Abstract
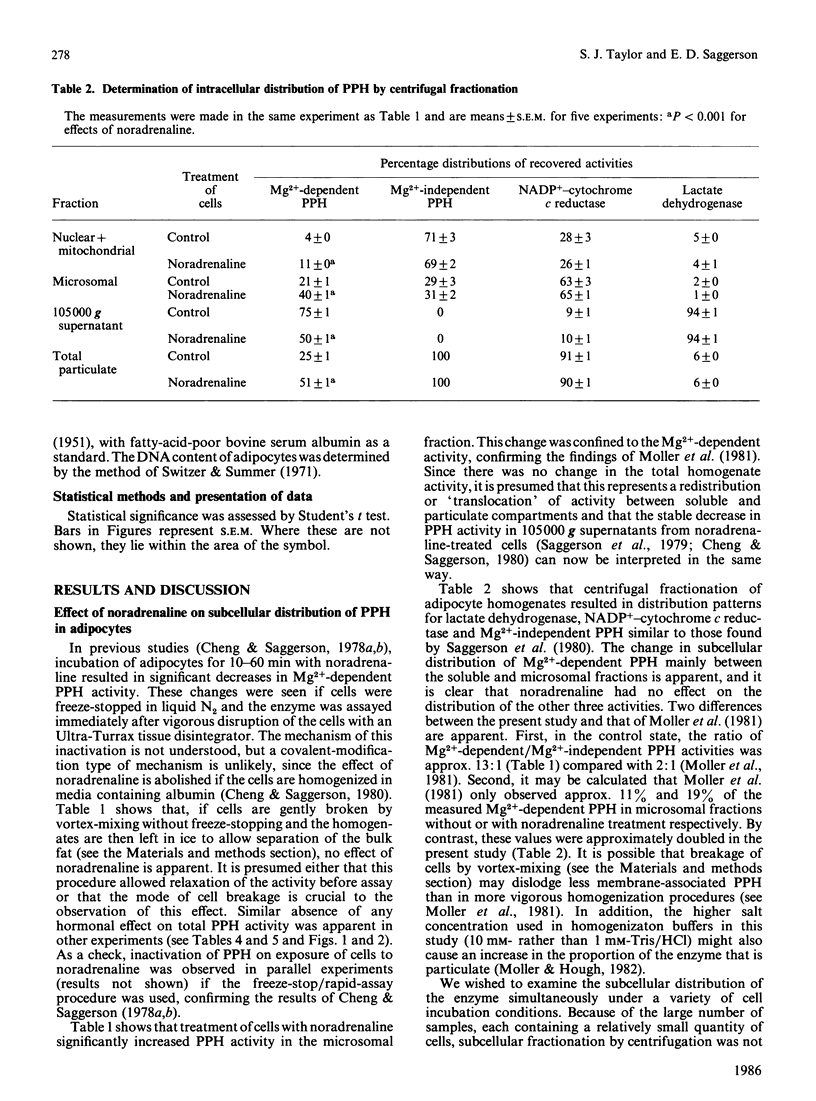
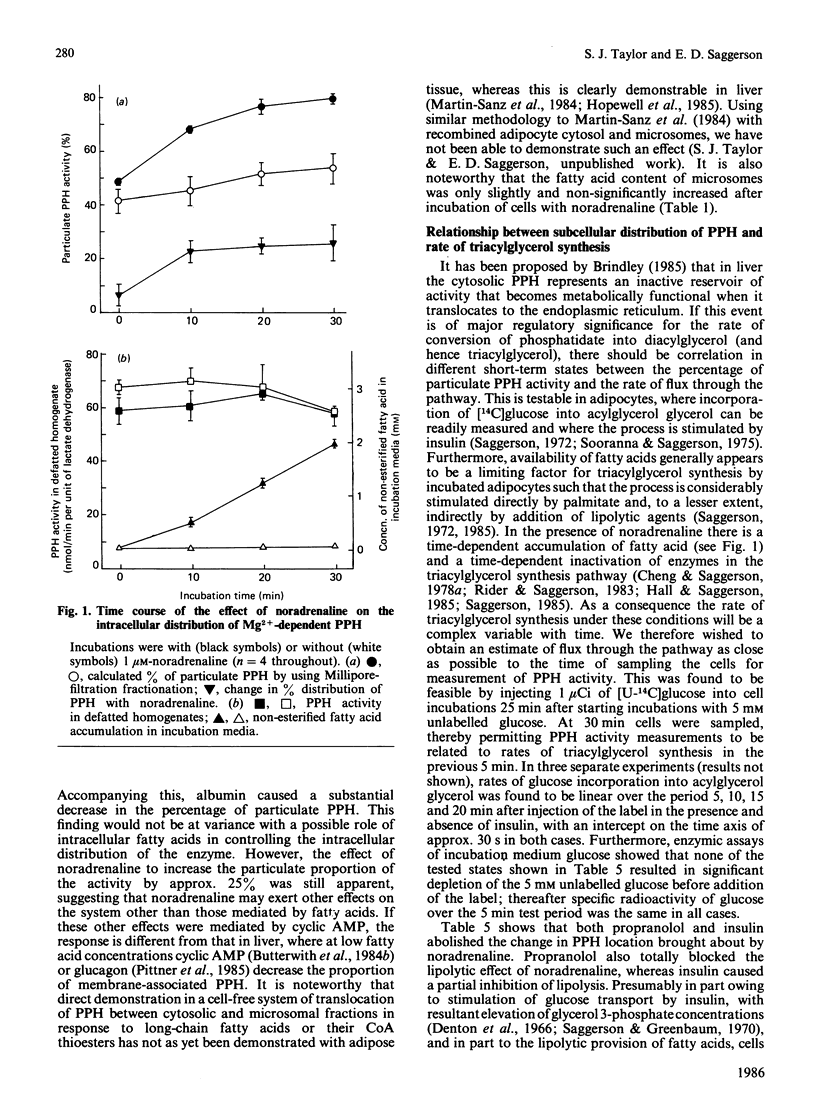
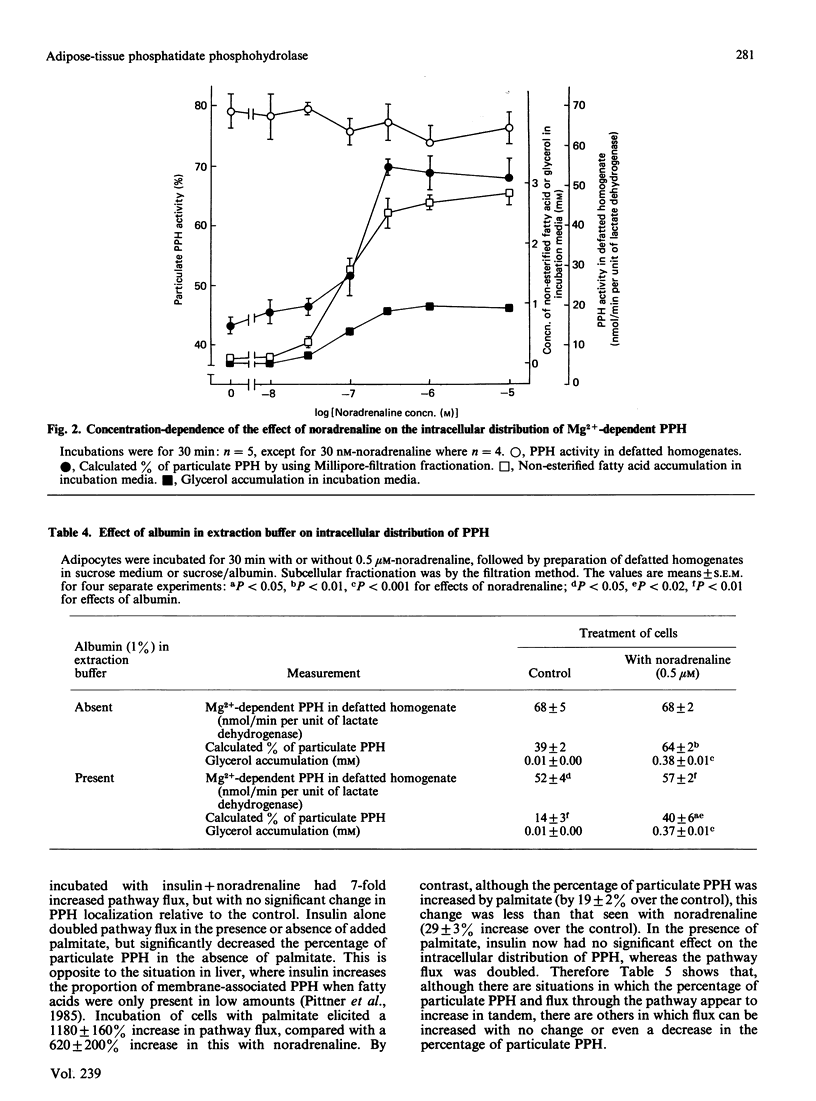
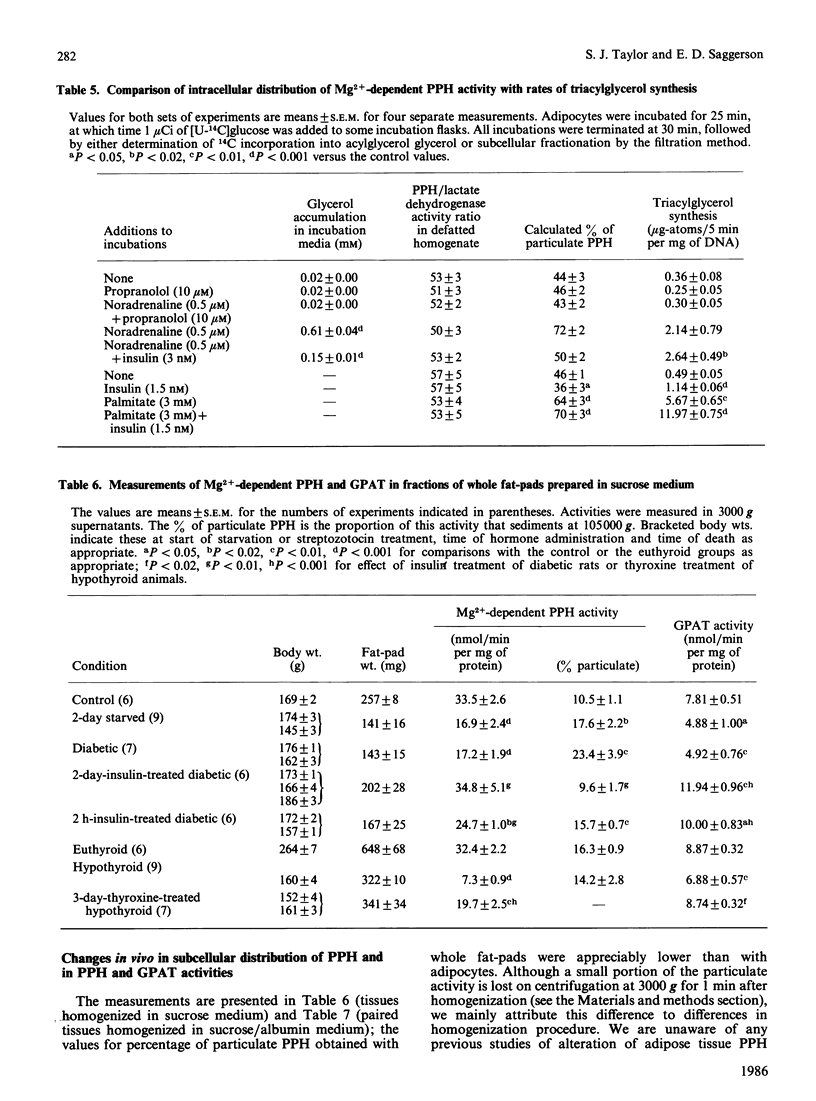
The subcellular distribution of Mg2+-dependent phosphatidate phosphohydrolase in rat adipocytes between a soluble and a membrane-bound fraction was measured by using both centrifugal fractionation and a novel Millipore-filtration method. The relative proportion of the phosphohydrolase associated with the particulate fraction was increased on incubation of cells with noradrenaline or palmitate. Insulin on its own decreased the proportion of the phosphohydrolase that was particulate and abolished the effect of noradrenaline, but not that of palmitate. The effect of noradrenaline on phosphohydrolase distribution was rapid, the effect being maximal within 10 min. Noradrenaline exerted this effect with a similar concentration-dependence to its lipolytic effect. Inclusion of albumin in homogenization buffers decreased the proportion of the phosphohydrolase that was particulate, but did not abolish the effect of noradrenaline. There was limited correlation between the proportion of the phosphohydrolase that was particulate and the measured rate of triacylglycerol synthesis in adipocytes incubated under a variety of conditions. Starvation, streptozotocin-diabetes and hypothyroidism decreased the specific activities of the phosphohydrolase and glycerolphosphate acyltransferase in homogenates from epididymal fat-pads. Restoration of these activities in the diabetic state was seen after administration of insulin over 2 days or, in the short term, within 2 h after a single administration of insulin. Administration of thyroxine over 3 days caused restoration of these activities in the hypothyroid state. Starvation and diabetes increased the proportion of the phosphohydrolase found in the microsomal fraction. This change was not seen when albumin was present in homogenization buffers. The possible role of fatty acids as regulators of the intracellular translocation of the phosphohydrolase, together with the role of this enzyme in the regulation of triacylglycerol synthesis in adipose tissue, is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Aas M., Daae L. N. Fatty acid activation and acyl transfer in organs from rats in different nutritional states. Biochim Biophys Acta. 1971 Jul 13;239(2):208–216. doi: 10.1016/0005-2760(71)90166-4. [DOI] [PubMed] [Google Scholar]
- Angel A., Desai K. S., Halperin M. L. Intracellular accumulation of free fatty acids in isolated white adipose cells. J Lipid Res. 1971 Jan;12(1):104–111. [PubMed] [Google Scholar]
- Angel A., Desai K. S., Halperin M. L. Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res. 1971 Mar;12(2):203–213. [PubMed] [Google Scholar]
- Angel A., Roncari D. A. The control of fatty acid esterification in a subcellular preparation of rat adipose tissue. Biochim Biophys Acta. 1967 Jun 6;137(3):464–474. doi: 10.1016/0005-2760(67)90127-0. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson D. A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett. 1977 Dec 15;84(2):229–232. doi: 10.1016/0014-5793(77)80694-7. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson E. D. A study of the glycerol phosphate acyltransferase and dihydroxyacetone phosphate acyltransferase activities in rat liver mitochondrial and microsomal fractions. Relative distribution in parenchymal and non-parenchymal cells, effects of N-ethylmaleimide, palmitoyl-coenzyme A concentration, starvation, adrenalectomy and anti-insulin serum treatment. Biochem J. 1979 Sep 15;182(3):751–762. doi: 10.1042/bj1820751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund L., Björkhem I., Einarsson K. Apparent phosphorylation - dephosphorylation of soluble phosphatidic acid phosphatase in rat liver. Biochem Biophys Res Commun. 1982 Mar 15;105(1):288–295. doi: 10.1016/s0006-291x(82)80043-0. [DOI] [PubMed] [Google Scholar]
- Brindley D. N. Intracellular translocation of phosphatidate phosphohydrolase and its possible role in the control of glycerolipid synthesis. Prog Lipid Res. 1984;23(3):115–133. doi: 10.1016/0163-7827(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Butterwith S. C., Hopewell R., Brindley D. N. Partial purification and characterization of the soluble phosphatidate phosphohydrolase of rat liver. Biochem J. 1984 Jun 15;220(3):825–833. doi: 10.1042/bj2200825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterwith S. C., Martin A., Brindley D. N. Can phosphorylation of phosphatidate phosphohydrolase by a cyclic AMP-dependent mechanism regulate its activity and subcellular distribution and control hepatic glycerolipid synthesis? Biochem J. 1984 Sep 1;222(2):487–493. doi: 10.1042/bj2220487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales C., Mangiapane E. H., Brindley D. N. Oleic acid promotes the activation and translocation of phosphatidate phosphohydrolase from the cytosol to particulate fractions of isolated rat hepatocytes. Biochem J. 1984 May 1;219(3):911–916. doi: 10.1042/bj2190911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzipanteli K., Saggerson D. Streptozotocin diabetes results in increased responsiveness of adipocyte lipolysis to glucagon. FEBS Lett. 1983 May 2;155(1):135–138. doi: 10.1016/0014-5793(83)80225-7. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cheng C. H., Saggerson E. D. Rapid antagonistic actions of noradrealine and insulin on rat adipocyte phosphatidate phosphohydrolase activity. FEBS Lett. 1978 Sep 1;93(1):120–124. doi: 10.1016/0014-5793(78)80818-7. [DOI] [PubMed] [Google Scholar]
- Cheng C. H., Saggerson E. D. Rapid effects of noradrenaline on Mg2+-dependent phosphatidate phosphohydrolase activity in rat adipocytes. FEBS Lett. 1978 Mar 1;87(1):65–68. doi: 10.1016/0014-5793(78)80134-3. [DOI] [PubMed] [Google Scholar]
- Cheng C. H., Saggerson E. D. The inactivation of rat adipocyte Mg2+-dependent phosphatidate phosphohydrolase by noradrenaline. Biochem J. 1980 Sep 15;190(3):659–662. doi: 10.1042/bj1900659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. H., Sooranna S. R., Saggerson E. D. Effects of noradrenaline and N6,O2'-dibutyryl 3',5'-cyclic AMP on adipocyte glycerolipid-synthesizing enzymes. Int J Biochem. 1980;12(4):667–670. doi: 10.1016/0020-711x(80)90025-7. [DOI] [PubMed] [Google Scholar]
- Chohan P., Carpenter C., Saggerson E. D. Changes in the anti-lipolytic action and binding to plasma membranes of N6-L-phenylisopropyladenosine in adipocytes from starved and hypothyroid rats. Biochem J. 1984 Oct 1;223(1):53–59. doi: 10.1042/bj2230053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang A. Q., Faas F. H., Carter W. J. Effects of streptozotocin-induced diabetes on phosphoglyceride metabolism of the rat liver. Lipids. 1984 Oct;19(10):738–748. doi: 10.1007/BF02534467. [DOI] [PubMed] [Google Scholar]
- Dang A. Q., Faas F. H., Carter W. J. Influence of hypo- and hyperthyroidism on rat liver glycerophospholipid metabolism. Lipids. 1985 Dec;20(12):897–902. doi: 10.1007/BF02534774. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Yorke R. E., Randle P. J. Measurement of concentrations of metabolites in adipose tissue and effects of insulin, alloxan-diabetes and adrenaline. Biochem J. 1966 Aug;100(2):407–419. doi: 10.1042/bj1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. H., MUELLER P. S. EFFECTS OF PALMITATE ON THE METABOLISM OF LEUKOCYTES FROM GUINEA PIG EXUDATE. J Lipid Res. 1963 Jan;4:39–45. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Hall M., Saggerson E. D. Reversible inactivation by noradrenaline of long-chain fatty acyl-CoA synthetase in rat adipocytes. Biochem J. 1985 Feb 15;226(1):275–282. doi: 10.1042/bj2260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper R. D., Saggerson E. D. Factors affecting fatty acid oxidation in fat cells isolated from rat white adipose tissue. J Lipid Res. 1976 Sep;17(5):516–526. [PubMed] [Google Scholar]
- Heindel J. J., Cushman S. W., Jeanrenaud B. Cell-associated fatty acid levels and energy-requiring processes in mouse adipocytes. Am J Physiol. 1974 Jan;226(1):16–24. doi: 10.1152/ajplegacy.1974.226.1.16. [DOI] [PubMed] [Google Scholar]
- Hopewell R., Martin-Sanz P., Martin A., Saxton J., Brindley D. N. Regulation of the translocation of phosphatidate phosphohydrolase between the cytosol and the endoplasmic reticulum of rat liver. Effects of unsaturated fatty acids, spermine, nucleotides, albumin and chlorpromazine. Biochem J. 1985 Dec 1;232(2):485–491. doi: 10.1042/bj2320485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Jamdar S. C., Fallon H. J. Glycerolipid synthesis in rat adipose tissue. II. Properties and distribution of phosphatidate phosphatase. J Lipid Res. 1973 Sep;14(5):517–524. [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J. Glycerolipid biosynthesis in rat adipose tissue. 10. Changes during a starvation and re-feeding cycle. Biochim Biophys Acta. 1982 Dec 13;713(3):647–656. doi: 10.1016/0005-2760(82)90325-3. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J., Wells G. N. Glycerolipid biosynthesis in rat adipose tissue 12. Properties of Mg2+-dependent and -independent phosphatidate phosphohydrolase. Arch Biochem Biophys. 1984 Sep;233(2):370–377. doi: 10.1016/0003-9861(84)90458-2. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J., Zeigler J. A. Glycerolipid biosynthesis in rat adipose tissue. Influence of adipocyte size. Biochem J. 1981 Jan 15;194(1):293–298. doi: 10.1042/bj1940293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula V. L., Savolainen M. J., Hassinen I. Hepatic triacylglycerol and fatty-acid biosynthesis during hypoxia in vivo. Acta Physiol Scand. 1978 Oct;104(2):148–155. doi: 10.1111/j.1748-1716.1978.tb06261.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawson N., Pollard A. D., Jennings R. J., Gurr M. I., Brindley D. N. The activities of lipoprotein lipase and of enzymes involved in triacylglycerol synthesis in rat adipose tissue. Effects of starvation, dietary modification and of corticotropin injection. Biochem J. 1981 Nov 15;200(2):285–294. doi: 10.1042/bj2000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiapane E. H., Lloyd-Davies K. A., Brindley D. N. A study of some enzymes of glycerolipid biosynthesis in rat liver after subtotal hepatectomy. Biochem J. 1973 May;134(1):103–112. doi: 10.1042/bj1340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanz P., Hopewell R., Brindley D. N. Long-chain fatty acids and their acyl-CoA esters cause the translocation of phosphatidate phosphohydrolase from the cytosolic to the microsomal fraction of rat liver. FEBS Lett. 1984 Oct 1;175(2):284–288. doi: 10.1016/0014-5793(84)80752-8. [DOI] [PubMed] [Google Scholar]
- Martin-Sanz P., Hopewell R., Brindley D. N. Spermine promotes the translocation of phosphatidate phosphohydrolase from the cytosol to the microsomal fraction of rat liver and it enhances the effects of oleate in this respect. FEBS Lett. 1985 Jan 7;179(2):262–266. doi: 10.1016/0014-5793(85)80531-7. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller F., Green P., Harkness E. J. Soluble rat adipocyte phosphatidate phosphatase activity: characterization and effects of fasting and various lipids. Biochim Biophys Acta. 1977 Feb 23;486(2):359–368. doi: 10.1016/0005-2760(77)90032-7. [DOI] [PubMed] [Google Scholar]
- Moller F., Hough M. R. Effect of salts on membrane binding and activity of adipocyte phosphatidate phosphohydrolase. Biochim Biophys Acta. 1982 Jun 11;711(3):521–531. doi: 10.1016/0005-2760(82)90068-6. [DOI] [PubMed] [Google Scholar]
- Moller F., Wong K. H., Green P. Control of fat cell phosphohydrolase by lipolytic agents. Can J Biochem. 1981 Jan;59(1):9–15. doi: 10.1139/o81-002. [DOI] [PubMed] [Google Scholar]
- Murthy V. K., Shipp J. C. Synthesis and accumulation of triglycerides in liver of diabetic rats. Effects of insulin treatment. Diabetes. 1979 May;28(5):472–478. doi: 10.2337/diab.28.5.472. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Interactions of insulin, glucagon and dexamethasone in controlling the activity of glycerol phosphate acyltransferase and the activity and subcellular distribution of phosphatidate phosphohydrolase in cultured rat hepatocytes. Biochem J. 1985 Sep 1;230(2):525–534. doi: 10.1042/bj2300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin S., Martin J. B., Boshans R. L., Schalch D. S., Pierce J. G., Bollinger J. Measurement of TSH in plasma and pituitary of the rat by a radioimmunoassay utilizing bovine TSH: effect of thyroidectomy or thyroxine administration on plasma TSH levels. Endocrinology. 1970 Nov;87(5):1022–1031. doi: 10.1210/endo-87-5-1022. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Saggerson E. D. Regulation by noradrenaline of the mitochondrial and microsomal forms of glycerol phosphate acyltransferase in rat adipocytes. Biochem J. 1983 Jul 15;214(1):235–246. doi: 10.1042/bj2140235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase in liver and five extrahepatic tissues in the rat. Inhibition by DL-2-bromopalmitoyl-CoA and effect of hypothyroidism. Biochem J. 1986 May 15;236(1):137–141. doi: 10.1042/bj2360137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Cheng C. H., Sooranna S. R. Subcellular distribution and some properties of N-ethylmaleimide-sensitive and-insensitive forms of glycerol phosphate acyltransferase in rat adipocytes. Biochem J. 1980 Jul 15;190(1):183–189. doi: 10.1042/bj1900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Tomassi G. The regulation of glyceride synthesis from pyruvate in isolated fat cells. The effects of palmitate and alteration of dietary status. Eur J Biochem. 1971 Nov 11;23(1):109–117. doi: 10.1111/j.1432-1033.1971.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Studies on the role of insulin in the regulation of glyceride synthesis in rat epididymal adipose tissue. Biochem J. 1975 Sep;150(3):441–451. doi: 10.1042/bj1500441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]
- Vavrecka M., Mitchell M. P., Hübscher G. The effect of starvation on the incorporation of palmitate into glycerides and phospholipids of rat liver homogenates. Biochem J. 1969 Nov;115(2):139–145. doi: 10.1042/bj1150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. H., Bowley M., Sturton R. G., Pritchard P. H., Brindley D. N., Hawthorne J. N. The effect of chronic diabetes, induced by streptozotocin, on the activities of some enzymes of glycerolipid synthesis in rat liver. Biochem J. 1977 Nov 15;168(2):147–153. doi: 10.1042/bj1680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase, the prototype ambiquitous enzyme. Curr Top Cell Regul. 1980;16:1–54. doi: 10.1016/b978-0-12-152816-4.50005-4. [DOI] [PubMed] [Google Scholar]
- Woods J. A., Knauer T. E., Lamb R. G. The acute effects of streptozotocin-induced diabetes on rat liver glycerolipid biosynthesis. Biochim Biophys Acta. 1981 Dec 23;666(3):482–492. doi: 10.1016/0005-2760(81)90310-6. [DOI] [PubMed] [Google Scholar]


