Abstract
Many viruses have evolved strategies that target crucial components within the apoptotic cascade. One of the best studied is the caspase 8 inhibitor, crmA/Spi-2, encoded by members of the poxvirus family. Since many proapoptotic stimuli induce apoptosis through a mitochondrion-dependent, caspase 8-independent pathway, we hypothesized that vaccinia virus would encode a mechanism to directly modulate the mitochondrial apoptotic pathway. In support of this, we observed that Jurkat cells, which undergo Fas-mediated apoptosis exclusively through the mitochondrial route, were resistant to Fas-induced death following infection with a crmA/Spi-2-deficient strain of vaccinia virus. In addition, vaccinia virus-infected cells subjected to the proapoptotic stimulus staurosporine exhibited decreased levels of both cytochrome c released from the mitochondria and caspase 3 activation. In all cases we found that the loss of the mitochondrial membrane potential, which occurs as a result of opening the multimeric permeability transition pore complex, was prevented in vaccinia virus-infected cells. Moreover, vaccinia virus infection specifically inhibited opening of the permeability transition pore following treatment with the permeability transition pore ligand atractyloside and t-butylhydroperoxide. These studies indicate that vaccinia virus infection directly impacts the mitochondrial apoptotic cascade by influencing the permeability transition pore.
An important function of the cell-mediated immune response is the detection and elimination of virus-infected cells as a means to arrest viral propagation. To do this, immune effector cells rely on the production of cytokines and the recognition of virus infected cells by cytotoxic T lymphocytes (CTL) to induce programmed cell death, or apoptosis (31). Apoptosis results in a variety of cellular changes including cell shrinkage, DNA fragmentation, chromatin condensation, and finally the formation of apoptotic bodies. These changes are mediated by biochemical events involving a family of cysteine proteases termed caspases (45, 61). Following an apoptotic stimulus, caspases become proteolytically activated and function to cleave cellular proteins, including other members of the caspase family. Recently, it has also become clear that within cells instructed to die, mitochondria play a central role in the execution of apoptosis (13). The induction of apoptosis results in both structural and physiological alterations to mitochondria, including disruption of electron transport and energy metabolism, production of reactive oxygen species, loss of the membrane potential, and release of proapoptotic proteins, including cytochrome c (37).
In order for a virus to replicate and disseminate within a host, manipulation of the apoptotic process is essential (2, 48, 64). To ensure their survival, viruses have evolved strategies that target crucial components within the apoptotic cascade. For example, virus-encoded inhibitors of apoptosis have been identified that either directly or indirectly modulate caspase activation. One of the best-studied viral caspase inhibitors is the cowpox virus-encoded cytokine response modifier A (crmA), also known as Spi-2. crmA/Spi-2 inhibits both Fas- and tumor necrosis factor (TNF)-induced apoptosis via interaction with caspase 8 (33, 59, 71). In a similar manner, baculoviruses encode P35, a broad-spectrum caspase inhibitor that protects infected cells from apoptosis (8, 70). As well, baculoviruses and African swine fever virus modulate the activation and activity of caspases through the expression of inhibitors of apoptosis (IAPs) (11, 14, 46). In addition to modulating caspase activity, viruses have also developed strategies that interfere with other components of the death pathway. For example, poxviruses encode secreted TNF receptors that inhibit TNF-α-induced apoptosis by blocking ligand-receptor interactions (27, 52, 55). Gammaherpesviruses and the poxvirus molluscum contagiosum encode viral Fas-associated death domain-like interleukin-1β-converting enzyme inhibitory proteins (vFLIPs), which interfere with recruitment of caspase 8 to the cytoplasmic domains of Fas and TNF receptor 1 (5, 28, 60). Additionally, adenovirus has evolved an elaborate scheme to stimulate the internalization of cell surface Fas (53, 62).
Since mitochondria play a central role in cell death, viruses have also established mechanisms to modulate the mitochondrial component of the apoptotic pathway. Members of the cellular Bcl-2 family influence the integrity of the mitochondria (10, 21), and many viruses encoding Bcl-2-like proteins have been identified. Viral Bcl-2 homologues with antiapoptotic function have been found in adenovirus (65) and African swine fever virus (1, 7) as well as in members of the gammaherpesvirus family including Epstein-Barr virus, equine herpesvirus 2, herpesvirus saimiri, Kaposi's sarcoma-associated herpesvirus, bovine herpesvirus, herpesvirus ateles, alcelaphine herpesvirus 1, and murine gammaherpesvirus 68 (64). In addition, novel virus gene products that act at the mitochondrial checkpoint but lack homology to Bcl-2 have also been identified. VMIA, encoded by human cytomegalovirus (HCMV), inhibits apoptosis and the release of cytochrome c in HeLa cells through interaction with the adenine nucleotide translocator (ANT) subunit of the permeability transition (PT) pore (20). M11L, encoded by the rabbit-specific poxvirus myxoma virus, localizes to the mitochondria and inhibits staurosporine-induced loss of mitochondrial membrane potential and apoptosis (17).
The large number of viruses encoding proteins that function to maintain the integrity of the mitochondria led us to hypothesize that vaccinia virus, a member of the poxvirus family, would employ a mechanism to directly modulate the mitochondrial apoptotic pathway. To determine whether vaccinia virus infection could inhibit the mitochondrion-mediated apoptotic pathway, we monitored the ability of vaccinia virus strain Copenhagen, which is naturally devoid of the caspase 8 inhibitor crmA/Spi2, to inhibit Fas- and staurosporine-mediated apoptosis. We show here, for the first time, that vaccinia virus modulates the apoptotic mitochondrial pathway by inhibiting the PT pore, thereby preserving the mitochondrial membrane potential and retaining cytochrome c.
MATERIALS AND METHODS
Cell and viruses.
Jurkat cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (both from Gibco BRL Life Technologies, Inc.), 100 μM 2-mercaptoethanol, 50 U of penicillin/ml, and 50 μg of streptomycin/ml (RHFM). Stably transfected Jurkat cells were generated as previously described (3) and maintained in RHFM supplemented with 800 μg of G418/ml. Recombinant vaccinia virus strain Copenhagen expressing beta-galactosidase (VV65) was a gift from G. McFadden (Robarts Research Institute, London, Ontario, Canada) (26). VV65 was routinely propagated in baby green monkey kidney (BGMK) cells, a gift from S. Dales, and grown in Dulbecco's modified Eagle medium supplemented with 10% newborn calf serum (both from Gibco BRL Life Technologies, Inc.), 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 2 mM glutamine. Viruses were isolated as previously described (57).
Virus infection.
Jurkat cells were infected at a multiplicity of infection (MOI) of 10 PFU per cell in 200 μl of RHFM at 37°C. After 1 h, the cells were supplemented with additional RHFM for 4 h and incubated at 37°C under 5% CO2 before induction of apoptosis. The efficiency of virus infection was routinely quantified by colorimetric analysis using the lacZ gene. In all experiments, the efficiency of infection was found to be greater than 95%. When necessary, VV65 was UV inactivated for 60 min prior to infection and cytosine arabinoside (Sigma Chemical Co.) was added to a final concentration of 40 μg/ml.
Antibodies.
The p20 fragment of caspase 3 (C3p20) was amplified by PCR from pSKII:CPP32 using the forward oligonucleotide 5′-GGATCCTCTGGAATATCCCTGGAC-3′ containing a BamHI restriction site and the reverse oligonucleotide 5′-GTCGACGTCTGTCTCAATGCCACA-3′ containing a SalI restriction site. Amplified C3p20 was subcloned into pGex4T-3 (Pharmacia Biotech) to construct pGex4T-3:C3p20. pGex4T-3-C3p20 was transformed into BL21DE3, and protein expression was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Rose Scientific Ltd.). Glutathione S-transferase (GST)-C3p20 was purified utilizing glutathione Sepharose 4B according to the manufacturer's instructions (Pharmacia Biotech). Pet15b-Bid, a gift from X. Wang (University of Texas Southwestern Medical Center, Dallas, Tex.) was used to express His-tagged Bid (40). Recombinant His-tagged Bid was purified using a Ni2+ column according to the manufacturer's instructions (Novagen). Rabbits were immunized by injection of 500 μg of bacterially expressed GST-caspase 3 or His-Bid in Freund's complete adjuvant (Gibco BRL Life Technologies). At monthly intervals the animals were boosted with 500 μg of antigen in Freund's incomplete adjuvant (Gibco BRL Life Technologies), and antiserum was collected 10 days after the fourth boost. The anti-cytochrome c antibody (clone 7H8.2Cl2) was purchased from PharMingen. Anti-human Fas immunoglobulin M (IgM) (clone CH11) was purchased from Upstate Biotechnology. Goat anti-mouse and goat anti-rabbit horseradish peroxidase-conjugated antibodies were purchased from Bio-Rad and used at dilutions of 1:3,000 and 1:10,000, respectively.
Apoptosis induction.
Cells were induced to undergo apoptosis by addition of either 250 ng of activating anti-Fas/ml or 1 to 5 μM staurosporine (Sigma Chemical Co.) as outlined in Results.
Chromium release assay.
51Cr release assays were performed as previously described (4). Briefly, cells were labeled with 100 μCi of 51Cr at 37°C for 1 h. Labeled target cells were incubated with 250 ng of anti-Fas clone CH11/ml, and 51Cr release was quantitated after 8 h. 51Cr release was calculated by the following equation: percent lysis = 100 × (sample release − spontaneous release)/(total release − spontaneous release). Standard deviations were generated from three replicates.
Cytochrome c release assay.
Cytochrome c release was monitored as previously described (25, 49). Following apoptosis treatment, 2 × 106 or 5 × 106 Jurkat cells were permeabilized by incubation in digitonin lysis buffer containing 75 mM NaCl, 1 mM NaH2PO4, 8 mM Na2HPO4, 250 mM sucrose, and 190 μg of digitonin (Sigma Chemical Co.)/ml. Cells were incubated on ice for 10 min, after which the mitochondria-containing pellet and the cytosolic supernatant were separated by centrifugation at 10,000 × g for 5 min. Mitochondrial pellets were resuspended in 0.1% Triton X-100–25 mM Tris (pH 8.0) prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
Measurement of mitochondrial membrane potential.
Changes in mitochondrial membrane potential were quantified by staining cells with tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes) (16, 18, 43). Cells were loaded with TMRE by a 30-min incubation (at 37°C, under 5% CO2) in RHFM containing 0.2 μM TMRE. As a control, cells were also treated with a membrane uncoupler, carbonyl cyanide m-chlorophenylhydrazone (ClCCP) (Sigma Chemical Co.), at a final concentration of 50 μM, for 15 min at 37°C under 5% CO2. To trigger the permeability transition, cells were treated with either 1 μM staurosporine or 300 μM t-butylhydroperoxide (both from Sigma Chemical Co.) for 1 or 2 h, respectively. Prior to flow cytometric analysis, cells were washed with phosphate-buffered saline (PBS) containing 1% fetal calf serum. TMRE fluorescence was acquired through the FL-2 channel equipped with a 585-nm filter (band pass, 42 nm). Data were acquired on either 10,000 or 20,000 cells per sample with fluorescence signals at logarithmic gain. Data were analyzed with CellQuest software, and standard deviations were generated from three independent experiments.
Detection of DNA fragmentation.
DNA fragmentation was assessed using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) kit (Roche Diagnostics Co.). Briefly, cells were harvested, washed in PBS containing 1% fetal calf serum, fixed in 2% paraformaldehyde, and permeabilized in 0.1% Triton X-100. Fixed and permeabilized cells were incubated for 1 h at 37°C in a solution containing 25 mM Tris (pH 6.6), 200 mM cacodylate, 1 mM CoCl2, 0.6 nM fluorescein-12-dUTP, and 25 U of terminal deoxynucleotidyltransferase (Roche Diagnostics Co.). Analysis was performed on a Becton Dickinson FACScan equipped with an argon laser at 15 mV with an excitation wavelength of 488 nm. Emission wavelengths were detected through the FL-1 channel equipped with a 530-nm filter (band pass, 20 nm). Data was acquired on 10,000 cells per sample with light scatter signals at linear gain and fluorescence signals at logarithmic gain.
In vitro reconstitution assay.
Mitochondria were purified as previously described (24, 49). For each test sample, 4 × 107 cells were washed in buffer A containing 20 mM morpholinepropanesulfonic acid (MOPS; pH 7.4), 100 mM sucrose, and 1 mM EGTA. Cells were resuspended in buffer B containing 20 mM MOPS (pH 7.4), 100 mM sucrose, 1 mM EGTA, 5% Percoll (Sigma Chemical Co.), and 190 μg of digitonin/ml. Following a 15-min incubation on ice with intermittent inversion, nuclei were pelleted at 2,500 × g for 10 min at 4°C. The pellet was discarded, and the supernatant was centrifuged at 15,000 × g for 15 min at 4°C. The mitochondrial fraction was collected and washed three times in buffer A and resuspended in buffer C containing 20 mM MOPS (pH 7.4), 300 mM sucrose, and 1.0 mM EGTA. The protein concentration of the mitochondrial fraction was determined using the bicinchoninic acid (BCA) kit from Pierce Chemical Company. For the in vitro assay, 6 μg of purified mitochondria was either incubated with 2, 5, or 10 ng of recombinant Bid in the presence or absence of 0.25 μg of purified granzyme B or treated with 5, 10, or 15 mM atractyloside (Sigma Chemical Co.) for 40 min at 37°C. Granzyme B was purified from YT-Indy cells was as previously described (9, 23). Following the addition of granzyme B and His-tagged Bid, the samples were incubated at 37°C for 60 min. Samples were then centrifuged at 15,000 × g to separate the mitochondrial pellet from the supernatant prior to SDS-PAGE analysis.
Immunoblotting.
Cellular lysates were analyzed by electrophoresis on an SDS–15% polyacrylamide gel. Proteins were transferred to nitrocellulose membranes (Osmonics Inc.) using a semidry transfer apparatus (Tyler Research Instruments) for 2.5 h at 500 mA. Membranes were blocked for at least 3 h in PBS containing 0.1% Tween and 5% skim milk. Caspase 3 and Bid were detected using polyclonal rabbit anti-caspase 3 and anti-Bid at a dilution of 1:10,000. Cytochrome c was detected using a monoclonal antibody at a 1:1,000 dilution. All primary antibodies were incubated with the membranes overnight at 4°C. Membranes were probed with either a goat anti-mouse (1:3,000) or a goat anti-rabbit (1:10,000) horseradish peroxidase-conjugated antibody. Proteins were visualized with a chemiluminescence detection system according to the manufacturer's directions (Amersham Pharmacia Biotech).
RESULTS
Vaccinia virus strain Copenhagen-infected cells are resistant to anti-Fas-mediated apoptosis.
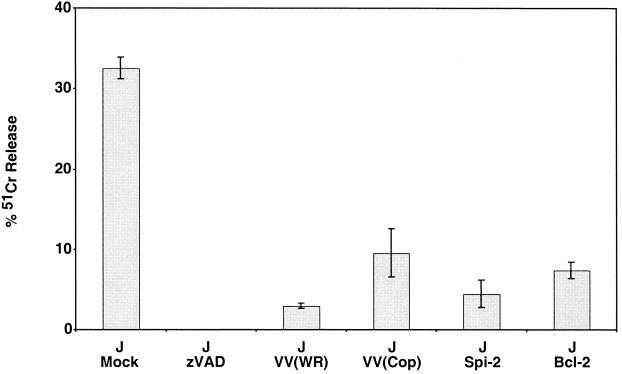
To determine whether vaccinia virus infection could inhibit the mitochondrion-mediated apoptotic pathway, we monitored the ability of vaccinia virus strain Copenhagen to inhibit anti-Fas-induced apoptosis of Jurkat cells. We utilized Jurkat cells, since Fas-induced apoptosis occurs exclusively via the mitochondrial pathway in this cell line (51). In addition, we utilized a strain of vaccinia virus, strain Copenhagen, which is naturally devoid of the caspase 8 inhibitor crmA/Spi-2 (19). Jurkat cells were either mock infected or infected with vaccinia virus strain Copenhagen. At 5 h postinfection, apoptosis was triggered by the addition of anti-Fas and cell death was measured by 51Cr release. As shown in Fig. 1, mock-infected cells treated with anti-Fas displayed approximately 30% 51Cr release. This release was completely inhibited by pretreating the cells with the broad-spectrum caspase inhibitor zVAD.fmk, indicating that cytolysis was directly dependent on caspase activation. Infection of Jurkat cells with vaccinia virus strain Western Reserve, which encodes a functional Spi-2, drastically reduced the levels of 51Cr released. As expected, Jurkat cells stably transfected with either Spi-2 or Bcl-2 were protected from anti-Fas-triggered death. Most significantly, Jurkat cells infected with vaccinia virus strain Copenhagen, lacking a functional crmA/Spi-2, also inhibited death mediated via the Fas pathway, clearly suggesting that vaccinia virus strain Copenhagen employs an additional antiapoptotic mechanism.
FIG. 1.
Vaccinia virus strain Copenhagen protects cells from anti-Fas-mediated death. Jurkat cells were either mock infected or infected with either vaccinia virus strain Copenhagen or vaccinia virus strain Western Reserve at an MOI of 10. Following 5 h of infection, cells were treated with 250 ng of anti-Fas antibody/ml to induce apoptosis, and cell death was monitored 8 h later by 51Cr release. As controls, Jurkat cells that overexpress SPI-2 and Bcl-2 were also treated with anti-Fas, and Jurkat cells were pretreated for 30 min with 100 μM zVAD.fmk prior to addition of anti-Fas. Standard deviations were generated from three replicates.
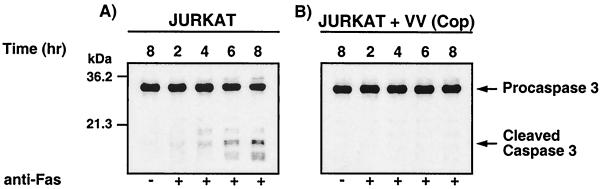
To begin to determine the point at which vaccinia virus strain Copenhagen-infected Jurkat cells were resistant to cell death, we monitored anti-Fas-mediated caspase 3 activation. Caspase 3 activation was assessed by Western blot analysis using an antibody raised against the large subunit of the active caspase. Over 8 h, uninfected cells treated with anti-Fas showed processing of caspase 3 from the full-length 32-kDa procaspase to the mature 19- and 17-kDa forms (Fig. 2A). In contrast, cells infected with vaccinia virus strain Copenhagen and treated with the anti-Fas antibody exhibited only minor amounts of active caspase 3 (Fig. 2B), indicating that infected cells were protected from apoptosis.
FIG. 2.
Vaccinia virus strain Copenhagen infection inhibits activation of caspase 3. Jurkat cells were either mock infected or infected with vaccinia virus strain Copenhagen at an MOI of 10 and treated with 250 ng of anti-Fas antibody/ml for 2, 4, 6, or 8 h. At the times indicated, cells were permeabilized with digitonin, and caspase 3 processing was monitored by Western blot analysis. (A) Mock-infected Jurkat cells; (B) Jurkat cells infected with vaccinia virus strain Copenhagen.
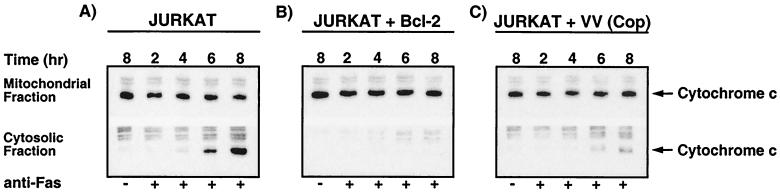
Since infection of Jurkat cells with vaccinia virus strain Copenhagen inhibited the activation of caspase 3, and since mitochondrial release of cytochrome c is necessary for caspase 3 activation, we assessed the ability of vaccinia virus strain Copenhagen to inhibit the release of cytochrome c following treatment with anti-Fas (13, 37, 39). To detect the release of cytochrome c, cells were fractionated into mitochondrial and cytosolic fractions and the release of cytochrome c was detected by Western blot analysis. Using this approach, apoptotic extracts demonstrated the translocation of cytochrome c from the mitochondria to the cytosolic fraction (Fig. 3A). Translocation of cytochrome c was first detected as early as 4 h following the addition of anti-Fas and was found to increase over time (Fig. 3A). As a control, we also monitored cytochrome c release in Jurkat cells that overexpress the antiapoptotic protein Bcl-2. As previously documented, Bcl-2 expression inhibited the translocation of cytochrome c to the cytosol (Fig. 3B) (35, 66). Fas-mediated release of cytochrome c was also monitored in Jurkat cells infected with vaccinia virus strain Copenhagen. As shown in Fig. 3C, infection with vaccinia virus strain Copenhagen significantly interfered with the release of cytochrome c, indicating that vaccinia virus strain Copenhagen inhibits the Fas-mediated apoptotic pathway upstream of cytochrome c release.
FIG. 3.
Vaccinia virus strain Copenhagen inhibits cytochrome c translocation. Jurkat cells were either mock infected or infected with virus. Following infection, cells were treated with 250 ng of anti-Fas/ml for 2, 4, 6, or 8 h to induce cytochrome c translocation. At the times indicated, cells were permeabilized with digitonin and fractionated into the mitochondrion-containing membranous fraction and the soluble cytoplasmic fraction, and cytochrome c was assessed by Western blot analysis. (A) Mock-infected Jurkat cells; (B) mock infected Jurkat cells that overexpress Bcl-2; (C) Jurkat cells infected with vaccinia virus strain Copenhagen.
Vaccinia virus strain Copenhagen infection inhibits cytochrome c release in isolated mitochondria but not cleavage of Bid.
The ability of vaccinia virus strain Copenhagen to inhibit Fas-induced cytochrome c release suggested to us that the virus could potentially hinder apoptosis by interfering with activation of the proapoptotic Bcl-2 family member Bid. During Fas-mediated apoptosis, Bid is cleaved by caspase 8, prompting the translocation of truncated Bid to the mitochondria, resulting in the release of cytochrome c (38, 40). Additionally, the serine proteinase granzyme B, which is released from activated CTL, is also able to induce apoptosis through the cleavage of Bid (3, 24). To investigate the potential inhibition of Bid activation by vaccinia virus strain Copenhagen, we performed an in vitro apoptotic reconstitution assay involving isolated mitochondria, recombinant Bid, and purified granzyme B. Mitochondria were purified from both mock-infected and virus-infected Jurkat cells as well as from Jurkat cells stably expressing Bcl-2. To ensure mitochondrial purity, virus-infected samples were subjected to Western blot analysis to detect Spi-1, a known vaccinia virus cytoplasmic protein (unpublished data) (34). Isolated mitochondria were incubated with increasing amounts of recombinant Bid, either in the presence or in the absence of purified granzyme B, and proteolytic cleavage of Bid was monitored by Western blot analysis. Figure 4A demonstrates that in the presence of purified mitochondria and granzyme B, recombinant Bid underwent proteolytic cleavage and activation in this assay. In agreement with previously published results, Bid was also processed in the presence of isolated mitochondria from cells overexpressing Bcl-2 (22, 44) (Fig. 4B). The processing of Bid was found to be unaltered when mitochondria from vaccinia virus-infected cells were used in the same assay, indicating that vaccinia virus infection does not inhibit the proteolytic processing of Bid (Fig. 4C).
FIG. 4.
Vaccinia virus infection protects against granzyme B-mediated cytochrome c release from isolated mitochondria by a mechanism downstream of Bid activation. Mitochondria were isolated from mock-infected Jurkat cells, Jurkat cells overexpressing Bcl-2, and Jurkat cells infected with vaccinia virus strain Copenhagen. Purified mitochondria were incubated for 60 min at 37°C with 2, 5, or 10 ng of recombinant Bid either in the presence or in the absence of granzyme B. Following treatment, samples were fractionated into mitochondria-containing and soluble fractions and the proteins were resolved by SDS-PAGE. (A through C)Western blot analysis of Bid cleavage in mitochondria isolated either from mock-infected cells (A), from cells overexpressing Bcl-2 (B), or from vaccinia virus strain Copenhagen-infected cells (C). (D through F) Western blot analysis of cytochrome c translocation from purified mitochondria to supernatant fractions in mitochondria isolated either from mock-infected cells (D), from mock-infected cells overexpressing Bcl-2 (E), or from vaccinia virus strain Copenhagen-infected cells (F).
Since vaccinia virus infection did not suppress granzyme B-induced Bid cleavage, we next asked if mitochondria isolated from vaccinia virus-infected cells could inhibit granzyme B-induced cytochrome c release in the presence of Bid. Mitochondria isolated from mock-infected Jurkat cells were incubated with increasing amounts of recombinant Bid in the presence of granzyme B, resulting in the translocation of cytochrome c from the mitochondria to the supernatant (Fig. 4D). As shown in Fig. 4D, an increase in the amount of cytochrome c translocation was detected following the addition of granzyme B and increasing amounts of recombinant Bid. At 5 and 10 ng of Bid, we found that some cytochrome c translocation was independent of Bid cleavage, as previously reported (40, 67) (Fig. 4D). The release of cytochrome c was completely abolished in mitochondria isolated from Jurkat cells overexpressing the antiapoptotic protein Bcl-2 (Fig. 4E). Inhibition of cytochrome c translocation was also seen in mitochondria isolated from vaccinia virus-infected cells (Fig. 4F). Taken together, these results indicated that cytochrome c release was inhibited in mitochondria isolated from infected cells and suggested that vaccinia virus infection directly modulated the mitochondrial arm of the apoptotic pathway.
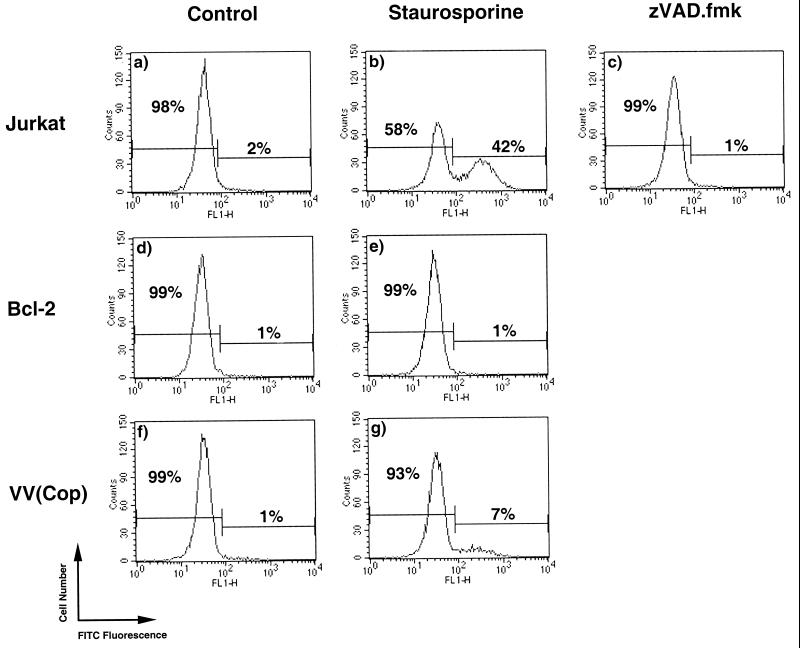
Vaccinia virus strain Copenhagen-infected cells are resistant to staurosporine-mediated apoptosis.
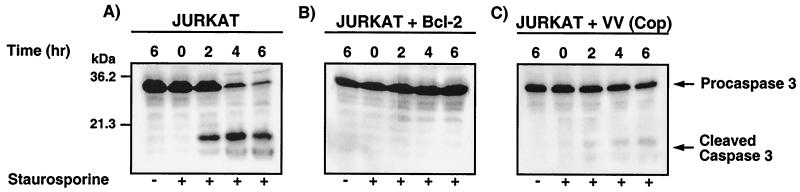
In view of our findings demonstrating that vaccinia virus strain Copenhagen could inhibit Fas-mediated cytochrome c release and cytochrome c release from isolated mitochondria, we assessed the ability of vaccinia virus strain Copenhagen to directly inhibit the mitochondrial route to apoptotic death. To determine if vaccinia virus strain Copenhagen could directly inhibit the mitochondrial cascade, we treated cells with the proapoptotic reagent staurosporine, which triggers the mitochondrion-mediated apoptotic pathway (6, 58). Mock-infected or virus-infected Jurkat cells were treated with staurosporine, and the levels of DNA fragmentation were measured using the TUNEL assay and flow cytometry. As shown in Fig. 5, untreated cells demonstrated low levels of DNA fragmentation (Fig. 5a, d, and f). Upon staurosporine treatment, 42% of the mock-infected Jurkat cell population showed DNA fragmentation (Fig. 5b). Preincubation with the broad-spectrum caspase inhibitor zVAD.fmk completely inhibited staurosporine-induced DNA fragmentation, clearly demonstrating that staurosporine-induced DNA fragmentation occurred via caspase activation, as expected (Fig. 5c). In addition, stably transfected Jurkat cells that overexpress Bcl-2 were also found to be resistant to apoptosis, indicating that staurosporine-mediated cell death occurred via the mitochondrial pathway (Fig. 5d). Most importantly, vaccinia virus strain Copenhagen-infected Jurkat cells treated with staurosporine displayed clear protection from apoptosis, with only 7% of the cells showing DNA fragmentation (Fig. 5g). To determine if the block was upstream of caspase 3 activation, cells were treated with staurosporine for 2, 4, or 6 h and caspase 3 processing was monitored by Western blot analysis. As shown in Fig. 6A, mock-infected Jurkat cells treated with staurosporine displayed rapid conversion of the 32-kDa procaspase 3 to the active fragments. This conversion was significantly inhibited both in Bcl-2-overexpressing cells and in cells infected with vaccinia virus strain Copenhagen (Fig. 6B and C). Compared to mock-infected cells, vaccinia virus strain Copenhagen-infected Jurkat cells treated with staurosporine displayed drastic reductions in levels of the 19- and 17-kDa caspase 3 fragments and maintenance of the full-length 32-kDa proform, indicating that apoptosis inhibition occurred upstream of caspase 3 activation (Fig. 6A and B).
FIG. 5.
DNA fragmentation is blocked by vaccinia virus strain Copenhagen infection. Jurkat cells were either mock infected or infected with vaccinia virus strain Copenhagen. Following infection, cells were treated with 2.5 μM staurosporine for 2 h, and DNA fragmentation was assessed by TUNEL as outlined in Materials and Methods. (a) Untreated Jurkat cells; (b) Jurkat cells treated with staurosporine; (c) Jurkat cells treated with staurosporine in the presence of 100 μM zVAD.fmk; (d) Jurkat cells overexpressing Bcl-2; (e) Jurkat cells overexpressing Bcl-2 treated with staurosporine; (f) Jurkat cells infected with vaccinia virus strain Copenhagen; (g) Jurkat cells infected with vaccinia virus and treated with staurosporine.
FIG. 6.
Staurosporine-induced caspase 3 activation is inhibited by vaccinia virus strain Copenhagen infection. Jurkat cells were either mock infected or infected with virus; following infection, they were treated with 5 μM staurosporine for 0, 2, 4, or 6 h. At the times indicated, cells were permeabilized with digitonin and the proteins were resolved by SDS-PAGE. The activation of caspase 3 was monitored by Western blot analysis. (A) Mock-infected Jurkat cells; (B) mock-infected Jurkat cells that overexpress Bcl-2; (C) Jurkat cells infected with vaccinia virus strain Copenhagen.
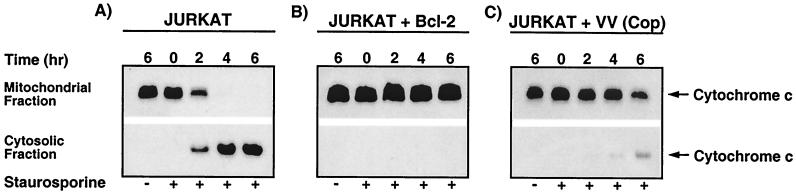
Since Jurkat cells infected with vaccinia virus strain Copenhagen and induced to undergo apoptosis with anti-Fas inhibited cytochrome c translocation from mitochondria to the cytosol, we investigated the possibility that staurosporine-induced cytochrome c translocation would be inhibited by virus infection as well. Mock-infected cells treated with staurosporine exhibited a dramatic loss of cytochrome c from the mitochondrial fraction and subsequent accumulation in the cytoplasmic fraction (Fig. 7A). In contrast to cells treated with anti-Fas antibody, cells treated with staurosporine displayed cytochrome c translocation as early as 2 h posttreatment, and we routinely detected complete translocation of cytochrome c to the cytoplasmic fraction after 6 h of treatment. As anticipated, staurosporine-induced translocation of cytochrome c was completely inhibited in Jurkat cells engineered to overexpress Bcl-2 (35, 66) (Fig. 7B). Most importantly, in cells infected with vaccinia virus strain Copenhagen, cytochrome c release was drastically reduced (Fig. 7C). In contrast to the situation in mock-infected cells, complete translocation of cytochrome c from the mitochondrial to the cytosolic fraction was inhibited by vaccinia virus infection, and cytosolic cytochrome c was only partially evident at 4 and 6 h post-staurosporine treatment.
FIG. 7.
Staurosporine-induced cytochrome c release is inhibited by vaccinia virus strain Copenhagen infection. Jurkat cells were either mock infected or infected with virus and were treated with 5 μM staurosporine for 0, 2, 4, or 6 h. At the times indicated, cells were fractionated into mitochondria-containing membrane fractions and cytoplasmic fractions by the addition of digitonin. The translocation of cytochrome c was monitored by Western blot analysis. (A) Mock-infected Jurkat cells; (B) mock-infected Jurkat cells that overexpress Bcl-2; (C) Jurkat cells infected with vaccinia virus strain Copenhagen.
Vaccinia virus infection inhibits disruption of the mitochondrial inner membrane potential and opening of the PT pore.
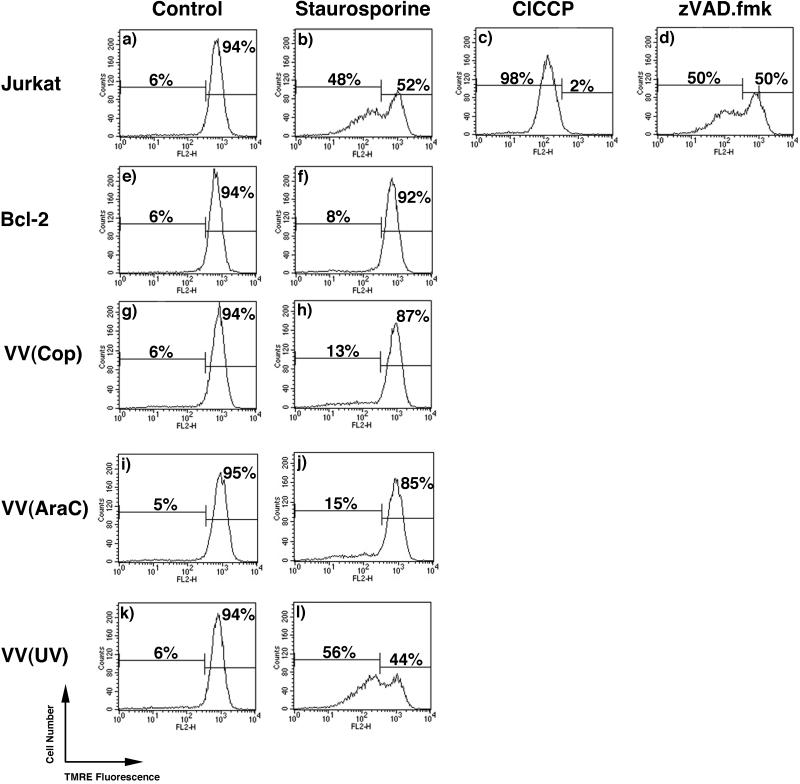
During apoptosis the release of cytochrome c coincides with loss of the inner mitochondrial membrane potential (41, 69). Disruption of the inner mitochondrial membrane potential is thought to occur due to the opening of the PT pore (12, 36, 37). Since vaccinia virus strain Copenhagen infection inhibited cytochrome c translocation and apoptosis, we asked if vaccinia virus infection was able to inhibit apoptosis by maintaining mitochondrial integrity and the inner mitochondrial membrane potential. Changes in the membrane potential were monitored by assaying the uptake of TMRE, a fluorescent mitochondrion-specific dye (18, 50). Disruption of the membrane potential in mock-infected and infected cells following staurosporine treatment was monitored by TMRE fluorescence. In untreated Jurkat cells, 94% of the cells demonstrated TMRE fluorescence, indicating an intact mitochondrial membrane potential (Fig. 8a). Upon staurosporine treatment, 48% of the cells exhibited a reduction in TMRE fluorescence (Fig. 8b). As a control, Jurkat cells were treated with a membrane uncoupler, ClCCP, resulting in the reduction of TMRE fluorescence in all cells (Fig. 8c). Jurkat cells treated with staurosporine in the presence of the caspase inhibitor zVAD.fmk still demonstrated a loss in membrane potential (Fig. 8d), indicating that staurosporine-induced loss of the mitochondrial membrane potential occurred in a caspase-independent manner. This is in contrast to what was observed with DNA fragmentation (Fig. 5c), because staurosporine directly induces the loss of the PT in a caspase-independent manner whereas DNA fragmentation requires caspase activation (58). As expected, Jurkat cells overexpressing Bcl-2 were completely resistant to the staurosporine-induced collapse of the inner membrane potential (Fig. 8e and f). Similarly, upon treatment with staurosporine, 87% of Jurkat cells infected with vaccinia virus maintained a TMRE-positive state (Fig. 8g and h), indicating that vaccinia virus infection inhibited staurosporine-induced loss of the inner mitochondrial membrane potential. The addition of cytosine arabinoside (araC), an inhibitor of virus replication and late gene expression, had no effect on the ability of vaccinia virus to inhibit staurosporine-induced loss of the inner mitochondrial membrane potential, indicating that virus replication and late gene expression were not necessary (Fig. 8i and j). In contrast, UV inactivation of the virus resulted in reversal of this observation, clearly showing that a productive vaccinia virus infection was necessary for the inhibition (Fig. 8k and l).
FIG. 8.
Vaccinia virus strain Copenhagen infection inhibits staurosporine-induced disruption of the mitochondrial membrane potential. Jurkat cells were either mock infected or infected with vaccinia virus and treated with 1 μM staurosporine for 1 h, and the mitochondrial membrane potential was determined using TMRE fluorescence. (a) Untreated Jurkat cells; (b) Jurkat cells treated with staurosporine; (c) Jurkat cells treated with the membrane uncoupler ClCCP; (d) Jurkat cells treated with staurosporine in the presence of 100 μM zVAD.fmk; (e) untreated Jurkat cells overexpressing Bcl-2; (f) Jurkat cells overexpressing Bcl-2 treated with staurosporine; (g) untreated Jurkat cells infected with vaccina virus strain Copenhagen; (h) vaccinia virus-infected cells treated with staurosporine; (i) Jurkat cells infected with vaccinia virus strain Copenhagen in the presence of 40 μg of araC/ml; (j) Jurkat cells infected with vaccinia virus strain Copenhagen in the presence of araC and staurosporine; (k) untreated cells infected with UV-inactivated vaccinia virus strain Copenhagen; (l) Jurkat cells infected with UV-inactivated vaccinia virus and treated with staurosporine.
Controlled permeabilization of the inner and outer mitochondrial membrane is known to occur as a result of opening a mitochondrial multiprotein complex known as the PT pore (12, 36, 37). The PT pore consists of the outer-mitochondrial-membrane-localized voltage-dependent anion carrier (VDAC), the inner-membrane-localized ANT, and the matrix protein cyclophilin D (12, 36, 37). Two accessory proteins, hexokinase and the peripheral benzodiazepine receptor, are also found associated with the PT pore. Members of the Bcl-2 family associate with components of the pore and modulate pore activity, thereby inhibiting apoptosis (12, 37, 42).
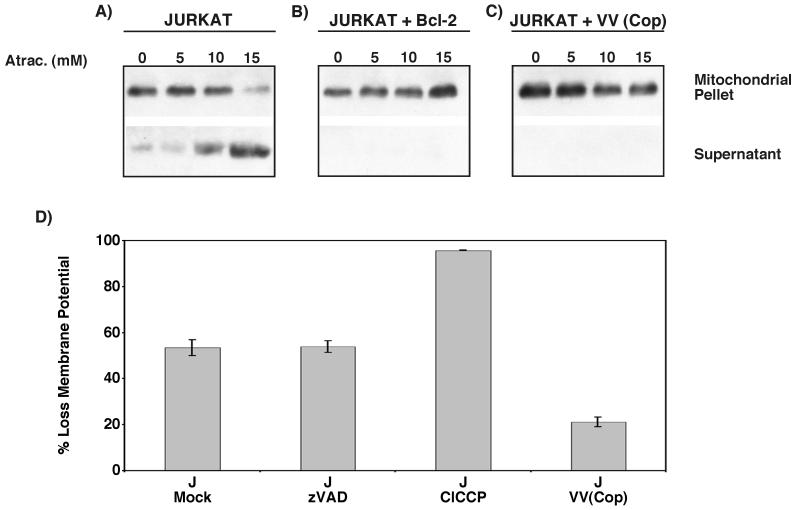
Since infection of cells with vaccinia virus renders them resistant to apoptosis and inhibits disruption of the mitochondrial inner-membrane potential, we asked if vaccinia virus strain Copenhagen infection regulated apoptosis by modulating the activity of the PT pore. Pore-specific ligands can act directly on components of the pore, resulting in dissipation of the inner-membrane potential and the release of cytochrome c (32, 68, 72). To ascertain if vaccinia virus infection could inhibit induction of the permeability transition and subsequent apoptosis, we treated purified mitochondria with the ANT ligand atractyloside and monitored cytochrome c release. Mitochondria were isolated from mock-infected, vaccinia virus-infected, or Bcl-2-overexpressing cells and induced to undergo permeability transition with various concentrations of atractyloside, and the translocation of cytochrome c was monitored by Western blot analysis. As shown in Fig. 9A, cytochrome c translocation from mock-infected mitochondria was detected following treatment with 5 mM atractyloside, and increasing levels of cytochrome c release were detected with higher concentrations of atractyloside. Atractyloside-induced cytochrome c translocation was completely inhibited in mitochondria isolated from cells overexpressing Bcl-2, a known PT pore-modulating protein (42) (Fig. 9B). As shown in Fig. 9C, atractyloside-induced cytochrome c release from mitochondria isolated from vaccinia virus-infected cells was also completely inhibited, indicating that vaccinia virus strain Copenhagen infection inhibited the onset of atractyloside-induced mitochondrial permeability transition and the subsequent translocation of cytochrome c. To further confirm that vaccinia virus infection inhibited the opening of the PT pore, we treated Jurkat cells with another permeability transition inducer, t-butylhydroperoxide, and monitored mitochondrial membrane potential using TMRE fluorescence (68, 72). As shown in Fig. 9D, treatment of mock-infected cells with t-butylhydroperoxide resulted in disruption of the mitochondrial membrane potential, as shown by a decrease in TMRE fluorescence. Disruption of the membrane potential following t-butylhydroperoxide treatment was not inhibited by zVAD.fmk, confirming previous findings that indicate that t-butylhydroperoxide does not disrupt membrane potential through caspase activation (68) (Fig. 9D). Infection with vaccinia virus strain Copenhagen, however, prevented loss of TMRE fluorescence, indicating that vaccinia virus infection regulates the PT pore (Fig. 9D).
FIG. 9.
Vaccinia virus strain Copenhagen inhibits opening of the PT pore. (A through C) Atractyloside-induced cytochrome c release is inhibited by vaccinia virus strain Copenhagen infection. Mitochondria were purified and incubated at 37°C with 5, 10, or 15 mM atractyloside (Atrac.) for 40 min. Following treatment, samples were fractionated into mitochondria-containing and soluble fractions, and translocation of cytochrome c was analyzed by Western blot analysis. (A) Mitochondria isolated from mock-infected Jurkat cells; (B) mitochondria isolated from mock-infected Jurkat cells overexpressing Bcl-2; (C) mitochondria isolated from vaccinia virus strain Copenhagen-infected Jurkat cells. (D) Vaccinia virus inhibits t-butylhydroperoxide-induced disruption of the mitochondrial membrane potential. Jurkat cells were either mock infected or infected with vaccinia virus and treated with 300 μM t-butylhydroperoxide for 2 h. The mitochondrial membrane potential was determined using TMRE fluorescence in the presence and absence of 100 μM zVAD.fmk. Standard deviations were generated from three independent experiments.
DISCUSSION
In order to survive and replicate within a host, viruses possess specific strategies to circumvent the multifaceted immune response, including a variety of strategies to inhibit apoptosis (63). The detection and apoptotic elimination of virus-infected cells is mediated by specialized classes of lymphocytic cells referred to as CTL and natural killer cells. The Poxviridae, of which vaccinia virus is the prototypic member, are large double-stranded DNA viruses that encode many proteins essential for evading the antiviral immune response (56). Until now, the modulation of apoptosis by vaccinia virus has been attributed primarily to expression of the serine proteinase inhibitor Spi-2 (33, 59, 71). Previous reports, however, have suggested that vaccinia virus may foster an additional mechanism to interfere with apoptosis (15, 33, 54). In this study we report for the first time that vaccinia virus strain Copenhagen regulates the mitochondrial apoptotic pathway by inhibiting the PT pore.
Since recent advances in apoptosis have implicated mitochondria as a central control point in cell death, we specifically asked if vaccinia virus employed a mechanism to interfere with the mitochondrial component of apoptotic death (13, 37). Considering that numerous viruses encode mediators of this cascade, we predicted that vaccinia virus infection would also result in maintenance of mitochondrial integrity following the addition of a proapoptotic stimulus. To perform our studies we utilized Jurkat cells, which, due to lower levels of caspase 8 activation, undergo Fas-mediated apoptosis exclusively through the mitochondrial route (51). In addition, we chose to utilize vaccinia virus strain Copenhagen, because it is naturally devoid of the caspase 8-inhibitor crmA/Spi-2 (19). Using this approach we found that vaccinia virus strain Copenhagen infection inhibited cell death mediated through the Fas surface receptor. Since this strain of vaccinia virus does not contain a functional Spi-2 protein, this result clearly indicated that vaccinia virus strain Copenhagen employed a novel, Spi-2-independent antiapoptotic mechanism. Prior to this study, other researchers have suggested the existence of a Spi-2-independent antiapoptotic effect conferred by vaccinia virus infection. Dobbelstein and Shenk found that some HeLa cells infected with a vaccinia virus lacking Spi-2 were still protected from apoptosis (15). In addition, Kettle and coworkers reported the existence of a Spi-2-independent mechanism that was capable of inhibiting cycloheximide-induced apoptosis (33). More recently, Shisler and Moss found that infection with a Spi-2-deficient virus inhibited cleavage of the caspase 3 substrate PARP (54).
To define more clearly the mechanism of vaccinia virus apoptosis inhibition, we monitored both the activation of caspase 3 and the translocation of cytochrome c in response to treatment with anti-Fas. Jurkat cells infected with vaccinia virus strain Copenhagen and treated with anti-Fas displayed reductions in both the activation of caspase 3 and the translocation of cytochrome c compared to mock-infected cells, clearly indicating that the block in apoptosis was upstream of cytochrome c release. Similarly, apoptosis induced by staurosporine was also dramatically inhibited in cells infected with vaccinia virus strain Copenhagen. Once again, both caspase 3 activation and cytochrome c release were inhibited, demonstrating that the novel mechanism employed by vaccinia virus occurred upstream of cytochrome c release. Analysis of Bid activation in an in vitro reconstitution assay indicated that vaccinia virus did not inhibit cleavage of Bid, allowing us to rule out the possiblity of interference with Bid activation. Western blot analysis of cytochrome c in the same in vitro reconstitution assay using mitochondria purified from infected and uninfected cells once again revealed that vaccinia virus interferes with the translocation of cytochrome c. Taken together, the data clearly suggest that vaccinia virus infection inhibited apoptosis by functioning directly at the mitochondria. Recently, M11L, encoded by the poxvirus myxoma virus, has been shown to localize to the mitochondria and inhibit apoptosis (17). Numerous virus-encoded Bcl-2 homologues and a novel protein from HCMV, vMIA, also inhibit apoptosis by functioning at the mitochondria (20, 64). Interestingly, no open reading frame exists in vaccinia virus strain Copenhagen that displays homology with known virus-encoded or cellular apoptotic inhibitors, suggesting that vaccinia virus has evolved a novel mechanism to inhibit cytochrome c release and apoptosis (19).
Although the exact mechanism of cytochrome c release during apoptosis is still controversial, the release of cytochrome c has been linked to disruption of the inner-mitochondrial-membrane potential (12, 36, 37, 41, 69). Dissipation of the inner-mitochondrial-membrane potential is a common and irreversible feature of apoptosis. We found that following treatment with staurosporine, cells infected with vaccinia virus strain Copenhagen retained the inner-mitochondrial-membrane potential, as monitored by TMRE fluorescence. This result supported our previous observations demonstrating that within infected cells the integrity of the mitochondria was maintained and cytochrome c translocation was inhibited. Loss of the membrane potential occurs by triggering opening of the PT pore, a phenomenon known as the “permeability transition” (12, 36, 37). Since disruption of the inner-mitochondrial-membrane potential and the induction of apoptosis occurs as a result of opening of the PT pore, our data suggested that perhaps vaccinia virus could maintain mitochondrial integrity by regulating the opening of the PT pore. In support of this we found that vaccinia virus infection inhibited cytochrome c release from mitochondria treated with increasing amounts of atractyloside, a pore agonist known to induce the permeability transition and the release of cytochrome c (32, 68, 72). In addition, we found that vaccinia virus-infected cells treated with the pro-oxidant t-butylhydroperoxide, which also causes disruption of the mitochondrial transmembrane potential, demonstrated preservation of the mitochondrial inner-membrane transmembrane potential (68). Thus, vaccinia virus infection inhibited the effects mediated by both atractyloside and t-butylhydroperoxide, two reagents that trigger the permeability transition. In addition, both Bid and staurosporine can induce apoptosis via a PT pore-dependent mechanism, and we found that vaccinia virus infection also inhibited apoptosis induced by these reagents (58, 67). Our data are therefore compatible with the idea that vaccinia virus has evolved a mechanism to directly modulate the permeability transition and thereby inhibit apoptosis (Fig. 10).
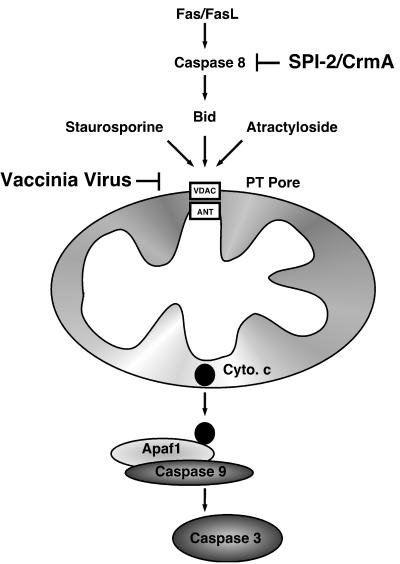
FIG. 10.
Model of vaccinia virus-mediated apoptosis inhibition. Fas initiated apoptosis occurs via activation of caspase 8 and by the subsequent proteolytic cleavage of Bid. Once cleaved, Bid translocates to the mitochondria, resulting in a loss of the inner-mitochondrial-membrane permeability transition and the release of cytochrome c. The release of cytochrome c (Cyto. c) results in activation of caspase 9 via interaction with the adapter molecule Apaf1 and the subsequent activation of caspase 3. Additionally, loss of the inner-membrane permeability transition can be triggered by staurosoporine and atractyloside. Vaccinia virus infection manipulates this pathway at two separate points. First, the vaccinia virus-encoded serine protease inhibitor SPI-2/crmA inhibits the activity of caspase 8. Second, vaccinia virus infection also inhibits apoptosis by modulating the PT pore, thereby preventing the loss of cytochrome c and activation of caspase 9.
Due to the multimeric nature of the PT pore, multiple targets for viral modulation are possible. Both pro- and antiapoptotic members of the Bcl-2 family associate with components of the PT pore and modulate apoptosis (12, 37, 42). At least one component of the PT pore has now been directly associated with virus-mediated apoptotic inhibition. The vMIA protein from HCMV associates with the ANT and inhibits apoptosis (20). Alternatively, proapoptotic viral proteins such as Vpr encoded by human immunodeficiency virus and HBx encoded by hepatitis B virus induce apoptosis by interaction with ANT and VDAC, respectively (29, 30, 47). Collectively, Vpr, HBx, and vMIA modulate apoptosis by interacting with components of the PT pore and regulating the pore complex. Thus, a similar scenario to account for vaccinia virus interference with the mitochondrial apoptotic pathway is likely, and we are currently looking for vaccinia virus proteins that interact with known components of the pore. At present, however, the exact composition of the PT pore is still controversial, and the identification of a novel vaccinia virus-encoded protein and its exact mechanism of action will lead to a clearer understanding of the PT pore and its regulation in the future. In addition, further investigation into vaccinia virus modulation of the PT pore and apoptosis will result in valuable information regarding virus-host interactions and the mechanisms of cell death.
ACKNOWLEDGMENTS
This work was supported by grants from the Canadian Institutes for Health Research and the Alberta Heritage Foundation for Medical Research (to M.B.). M.B. is the recipient of an Alberta Heritage Foundation for Medical Research Scholar Award, and A.F.A.M. is the recipient of a studentship from the Canadian Institutes for Health Research.
We thank D. Burshtyn and S. Slemko for critical review of the manuscript and H. Everett for helpful discussions.
REFERENCES
- 1.Afonso C L, Neilan J G, Kutish G F, Rock D L. An African swine fever virus Bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J Virol. 1996;70:4858–4863. doi: 10.1128/jvi.70.7.4858-4863.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry M, McFadden G. Apoptosis regulators from DNA viruses. Curr Opin Immunol. 1998;10:422–430. doi: 10.1016/s0952-7915(98)80116-7. [DOI] [PubMed] [Google Scholar]
- 3.Barry M, Heibein J A, Pinkoski M J, Lee S F, Moyer R W, Green D R, Bleackley R C. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20:3781–3794. doi: 10.1128/mcb.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry M, Heibein J, Pinkoski M, Bleackley R C. Quantitative measurement of apoptosis induced by cytotoxic T lymphocytes. Methods Enzymol. 2000;322:40–46. doi: 10.1016/s0076-6879(00)22006-5. [DOI] [PubMed] [Google Scholar]
- 5.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E M, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1772–1776. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand R, Solary E, O'Connor P, Kohn K W, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211:314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 7.Brun A, Rivas C, Esteban M, Escribano J M, Alonso C. African swine fever virus gene A179, a viral homologue of bcl-2, protects cells from programmed cell death. Virology. 1996;225:227–230. doi: 10.1006/viro.1996.0592. [DOI] [PubMed] [Google Scholar]
- 8.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mankovich J, Shi L, Greenberg A H, Miller L K, Wong W W. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 9.Caputo A, Parrish J C, James M N G, Powers J C, Bleackley R C. Electrostatic reversal of serine proteinase substrate specificity. Proteins. 1999;35:415–424. [PubMed] [Google Scholar]
- 10.Chao D T, Korsmeyer S J. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 11.Clem R J, Duckett C S. The iap genes: unique arbitrators of cell death. Trends Cell Biol. 1997;7:337–339. doi: 10.1016/S0962-8924(97)01088-X. [DOI] [PubMed] [Google Scholar]
- 12.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 13.Desagher S, Martinou J C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 14.Deveraux Q L, Reed J C. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 15.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrenberg B, Montana V, Wei M D, Wuskell J P, Loew L M. Membrane potential can be determined in individual cells from the Nernstian distribution of cationic dyes. Biophys J. 1988;53:785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett H, Barry M, Lee S F, Sun X, Graham K, Stone J, Bleackley R C, McFadden G. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J Exp Med. 2000;191:1487–1498. doi: 10.1084/jem.191.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farkas D L, Wei M D, Febbroriello P, Carson J H, Loew L M. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1998;56:1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goebel S J, Johnson G P, Perkus M E, Davis S W, Winslow J P, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 20.Goldmacher V S, Bartle L M, Skaletskaya A, Dionne C A, Kedersha N L, Vater C A, Han J, Lutz R J W, Watanabe S, Cahir McFarland E D, Kieff E D, Mocarski E S, Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross A, McDonnell J M, Korsmeyer S J. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 22.Gross A, Yin X M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved Bid targets mitochondria and is required for cytochrome c release, while Bcl-Xl prevents this release but not tumor necrosis factor-R1/Fas Death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 23.Hanna W L, Zhang X, Turbov J, Winkler U, Hudig D, Froelich C J. Rapid purification of cationic granule proteases: application to human granzymes. Protein Expr Purif. 1993;4:398–404. doi: 10.1006/prep.1993.1052. [DOI] [PubMed] [Google Scholar]
- 24.Heibein J A, Goping I S, Barry M, Pinkoski M J, Shore G C, Green D R, Bleackley R C. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid and Bax. J Exp Med. 2000;192:1391–1401. doi: 10.1084/jem.192.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heibein J A, Barry M, Motyka B, Bleackley R C. Granzyme B-induced loss of mitochondrial inner membrane potential (ΔΨm) and cytochrome c release are caspase-independent. J Immunol. 1999;163:4683–4693. [PubMed] [Google Scholar]
- 26.Hnatiuk S, Barry M, Zeng W, Liu L, Lucas A, Percy D, McFadden G. Role of the C-terminal RDEL motif of the myxoma virus M-T4 protein in terms of apoptosis regulation and viral pathogenesis. Virology. 1999;263:290–306. doi: 10.1006/viro.1999.9946. [DOI] [PubMed] [Google Scholar]
- 27.Hu F Q, Smith C A, Pickup D J. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204:343–356. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- 28.Hu S, Vincenz C, Buller M, Dixit V M. A novel family of viral death effector domain-containing molecules that inhibit both CD95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 29.Jacotot E, Ferri K F, El Hamel C, Brenner C, Druillennec S, Hoebeke J, Rustin P, Metivier D, Lenoir C, Geuskens M, Vieira H L, Loeffler M, Belzacq A S, Briand J P, Zamzami N, Edelman L, Xie Z H, Reed J C, Roques B P, Kroemer G. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J Exp Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacotot E, Ravagnan L, Loeffler M, Ferri K F, Vieira H L A, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand J P, Irinopoulou T, Daugas E, Susin S A, Cointe D, Xie Z H, Reed J C, Roques B P, Kroemer G. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–45. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kägi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Kantrow S P, Piantadosi C A. Release of cytochrome c from liver mitochondria during permeability transition. Biochem Biophys Res Commun. 1997;232:669–671. doi: 10.1006/bbrc.1997.6353. [DOI] [PubMed] [Google Scholar]
- 33.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith G L. Vaccinia virus serpin B13R (Spi-2) inhibits interleukin-1β converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1β-induced fever. J Gen Virol. 1997;78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 34.Kettle S, Blake N W, Law K M, Smith G L. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode Mr 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206:136–147. doi: 10.1016/s0042-6822(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 35.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 36.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 37.Kroemer G, Reed J C. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Zhu H, Xu C, Yuan J. Cleavage of Bid by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 40.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from the mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin S A, Masse B, Kroemer G. Mitochondrial permeability transition triggers lymphocyte apoptosis. J Immunol. 1996;157:4830–4836. [PubMed] [Google Scholar]
- 42.Marzo I, Brenner C, Zamzami N, Susin S A, Beutner G, Brdiczka D, Remy R, Xie Z-H, Reed J C, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and Bcl-2 related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin S A, Marzo I, Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett. 1998;61:157–163. doi: 10.1016/s0165-2478(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 44.Murphy K M, Streips U N, Lock R B. Bcl-2 inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. J Biol Chem. 2000;275:17225–17228. doi: 10.1074/jbc.C900590199. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 46.Nogal M L, Gonzalez de Buitrago G, Rodriguez C, Cubelos B, Carrascosa A L, Salas M L, Revilla Y. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J Virol. 2001;75:2535–2543. doi: 10.1128/JVI.75.6.2535-2543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahmani Z, Huh K W, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters the transmembrane potential. J Virol. 2000;74:2840–2846. doi: 10.1128/jvi.74.6.2840-2846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roulston A, Marcellus R C, Branton P E. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 49.Samali A, Cai J, Zhivotovsky B, Jones D P, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase 3, Hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scaduto R C, Jr, Grotyohann L W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreiber M, Rajarathnam K, McFadden G. Myxoma virus T2 protein, a tumor necrosis factor (TNF) receptor homolog, is secreted as a monomer and dimer that each bind rabbit TNFα, but the dimer is a more potent TNF inhibitor. J Biol Chem. 1996;271:13333–13341. doi: 10.1074/jbc.271.23.13333. [DOI] [PubMed] [Google Scholar]
- 53.Shisler J, Yang C, Walter B, Ware C F, Gooding L R. The adenovirus E3–10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol. 1997;71:8299–8306. doi: 10.1128/jvi.71.11.8299-8306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shisler J L, Moss B. Molluscum contagiosum virus inhibitors of apoptosis: the MC159 v-FLIP protein blocks Fas-induced activation of procaspases and degradation of the related MC160 protein. Virology. 2001;282:14–25. doi: 10.1006/viro.2001.0834. [DOI] [PubMed] [Google Scholar]
- 55.Smith C A, Davis T, Wignall J M, Din W S, Farrah T, Upton C, McFadden G, Goodwin R G. T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991;176:335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- 56.Smith G L, Symons J A, Khanna A, Vanderplasschen A, Alcami A. Vaccinia virus immune evasion. Immunol Rev. 1997;159:137–154. doi: 10.1111/j.1600-065x.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 57.Stuart D, Graham K, Schreiber M, Macaulay C, McFadden G. The target DNA sequence for resolution of provirus replicative intermediates is an active late promoter. J Virol. 1991;65:61–70. doi: 10.1128/jvi.65.1.61-70.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tafani M, Minchenko D A, Serroni A, Farber J L. Induction of the mitochondrial permeability transition mediates the killing of HeLa cells by staurosporine. Cancer Res. 2001;61:2459–2466. [PubMed] [Google Scholar]
- 59.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 60.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 61.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 62.Tollefson A E, Hermiston T W, Lichtenstein D L, Colle C F, Tripp R A, Dimitrov T, Toth K, Wells C E, Doherty P C, Wold W M. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature. 1998;392:726–730. doi: 10.1038/33712. [DOI] [PubMed] [Google Scholar]
- 63.Tortorella D, Gewurz B E, Furman M H, Schust D J, Ploegh H L. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 64.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 65.White E, Sabbatini P, Debbas M, Wold W S, Kusher D I, Gooding L R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 67.Zamzami N, El Hamel C, Maisse C, Brenner C, Munoz-Pinedo C, Belzacq A S, Costantini P, Vieira H, Loeffler M, Molle G, Kroemer G. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342–6350. doi: 10.1038/sj.onc.1204030. [DOI] [PubMed] [Google Scholar]
- 68.Zamzami N, Marzo I, Susin S A, Brenner C, Larochette N, Marchetti P, Reed J, Kofler R, Kroemer G. The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of Bcl-2 by enforcing mtiochondrial permeability transition. Oncogene. 1998;16:1055–1063. doi: 10.1038/sj.onc.1201864. [DOI] [PubMed] [Google Scholar]
- 69.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Q, Krebs J F, Snipas S J, Price A, Alnemri E S, Tomaselli K J, Salvesen G S. Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics, and characterization of the caspase/p35 complex. Biochemistry. 1998;37:10757–10765. doi: 10.1021/bi980893w. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA: analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 72.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]