Abstract
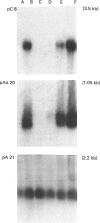
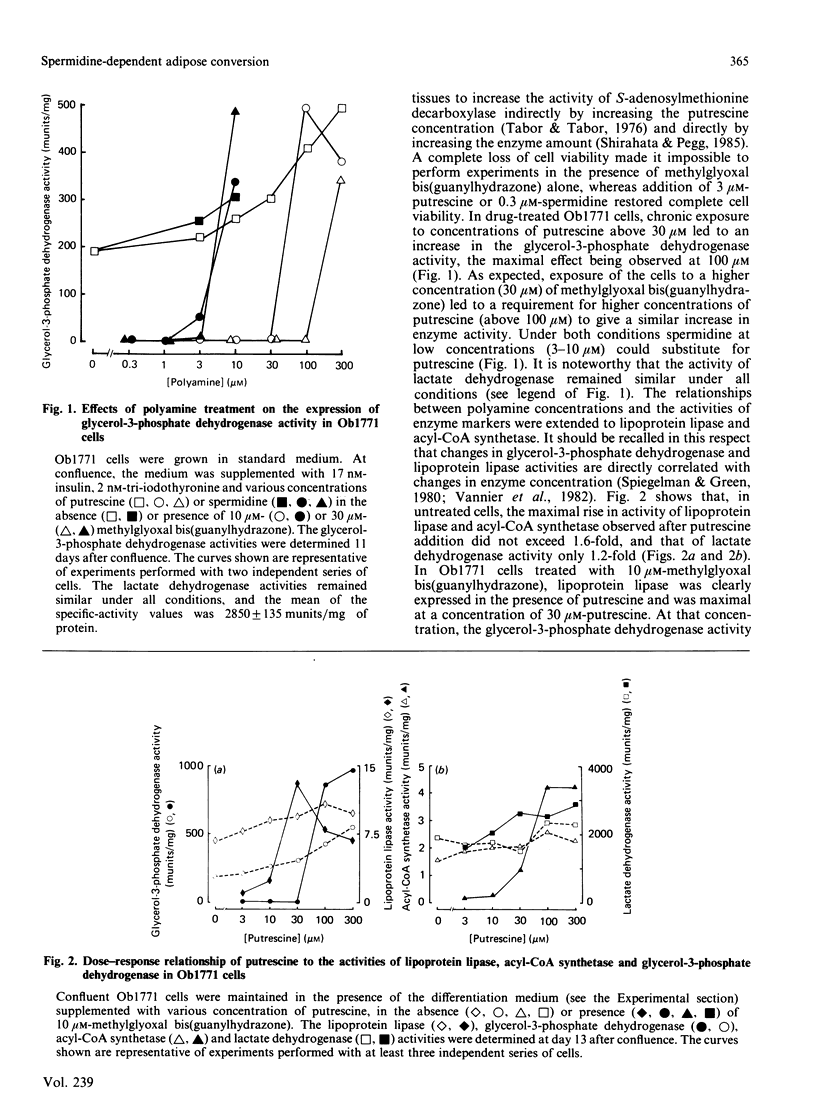
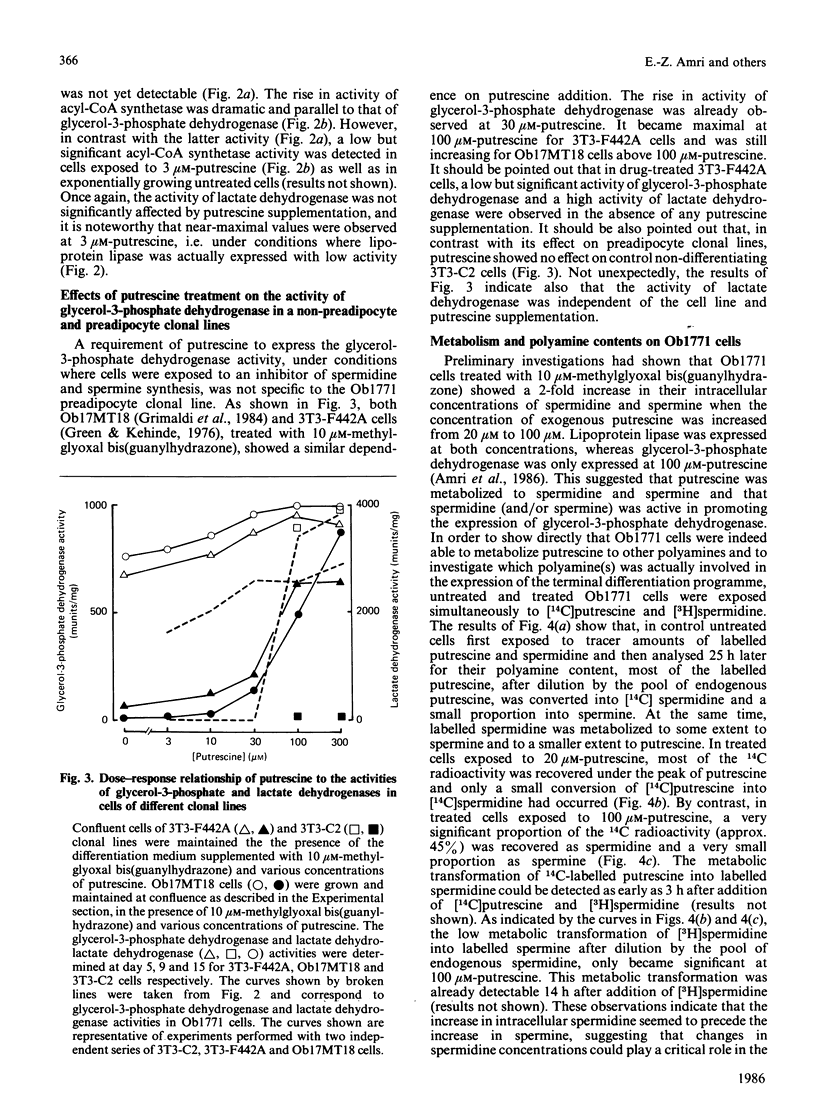
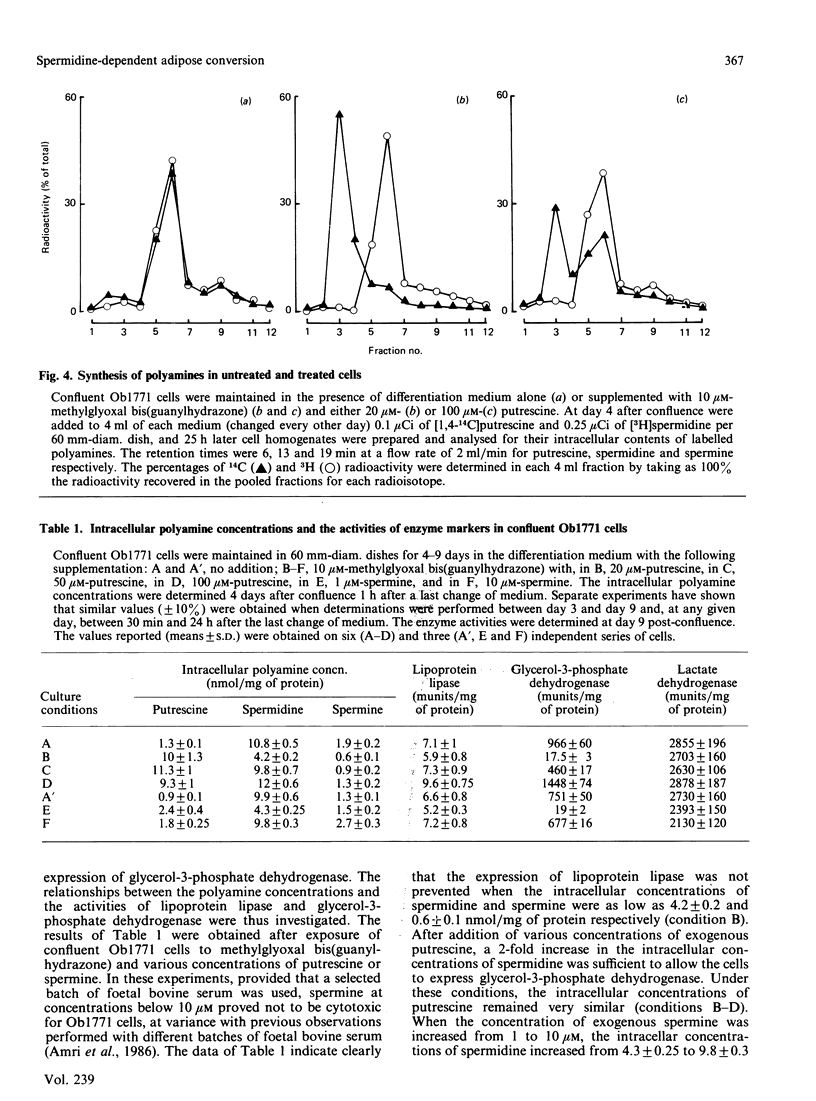
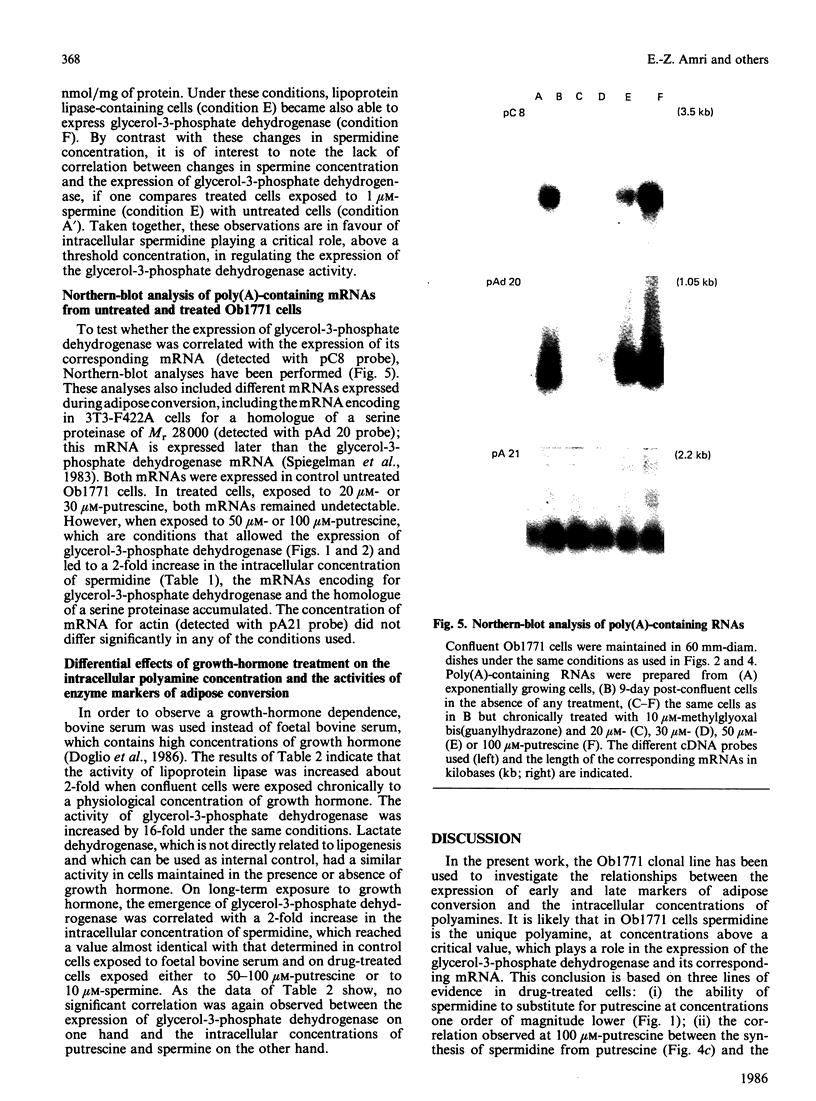
Confluent Ob1771 cells treated with an inhibitor of spermidine and spermine synthesis, methylglyoxyal bis(guanylhydrazone), were dependent on putrescine addition for the expression of glycerol-3-phosphate dehydrogenase and acyl-CoA synthetase, which behaved as late markers of adipose conversion. A similar dependence was observed with drug-treated Ob17MT18 and 3T3-F442A preadipocyte cells, but not with non-differentiating 3T3-C2 cells. Studies in drug-treated Ob1771 cells at the mRNA level showed that the parallel expression of mRNAs encoding for glycerol-3-phosphate dehydrogenase and an homologue of serine proteinases of Mr 28,000 [Cook, Groves, Min & Spiegelman (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 6480-6484] was also dependent on putrescine addition. Double-isotope experiments with [14C]putrescine and [3H]spermidine, as well as analysis of the polyamine content in drug-treated Ob1771 cells under various conditions, demonstrate after putrescine addition that the expression of late markers of adipose conversion was highly correlated with a 2-fold increase in the intracellular concentration of spermidine. No correlation was observed with changes in the intracellular concentrations of putrescine and spermine. Long-term exposure of untreated Ob1771 cells to growth hormone, which led to the expression of late markers of adipose conversion [Doglio, Dani, Grimaldi & Ailhaud (1986) Biochem. J. 238, 123-129] was also accompanied by the same increase in spermidine concentration, which attained values identical with those determined in drug-treated cells supplemented with putrescine. This observation suggests that the permissive effect of growth hormone on the terminal differentiation of adipose cells might e related to changes in the intracellular concentration of spermidine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amri E. Z., Dani C., Doglio A., Etienne J., Grimaldi P., Ailhaud G. Adipose cell differentiation: evidence for a two-step process in the polyamine-dependent Ob1754 clonal line. Biochem J. 1986 Aug 15;238(1):115–122. doi: 10.1042/bj2380115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri E. Z., Grimaldi P., Négrel R., Ailhaud G. Adipose conversion of ob17 cells. Insulin acts solely as a modulator in the expression of the differentiation program. Exp Cell Res. 1984 Jun;152(2):368–377. doi: 10.1016/0014-4827(84)90638-4. [DOI] [PubMed] [Google Scholar]
- Bethell D. R., Pegg A. E. Polyamines are needed for the differentiation of 3T3-L1 fibroblasts into adipose cells. Biochem Biophys Res Commun. 1981 Sep 16;102(1):272–278. doi: 10.1016/0006-291x(81)91517-5. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Cook K. S., Groves D. L., Min H. Y., Spiegelman B. M. A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6480–6484. doi: 10.1073/pnas.82.19.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doglio A., Dani C., Grimaldi P., Ailhaud G. Growth hormone regulation of the expression of differentiation-dependent genes in preadipocyte Ob1771 cells. Biochem J. 1986 Aug 15;238(1):123–129. doi: 10.1042/bj2380123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D., Ailhaud G., Négrel R. Fetuin modulates growth and differentiation of Ob17 preadipose cells in serum-free hormone-supplemented medium. Biochim Biophys Acta. 1985 Jul 30;846(1):185–191. doi: 10.1016/0167-4889(85)90125-9. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grimaldi P., Czerucka D., Rassoulzadegan M., Cuzin F., Ailhaud G. ob17 cells transformed by the middle-T-only gene of polyoma virus differentiate in vitro and in vivo into adipose cells. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5440–5444. doi: 10.1073/pnas.81.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murphy M. G., Négrel R., Ailhaud G. Lipoprotein lipase and monoacylglycerol lipase activities during maturation of ob17 preadipocytes. Biochim Biophys Acta. 1981 May 22;664(2):240–248. doi: 10.1016/0005-2760(81)90046-1. [DOI] [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Regulation of S-adenosylmethionine decarboxylase activity in rat liver and prostate. J Biol Chem. 1985 Aug 15;260(17):9583–9588. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Vannier C., Amri E. Z., Etienne J., Négrel R., Ailhaud G. Maturation and secretion of lipoprotein lipase in cultured adipose cells. I. Intracellular activation of the enzyme. J Biol Chem. 1985 Apr 10;260(7):4424–4431. [PubMed] [Google Scholar]
- Vannier C., Gaillard D., Grimaldi P., Amri E. Z., Djian P., Cermolacce C., Forest C., Etienne J., Negrel R., Ailhaud G. Adipose conversion of ob17 cells and hormone-related events. Int J Obes. 1985;9 (Suppl 1):41–53. [PubMed] [Google Scholar]
- Vannier C., Jansen H., Négrel R., Ailhaud G. Study of lipoprotein lipase content in Ob17 preadipocytes during adipose conversion. Immunofluorescent localization of the enzyme. J Biol Chem. 1982 Oct 25;257(20):12387–12393. [PubMed] [Google Scholar]