19):11846–11850. [PubMed] [Google Scholar]
- Tscherne J. S., Lanks K. W., Salim P. D., Grunberger D., Cantor C. R., Weinstein I. B. Studies on rat liver phenylalanyl transfer ribonucleic acid synthetase. II. Further purification, substrate specificity, and effects of substrates on heat inactivation. J Biol Chem. 1973 Jun 10;248(11):4052–4059. [PubMed] [Google Scholar]
- Tsui F. W., Andrulis I. L., Murialdo H., Siminovitch L. Amplification of the gene for histidyl-tRNA synthetase in histidinol-resistant Chinese hamster ovary cells. Mol Cell Biol. 1985 Sep;5(9):2381–2388. doi: 10.1128/mcb.5.9.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussery M. A., Tanaka W. K., Hardesty B. Subcellular distribution of aminoacyl-tRNA synthetases in various eukaryotic cells. Eur J Biochem. 1977 Feb;72(3):491–500. doi: 10.1111/j.1432-1033.1977.tb11272.x. [DOI] [PubMed] [Google Scholar]
- Van Dang C., Mawhinney T. P., Hilderman R. H. Characterization of a homogeneous arginyl- and lysyl-tRNA synthetase complex isolated from rat liver. Arginyl- and lysyl-tRNA synthetases contain carbohydrates. Biochemistry. 1982 Sep 28;21(20):4891–4895. doi: 10.1021/bi00263a010. [DOI] [PubMed] [Google Scholar]
- Van Dang C., Yang D. C. Disassembly and gross structure of particulate aminoacyl-tRNA synthetases from rat liver. Isolation and the structural relationship of synthetase complexes. J Biol Chem. 1979 Jun 25;254(12):5350–5356. [PubMed] [Google Scholar]
- Varshavsky A. Diadenosine 5', 5"'-P1, P4-tetraphosphate: a pleiotropically acting alarmone? Cell. 1983 Oct;34(3):711–712. doi: 10.1016/0092-8674(83)90526-3. [DOI] [PubMed] [Google Scholar]
- Vellekamp G., Sihag R. K., Deutscher M. P. Comparison of the complexed and free forms of rat liver arginyl-tRNA synthetase and origin of the free form. J Biol Chem. 1985 Aug 15;260(17):9843–9847. [PubMed] [Google Scholar]
- Wahab S. Z., Yang D. C. Influence of supramolecular structure on the enzyme mechanisms of rat liver lysyl-tRNA synthetase-catalyzed reactions. Synthesis of P1,P4-bis(5'-adenosyl)tetraphosphate. J Biol Chem. 1985 Oct 15;260(23):12735–12739. [PubMed] [Google Scholar]
- Wahab S. Z., Yang D. C. Synthesis of diadenosine 5',5''' -P1,P4-tetraphosphate by lysyl-tRNA synthetase and a multienzyme complex of aminoacyl-tRNA synthetases from rat liver. J Biol Chem. 1985 May 10;260(9):5286–5289. [PubMed] [Google Scholar]
- Walker E. J., Treacy G. B., Jeffrey P. D. Molecular weights of mitochondrial and cytoplasmic aminoacyl-tRNA synthetases of beef liver and their complexes. Biochemistry. 1983 Apr 12;22(8):1934–1941. doi: 10.1021/bi00277a030. [DOI] [PubMed] [Google Scholar]
- Webster T., Tsai H., Kula M., Mackie G. A., Schimmel P. Specific sequence homology and three-dimensional structure of an aminoacyl transfer RNA synthetase. Science. 1984 Dec 14;226(4680):1315–1317. doi: 10.1126/science.6390679. [DOI] [PubMed] [Google Scholar]
- Weinmann-Dorsch C., Hedl A., Grummt I., Albert W., Ferdinand F. J., Friis R. R., Pierron G., Moll W., Grummt F. Drastic rise of intracellular adenosine(5')tetraphospho(5')adenosine correlates with onset of DNA synthesis in eukaryotic cells. Eur J Biochem. 1984 Jan 2;138(1):179–185. doi: 10.1111/j.1432-1033.1984.tb07897.x. [DOI] [PubMed] [Google Scholar]
- Wetzel R. Aminoacyl-tRNA synthetase families and their significance to the origin of the genetic code. Orig Life. 1978 Sep;9(1):39–50. doi: 10.1007/BF00929712. [DOI] [PubMed] [Google Scholar]
- Yang D. C., Dang C. V., Arnett F. C. Rat liver histidyl-tRNA synthetase. Purification and inhibition by the myositis-specific anti-Jo-1 autoantibody. Biochem Biophys Res Commun. 1984 Apr 16;120(1):15–21. doi: 10.1016/0006-291x(84)91407-4. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. Diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem. 1983 Oct 1;134(1):1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]


