Abstract
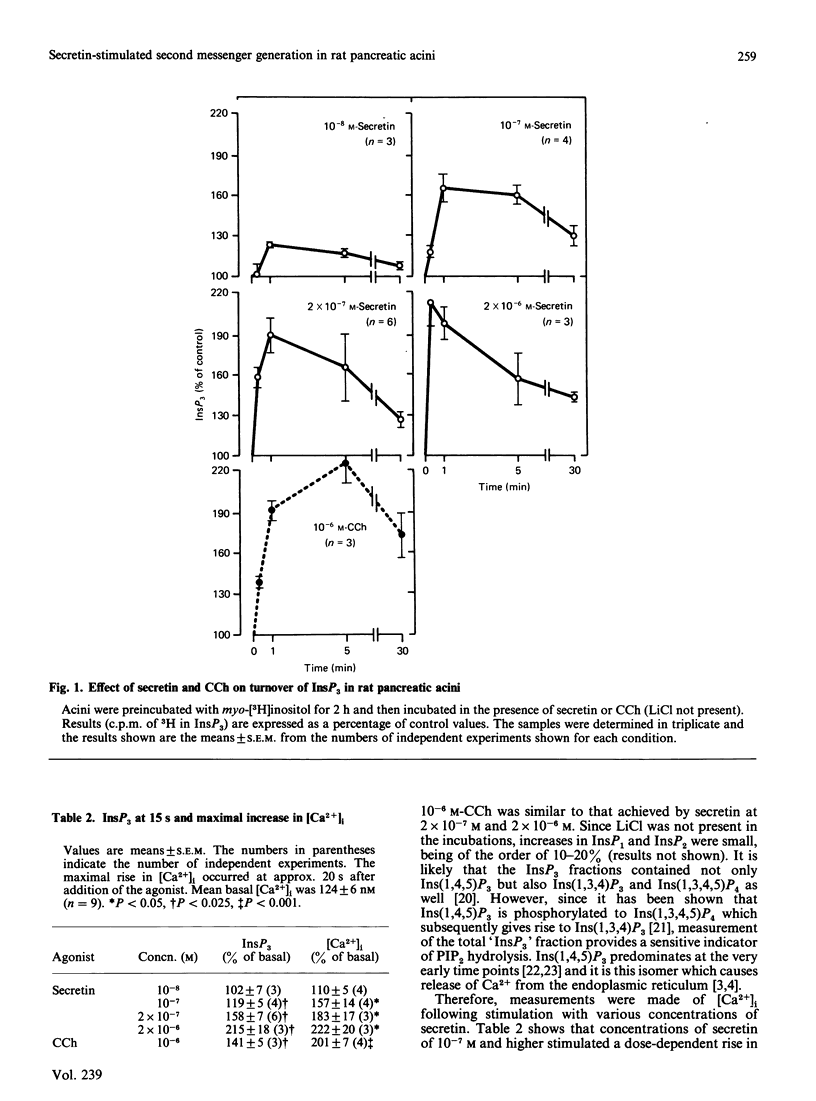
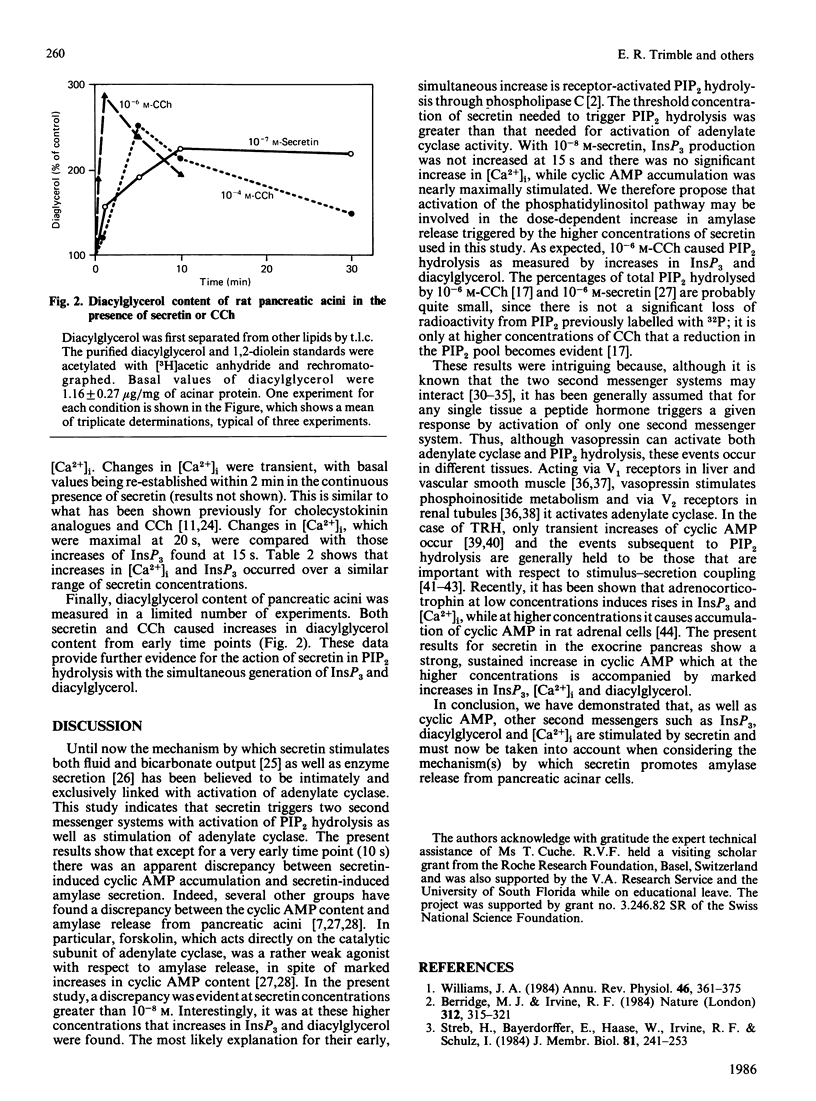
Previous studies have shown that the dose-response relationship for secretin-stimulated cyclic AMP accumulation is different from that for secretin-stimulated enzyme secretion in the rat exocrine pancreas. Here we show that secretin concentrations of 10(-10) M and higher stimulated a rise in cyclic AMP levels, with maximum effect on cyclic AMP accumulation being achieved already with 10(-8) M-secretin. However, at this concentration of secretin, enzyme secretion rates were approximately half-maximal. Unexpectedly, at concentrations of secretin greater than 10(-8) M there was evidence suggestive of phosphatidylinositol bisphosphate hydrolysis with rapid increases in inositol trisphosphate, cytosolic free calcium and diacylglycerol content of rat pancreatic acini. Furthermore, there was a dose-response relationship among secretin concentration (in the range 10(-8) M-2 X 10(-6) M), increases in inositol trisphosphate and increases in cytosolic free calcium ([Ca2+]i). Contrary to what has been previously believed, these results clearly indicate that in rat pancreatic acini secretin not only stimulates cyclic AMP accumulation but also raises inositol trisphosphate, [Ca2+]i and diacylglycerol. Thus, two second messenger systems may play a role in the regulation of secretin-induced amylase release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banschbach M. W., Geison R. L., O'Brien J. F. Use of (1-14C) aectic anhydride to quantitate diglycerides: a new analytical procedure. Anal Biochem. 1974 Jun;59(2):617–627. doi: 10.1016/0003-2697(74)90315-7. [DOI] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bissonnette B. M., Collen M. J., Adachi H., Jensen R. T., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on rat pancreatic acini. Am J Physiol. 1984 Jun;246(6 Pt 1):G710–G717. doi: 10.1152/ajpgi.1984.246.6.G710. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Pozzan T., Wollheim C. B. Caerulein and carbamoylcholine stimulate pancreatic amylase release at resting cytosolic free Ca2+. Biochem J. 1986 Apr 1;235(1):139–143. doi: 10.1042/bj2350139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannies P. S., Gautvik K. M., Tashjian A. H., Jr A possible role of cyclic AMP in mediating the effects of thyrotropin-releasing hormone on prolactin release and on prolactin and growth hormone synthesis in pituitary cells in culture. Endocrinology. 1976 May;98(5):1147–1159. doi: 10.1210/endo-98-5-1147. [DOI] [PubMed] [Google Scholar]
- Dehaye J. P., Gillard M., Poloczek P., Stievenart M., Winand J., Christophe J. Effects of forskolin on adenylate cyclase activity and amylase secretion in the rat exocrine pancreas. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(3):269–280. [PubMed] [Google Scholar]
- Farese R. V., Orchard J. L., Larson R. E., Sabir M. A., Davis J. S. Phosphatidylinositol hydrolysis and phosphatidylinositol 4',5-diphosphate hydrolysis are separable responses during secretagogue action in the rat pancreas. Biochim Biophys Acta. 1985 Aug 30;846(2):296–304. doi: 10.1016/0167-4889(85)90077-1. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Rosic N., Babischkin J., Farese M. G., Foster R., Davis J. S. Dual activation of the inositol-triphosphate-calcium and cyclic nucleotide intracellular signaling systems by adrenocorticotropin in rat adrenal cells. Biochem Biophys Res Commun. 1986 Mar 28;135(3):742–748. doi: 10.1016/0006-291x(86)90991-5. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Jensen R. T. Receptors and cell activation associated with pancreatic enzyme secretion. Annu Rev Physiol. 1986;48:103–117. doi: 10.1146/annurev.ph.48.030186.000535. [DOI] [PubMed] [Google Scholar]
- Gautvik K. M., Walaas E., Walaas O. Effect of thyroliberin on the concentration of adenosine 3':5'-phosphate and on the activity of adenosine 3':5'-phosphate-dependent protein kinase in prolactin-producing cells in culture. Biochem J. 1977 Feb 15;162(2):379–386. doi: 10.1042/bj1620379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S. Forskolin potentiates calcium-dependent amylase secretion from rat pancreatic acinar cells. Can J Physiol Pharmacol. 1983 Oct;61(10):1168–1176. doi: 10.1139/y83-174. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Kinsella A. R., Houslay M. D. The phorbol ester, TPA inhibits glucagon-stimulated adenylate cyclase activity. FEBS Lett. 1984 May 7;170(1):38–42. doi: 10.1016/0014-5793(84)81364-2. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litosch I., Lin S. H., Fain J. N. Rapid changes in hepatocyte phosphoinositides induced by vasopressin. J Biol Chem. 1983 Nov 25;258(22):13727–13732. [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orchard J. L., Davis J. S., Larson R. E., Farese R. V. Effects of carbachol and pancreozymin (cholecystokinin-octapeptide) on polyphosphoinositide metabolism in the rat pancreas in vitro. Biochem J. 1984 Jan 1;217(1):281–287. doi: 10.1042/bj2170281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985 Aug 25;260(18):10081–10086. [PubMed] [Google Scholar]
- Powers R. E., Saluja A. K., Houlihan M. J., Steer M. L. Inositol trisphosphate production and amylase secretion in mouse pancreatic acini. Biochem Biophys Res Commun. 1985 Aug 30;131(1):284–288. doi: 10.1016/0006-291x(85)91800-5. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebois R. V., Patel J. Phorbol ester causes desensitization of gonadotropin-responsive adenylate cyclase in a murine Leydig tumor cell line. J Biol Chem. 1985 Jul 5;260(13):8026–8031. [PubMed] [Google Scholar]
- Robberecht P., Waelbroeck M., Noyer M., Chatelain P., De Neef P., König W., Christophe J. Characterization of secretin and vasoactive intestinal peptide receptors in rat pancreatic plasma membranes using the native peptides, secretin-(7-27) and five secretin analogues. Digestion. 1982;23(3):201–210. doi: 10.1159/000198728. [DOI] [PubMed] [Google Scholar]
- Roy C. Regulation by adenosine of the vasopressin-sensitive adenylate cyclase in pig-kidney cells (LLC-PK1L) grown in defined media. Eur J Biochem. 1984 Sep 3;143(2):243–250. doi: 10.1111/j.1432-1033.1984.tb08365.x. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Godfrey P. P., Chapman D. A., Putney J. W., Jr Secretagogue-induced formation of inositol phosphates in rat exocrine pancreas. Implications for a messenger role for inositol trisphosphate. Biochem J. 1984 Apr 15;219(2):655–659. doi: 10.1042/bj2190655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Roduit C., Zahnd G. R. Polyphosphoinositide hydrolysis by phospholipase C is accelerated by thyrotropin releasing hormone (TRH) in clonal rat pituitary cells (GH3 cells). FEBS Lett. 1984 Mar 12;168(1):54–60. doi: 10.1016/0014-5793(84)80205-7. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Nambi P., Peters J. R., Lefkowitz R. J. Phorbol diesters promote beta-adrenergic receptor phosphorylation and adenylate cyclase desensitization in duck erythrocytes. Biochem Biophys Res Commun. 1984 Jun 29;121(3):973–979. doi: 10.1016/0006-291x(84)90772-1. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A. Regulatory mechanisms in pancreas and salivary acini. Annu Rev Physiol. 1984;46:361–375. doi: 10.1146/annurev.ph.46.030184.002045. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984 Feb 25;259(4):2262–2267. [PubMed] [Google Scholar]


