Abstract
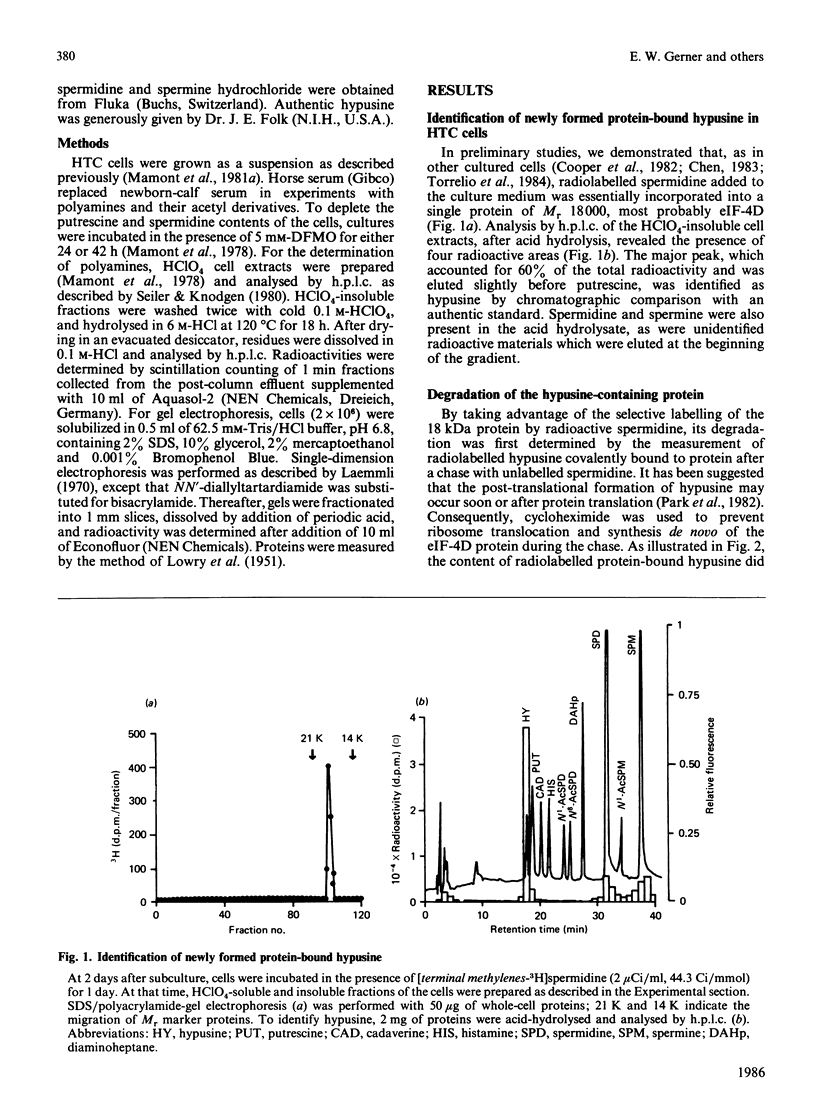
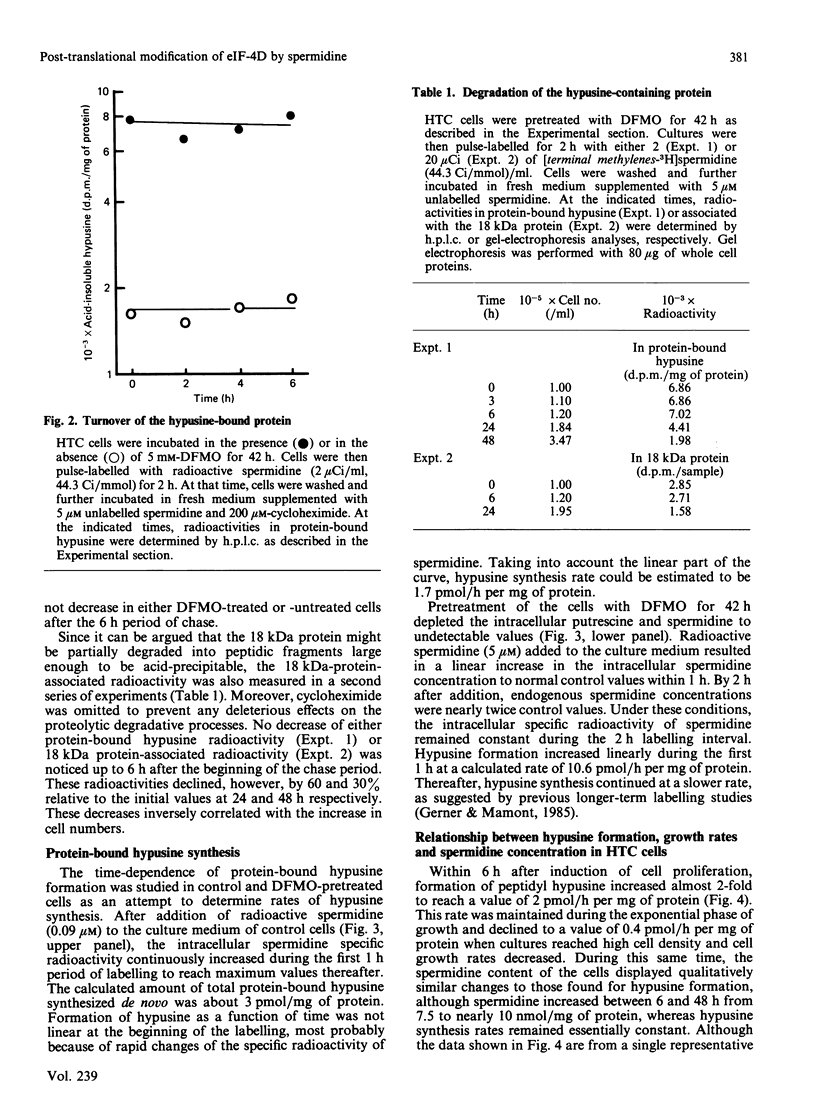
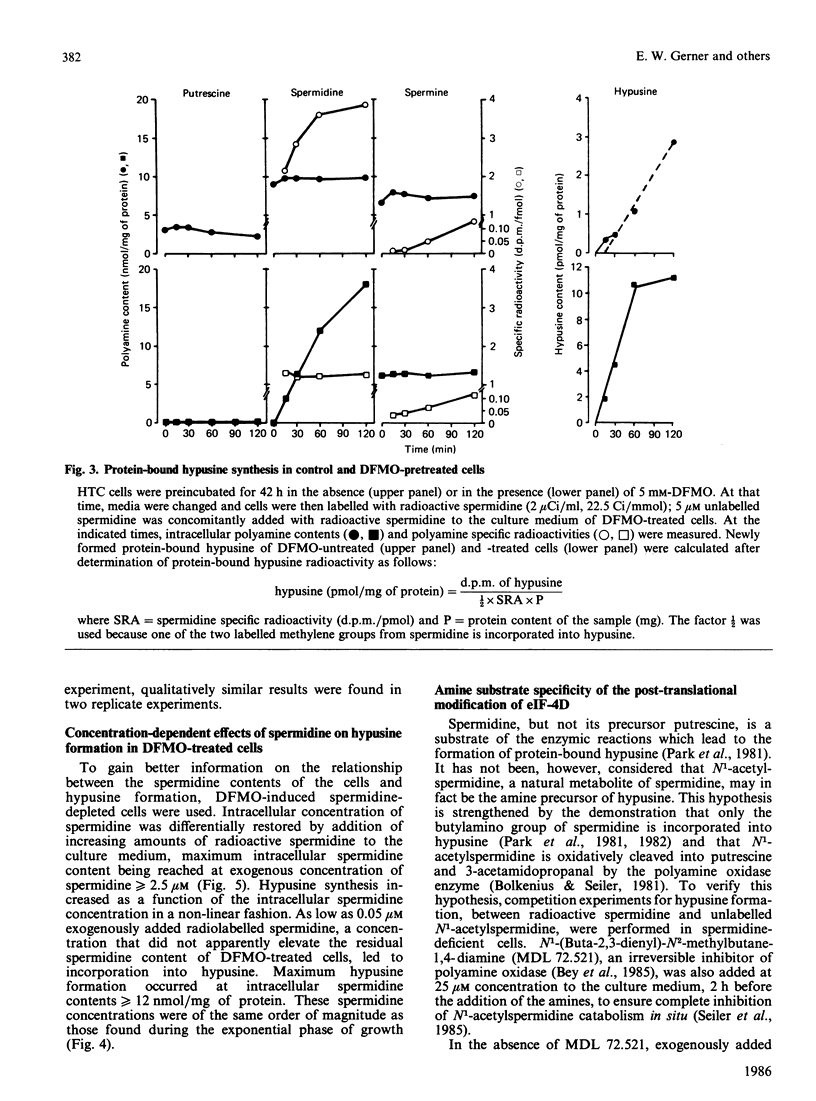
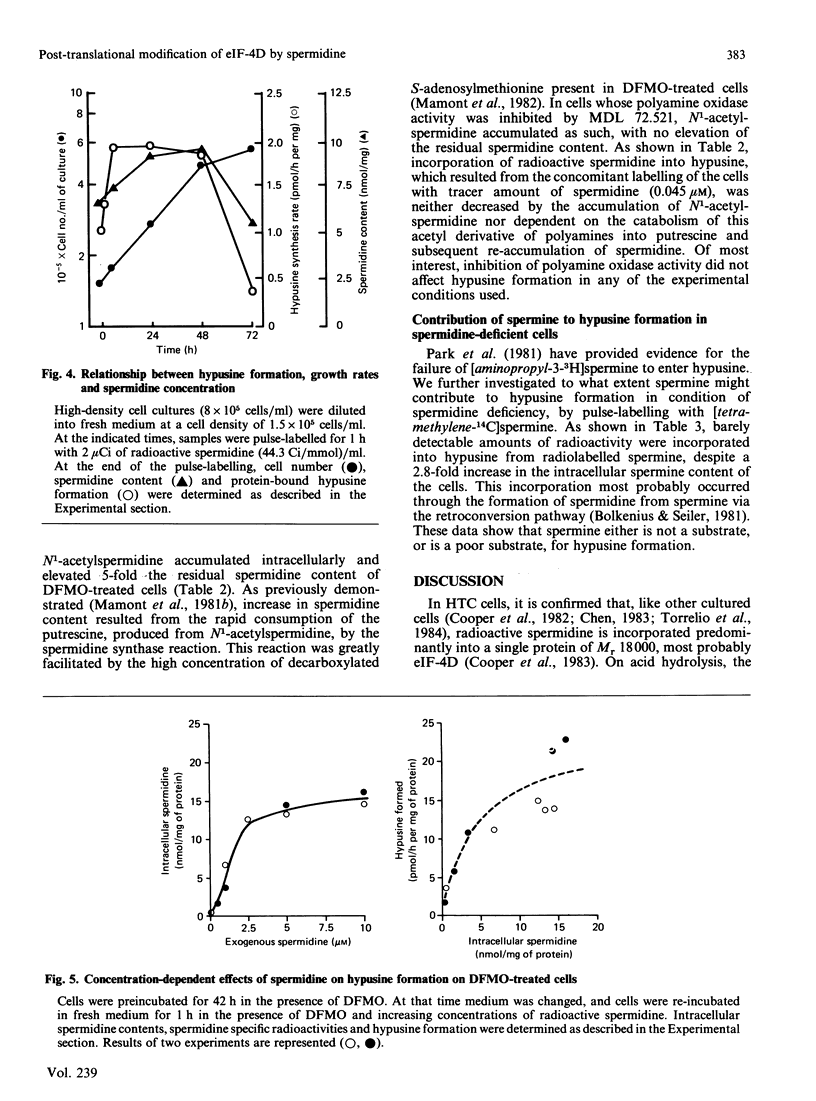
The rates of synthesis and turnover of the rare amino acid hypusine [N6-(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid] in protein were studied in relationship to polyamine metabolism and growth rates in rat hepatoma tissue-culture (HTC) cells. Hypusine is selectively formed in the eukaryotic translation initiation factor eIF-4D, by a post-translational mechanism involving spermidine [Cooper, Park, Folk, Safer & Braverman (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 1854-1857]. The half-life of the hypusine-containing protein was longer than 24 h. In cells whose intracellular spermidine pools had been initially depleted, by using DL-alpha-difluoromethylornithine (DFMO), maximum synthesis rates of hypusine in protein were 5-10 times higher, on restoration of endogenous spermidine contents by exogenous addition, than those observed in untreated exponential-phase cultures. In cells pretreated with DFMO, the rate of hypusine synthesis was constant for up to 1 h after the addition of 5 microM-spermidine, whereas endogenous spermidine contents varied from less than 1 to more than 10 nmol/mg of protein. However, the overall amount of hypusine formed, during the first 1 h after the addition of various concentrations of spermidine (0.05-10 microM) to the culture medium, was markedly dependent on the final endogenous spermidine content achieved at the end of the 1 h measurement interval. Early in exponential-phase growth, protein-bound hypusine was synthesized at a rate of 1-2 pmol/h per mg of protein. This rate decreased to less than 0.5 pmol/h per mg of protein when cell growth rates decreased as cultures reached high cell densities. Analysis of the polyamine substrate specificity for hypusine formation showed that N1-acetylspermidine did not compete with spermidine in the reaction, nor did N1-(buta-2,3-dienyl)-N2-methylbutane-1,4-diamine, and irreversible inhibitor of polyamine oxidase, block the reaction. On the basis of comparative radiolabelling experiments, spermine was either a poor substrate, or not a substrate, for hypusine formation. These results confirm that spermidine is the likely precursor of the aminohydroxybutyl moiety of hypusine, and show that overall hypusine formation, but not necessarily the synthesis rate, is dependent on the endogenous spermidine concentration, especially under conditions where spermidine concentrations are initially low, as is the case after DFMO treatment, and then increase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bey P., Bolkenius F. N., Seiler N., Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985 Jan;28(1):1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Chen K. Y. An 18000-dalton protein metabolically labeled by polyamines in various mammalian cell lines. Biochim Biophys Acta. 1983 Apr 20;756(3):395–402. doi: 10.1016/0304-4165(83)90350-1. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Park M. H., Folk J. E. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell. 1982 Jul;29(3):791–797. doi: 10.1016/0092-8674(82)90441-x. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Park M. H., Folk J. E., Safer B., Braverman R. Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1854–1857. doi: 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Hovi T. Polyamine depletion results in impairment of polyribosome formation and protein synthesis before onset of DNA synthesis in mitogen-activated human lymphocytes. Eur J Biochem. 1985 Oct 1;152(1):229–237. doi: 10.1111/j.1432-1033.1985.tb09188.x. [DOI] [PubMed] [Google Scholar]
- Imaoka N., Nakajima T. Hypusine, N6-(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid, in tissue proteins of mammals. Biochim Biophys Acta. 1973 Aug 17;320(1):97–103. doi: 10.1016/0304-4165(73)90170-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Wagner J., Siat M., Joder-Ohlenbusch A. M., Claverie N. Accumulation of decarboxylated S-adenosyl-L-methionine in mammalian cells as a consequence of the inhibition of putrescine biosynthesis. Eur J Biochem. 1982 Apr;123(3):499–504. doi: 10.1111/j.1432-1033.1982.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Seiler N., Siat M., Joder-Ohlenbusch A. M., Knödgen B. Metabolism of acetyl derivatives of polyamines in cultured polyamine-deficient rat hepatoma cells. Med Biol. 1981 Dec;59(5-6):347–353. [PubMed] [Google Scholar]
- Nakajima T., Matsubayashi T., Kakimoto Y., Sano I. Distribution of hypusine, N 6 -(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid, in mammalian organs. Biochim Biophys Acta. 1971 Oct;252(1):92–97. doi: 10.1016/0304-4165(71)90095-x. [DOI] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981 May;78(5):2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. The biosynthesis of protein-bound hypusine (N epsilon -(4-amino-2-hydroxybutyl)lysine). Lysine as the amino acid precursor and the intermediate role of deoxyhypusine (N epsilon -(4-aminobutyl)lysine). J Biol Chem. 1982 Jun 25;257(12):7217–7222. [PubMed] [Google Scholar]
- Park M. H., Liberato D. J., Yergey A. L., Folk J. E. The biosynthesis of hypusine (N epsilon-(4-amino-2-hydroxybutyl)lysine). Alignment of the butylamine segment and source of the secondary amino nitrogen. J Biol Chem. 1984 Oct 10;259(19):12123–12127. [PubMed] [Google Scholar]
- Rudkin B. B., Mamont P. S., Seiler N. Decreased protein-synthetic activity is an early consequence of spermidine depletion in rat hepatoma tissue-culture cells. Biochem J. 1984 Feb 1;217(3):731–741. doi: 10.1042/bj2170731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano A., Miyake M., Kakimoto Y. A rapid and sensitive method for the determination of hypusine in proteins and its distribution and developmental changes. Biochim Biophys Acta. 1984 Jul 30;800(2):135–139. doi: 10.1016/0304-4165(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Shiba T., Mizote H., Kaneko T., Nakajima T., Kakimoto Y. Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination. Biochim Biophys Acta. 1971 Sep 21;244(3):523–531. doi: 10.1016/0304-4165(71)90069-9. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. A., Benne R., Voorma H. O. Initiation of eukaryotic protein synthesis. FEBS Lett. 1981 Jun 15;128(2):177–185. doi: 10.1016/0014-5793(81)80076-2. [DOI] [PubMed] [Google Scholar]
- Torrelio B. M., Paz M. A., Gallop P. M. Cellular proliferation and hypusine synthesis. Exp Cell Res. 1984 Oct;154(2):454–463. doi: 10.1016/0014-4827(84)90169-1. [DOI] [PubMed] [Google Scholar]


