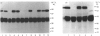Abstract
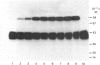
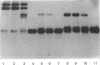
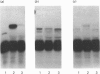
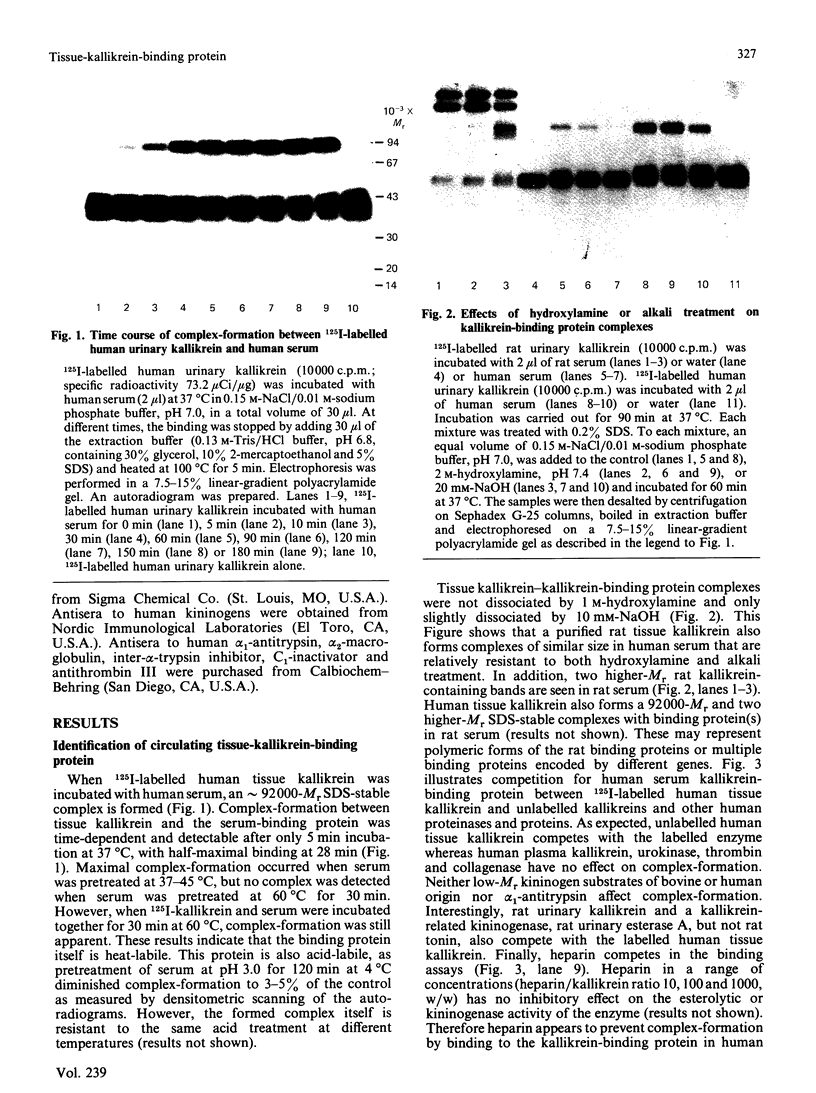
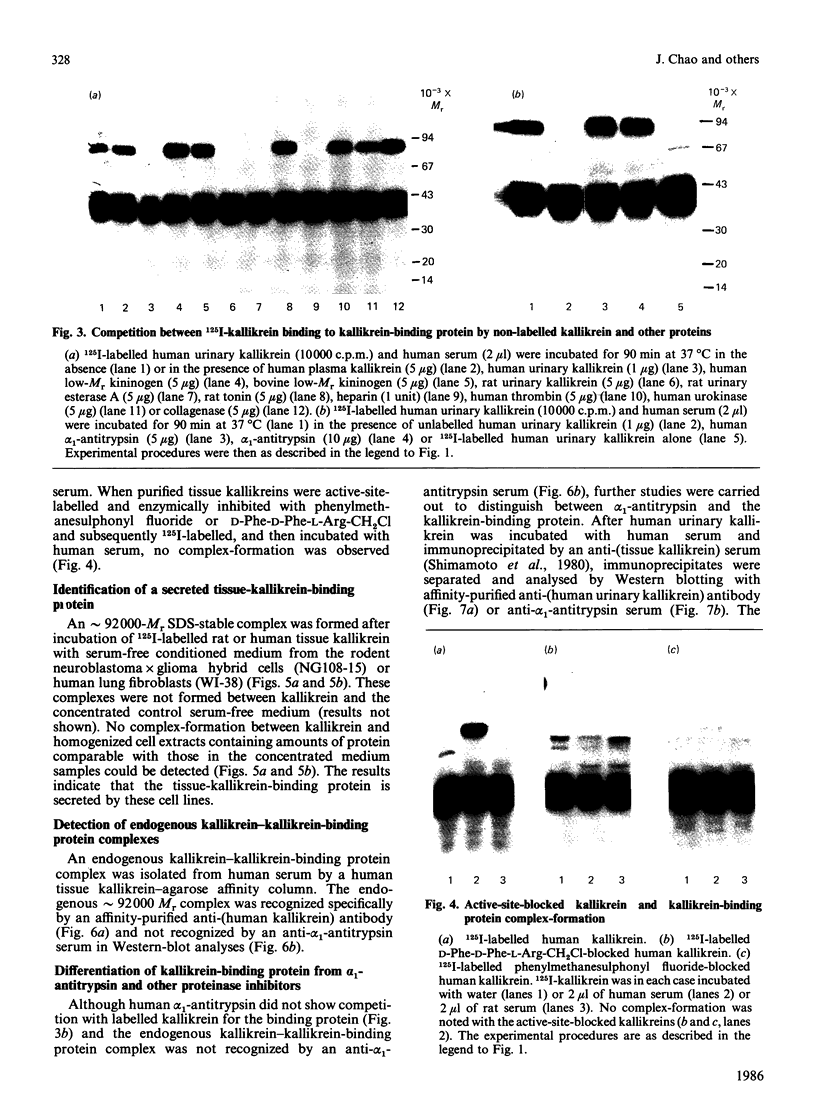
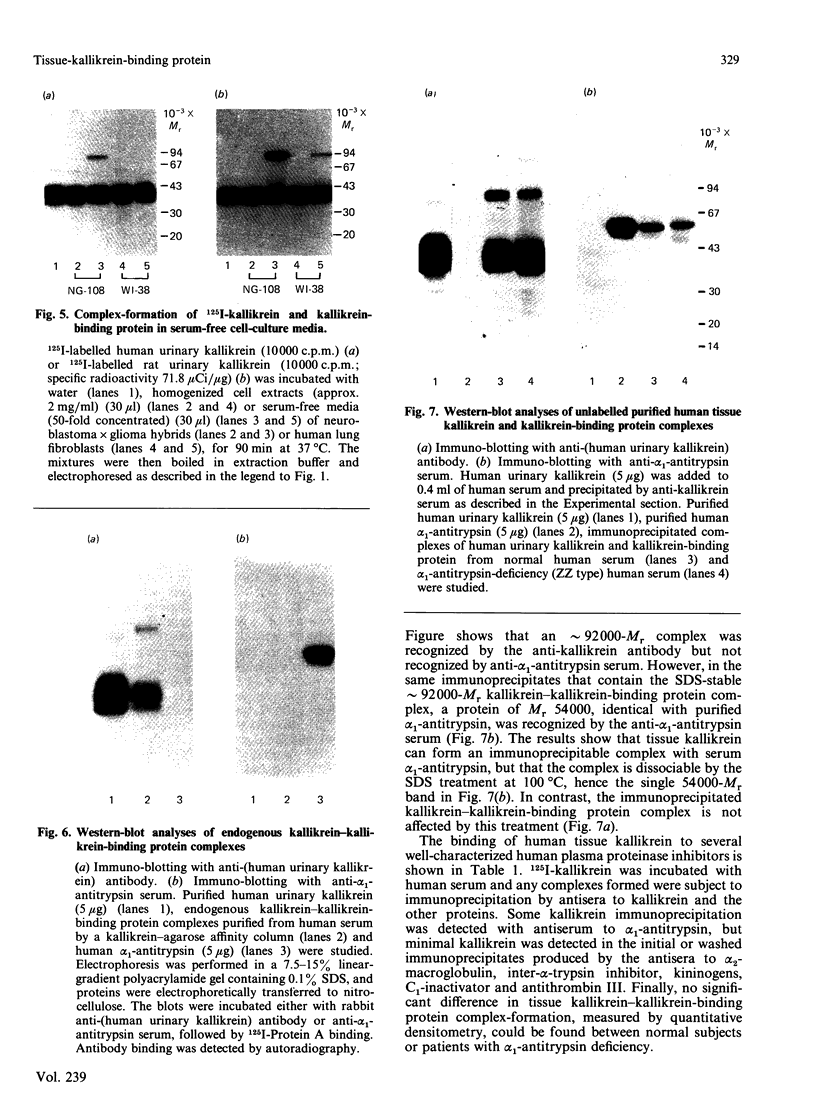
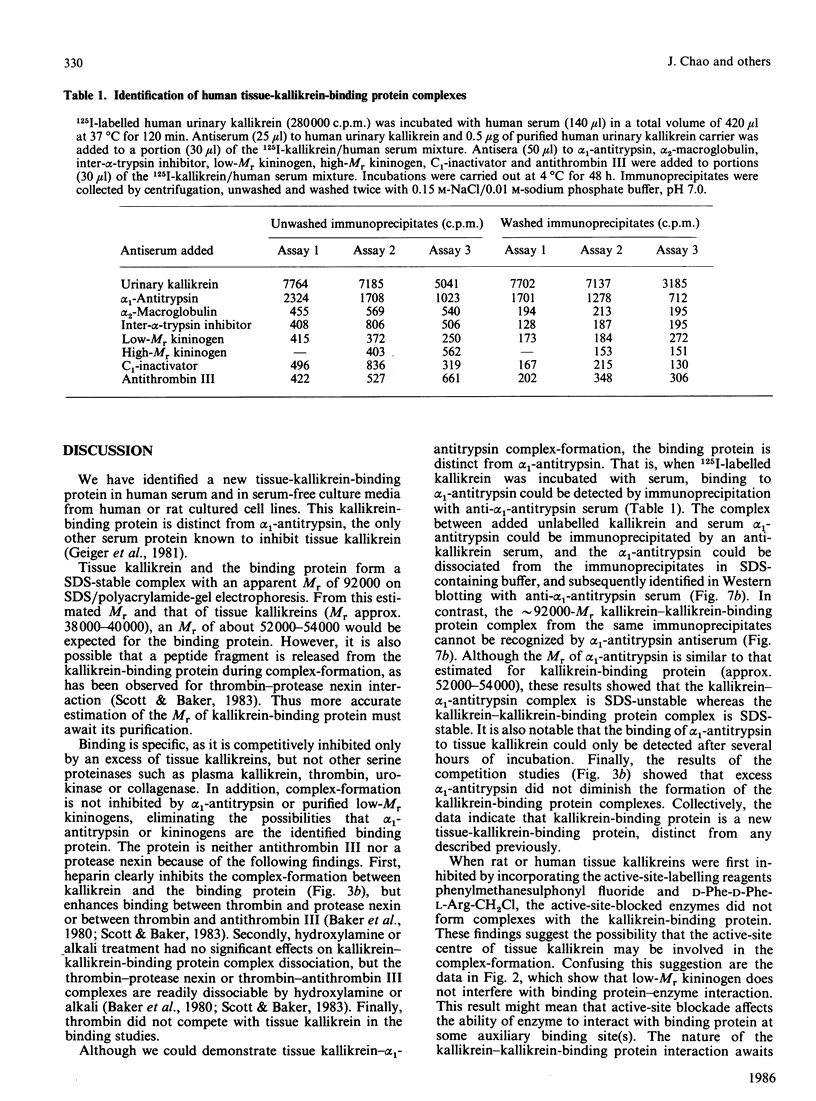
We have identified a tissue-kallikrein-binding protein in human serum and in the serum-free culture media from human lung fibroblasts (WI-38) and rodent neuroblastoma X glioma hybrid cells (NG108-15). Purified and 125I-labelled tissue kallikrein and human serum form an approximately 92,000-Mr SDS-stable complex. The relative quantity of this complex-formation is measured by densitometric scanning of autoradiograms. Complex-formation between tissue kallikrein and the serum binding protein was time-dependent and detectable after 5 min incubation at 37 degrees C, with half-maximal binding at 28 min. Binding of 125I-kallikrein to kallikrein-binding protein is temperature-dependent and can be inhibited by heparin or excess unlabelled tissue kallikrein but not by plasma kallikrein, collagenase, thrombin, urokinase, alpha 1-antitrypsin or kininogens. The kallikrein-binding protein is acid- and heat-labile, as pretreatment of sera at pH 3.0 or at 60 degrees C for 30 min diminishes complex-formation. However, the formed complexes are stable to acid or 1 M-hydroxylamine treatment and can only be partially dissociated with 10 mM-NaOH. When kallikrein was inhibited by the active-site-labelling reagents phenylmethanesulphonyl fluoride or D-Phe-D-Phe-L-Arg-CH2Cl no complex-formation was observed. An endogenous approximately 92,000-Mr kallikrein-kallikrein-binding protein complex was isolated from normal human serum by using a human tissue kallikrein-agarose affinity column. These complexes were recognized by anti-(human tissue kallikrein) antibodies, but not by anti-alpha 1-antitrypsin serum, in Western-blot analyses. The results show that the kallikrein-binding protein is distinct from alpha 1-antitrypsin and is not identifiable with any of the well-characterized plasma proteinase inhibitors such as alpha 2-macroglobulin, inter-alpha-trypsin inhibitor, C1-inactivator or antithrombin III. The functional role of this kallikrein-binding protein and its impact on kallikrein activity or metabolism in vivo remain to be investigated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cemassieux S., Boucher R., Crisé C., Genest J. Purification and characterization of tonin. Can J Biochem. 1976 Sep;54(9):788–795. doi: 10.1139/o76-113. [DOI] [PubMed] [Google Scholar]
- Chao J., Margolius H. S. Differential effects of testosterone, thyroxine, and cortisol on rat submandibular gland versus renal kallikrein. Endocrinology. 1983 Dec;113(6):2221–2225. doi: 10.1210/endo-113-6-2221. [DOI] [PubMed] [Google Scholar]
- Chao J., Margolius H. S. Isozymes of rat urinary kallikrein. Biochem Pharmacol. 1979 Jul 1;28(13):2071–2079. doi: 10.1016/0006-2952(79)90226-0. [DOI] [PubMed] [Google Scholar]
- Chao J. Purification and characterization of rat urinary esterase A, a plasminogen activator. J Biol Chem. 1983 Apr 10;258(7):4434–4439. [PubMed] [Google Scholar]
- Chao J., Woodley C., Chao L., Margolius H. S. Identification of tissue kallikrein in brain and in the cell-free translation product encoded by brain mRNA. J Biol Chem. 1983 Dec 25;258(24):15173–15178. [PubMed] [Google Scholar]
- Eaton D. L., Baker J. B. Evidence that a variety of cultured cells secrete protease nexin and produce a distinct cytoplasmic serine protease-binding factor. J Cell Physiol. 1983 Nov;117(2):175–182. doi: 10.1002/jcp.1041170207. [DOI] [PubMed] [Google Scholar]
- Gerald W. L., Chao J., Chao L. Sex dimorphism and hormonal regulation of rat tissue kallikrein mRNA. Biochim Biophys Acta. 1986 May 27;867(1-2):16–23. doi: 10.1016/0167-4781(86)90024-2. [DOI] [PubMed] [Google Scholar]
- Lawton W. J., Proud D., Frech M. E., Pierce J. V., Keiser H. R., Pisano J. J. Characterization and origin of immunoreactive glandular kallikrein in rat plasma. Biochem Pharmacol. 1981 Jul 1;30(13):1731–1737. doi: 10.1016/0006-2952(81)90002-2. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Chao J., Margolius H. S. Tissue kallikrein synthesis and its modification by testosterone or low dietary sodium. Biochem J. 1984 Feb 15;218(1):37–43. doi: 10.1042/bj2180037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolly H., Scicli A. G., Scicli G., Carretero O. A. Characterization of a kininogenase from rat vascular tissue resembling tissue kallikrein. Circ Res. 1985 Jun;56(6):816–821. doi: 10.1161/01.res.56.6.816. [DOI] [PubMed] [Google Scholar]
- Sakamoto W., Nishikaze O. Alpha 1-antitrypsin and alpha 2-macroglobulin do not inhibit the kinin-releasing activity of kallikreins from human urine and saliva. Biochim Biophys Acta. 1980 Dec 15;633(3):305–309. doi: 10.1016/0304-4165(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Baker J. B. Purification of human protease nexin. J Biol Chem. 1983 Sep 10;258(17):10439–10444. [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Shimamoto K., Chao J., Margolius H. S. The radioimmunoassay of human urinary kallikrein and comparisons with kallikrein activity measurements. J Clin Endocrinol Metab. 1980 Oct;51(4):840–848. doi: 10.1210/jcem-51-4-840. [DOI] [PubMed] [Google Scholar]
- Shimamoto K., Mayfield R. K., Margolius H. S., Chao J., Stroud W., Kaplan A. P. Immunoreactive tissue kallikrein in human serum. J Lab Clin Med. 1984 May;103(5):731–738. [PubMed] [Google Scholar]
- Simson J. A., Dom R., Chao J., Woodley C., Chao L., Margolius H. S. Immunocytochemical localization of tissue kallikrein in brain ventricular epithelium and hypothalamic cell bodies. J Histochem Cytochem. 1985 Sep;33(9):951–953. doi: 10.1177/33.9.3894505. [DOI] [PubMed] [Google Scholar]
- Vahtera E., Hamberg U. Absence of binding of pancreatic and urinary kallikreins to alpha 2-macroglobulin. Biochem J. 1976 Aug 1;157(2):521–524. doi: 10.1042/bj1570521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley C. M., Chao J., Margolius H. S., Chao L. Specific identification of tissue kallikrein in exocrine tissues and in cell-free translation products with monoclonal antibodies. Biochem J. 1985 Nov 1;231(3):721–728. doi: 10.1042/bj2310721. [DOI] [PMC free article] [PubMed] [Google Scholar]