Abstract
The American hoverfly Eupeodes americanus (Wiedemann) (Diptera: Syrphidae) is an aphidophagous predator during its larval stage and is currently being evaluated for inclusion in biocontrol programs as a new biocontrol agent. However, little is known about its reproductive aptitudes. The objective of the present study was to determine the reproductive parameters of E. americanus and to compare them with those of a commercialized and widely used biological control agent for aphids, the aphid midge Aphidoletes aphidimyza (Rondani) (Diptera: Cecidomyiidae). The preoviposition period, oviposition period, adult longevity, lifetime and daily fecundity, egg hatching rate, and fertility were determined for E. americanus females and compared to those of A. aphidimyza. Trials were conducted under laboratory conditions in rearing cages on the broad bean plant Vicia faba L. (Fabaceae), infested with pea aphids Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae). The results revealed that the preoviposition period, oviposition period, and adult longevity were significantly longer in E. americanus than in A. aphidimyza. The daily fecundity and egg-hatching rate were similar in both species. However, lifetime fecundity and fertility were considerably higher in E. americanus than in A. aphidimyza. This study demonstrates that the reproductive capacity of E. americanus is clearly superior to that of A. aphidimyza and therefore supports its inclusion in the aphid pest management program as a new biocontrol agent.
Keywords: preoviposition period, oviposition period, longevity, fecundity, fertility
Introduction
Aphids (Hemiptera: Aphididae) are among the most harmful pests of numerous crops around the world, including greenhouse-grown sweet pepper and cucumber (Ramakers 2004, Sanchez et al. 2007, Messelink et al. 2020) or cotton and soybeans among field crops (Blackman and Eastop 2007). Over the past decades, the intensive use of insecticides has favored not only the emergence of resistant populations of several aphid species (Herron et al. 2001, Kift et al. 2004, Bass et al. 2015), but has also led to the emergence of health and environmental issues (Cabrera 2017). With increased awareness linked to these problems, concepts such as sustainable agriculture and environmental conservation have been championed in recent times, which promote biological control as a better alternative to chemical control (van Lenteren et al. 2018).
Within aphidophagous guilds of natural enemies, many studies demonstrate that the predatory larvae of several species of Syrphidae can play important roles as natural control agents (Chambers 1986, Belliure and Michaud 2001, Putra and Yasuda 2006, Amorós-Jiménez et al. 2012, Arcaya et al. 2017, Dunn et al. 2020). In Europe, several species have been studied and 3 of them, namely Episyrphus balteatus (De Geer) (Diptera: Syrphidae), Sphaerophoria rueppellii (Wiedemann), and Eupeodes corollae (Fabricius) (Diptera: Syrphidae) are already commercialized (Almohamad et al. 2006, van Lenteren et al. 2018, Pekas et al. 2020). Recently, the American hoverfly, Eupeodes americanus (Wiedemann) has been commercialized in Canada and represents the first hoverfly species available to growers in North America. It is a generalist Nearctic aphid predator (Skevington 2019), feeding on more than 40 different aphid species (Rojo et al. 2003). Among their aphid hosts, are numerous crop pests such as the foxglove aphid Aulacorthum solani (Kaltenbach) (Hemiptera: Aphididae) on pepper (Bellefeuille et al. 2021), the melon aphid Aphis gossypii Glover on melon and cucumber (Heiss 1938, Fauteux et al. 2024), the soybean aphid Aphis glycines Matsumura on soya (Kaiser et al. 2007, Noma et al. 2010), and the green peach aphid Myzus persicae (Sulzer) on potatoes and pepper (Vockeroth 1992, Gonzalez et al., 2023a).
In a previous study, Bellefeuille et al. (2019) examined the efficacy of E. americanus, and highlighted its ability to fly, lay eggs and feed on aphids even at low temperatures (from 12 °C to 18 °C), which is not the case for most commercialized aphidophagous natural enemies such as parasitoids, coccinellids or the aphid midge Aphidoletes aphidimyza (Rondani) (Diptera: Cecidomyiidae) (Langer et al. 2004, Alotaibi 2008, Sørensen et al. 2013). Moreover, the larva of the American hoverfly is a furtive predator, as is the aphid midge (Lucas and Brodeur 2001) and can feed on aphids without triggering a defensive response (Meseguer et al. 2021).
In order to resolve aphid problems in greenhouses, it is important for biological control agents to become established within growing environments before pest aphids invade. To facilitate this, it is possible to establish a banker plant system within greenhouses (Frank 2010, Huang et al. 2011, Gonzalez et al. 2023b). The efficacy of E. americanus associated with a banker plant system was demonstrated in experimental and commercial greenhouses in spring production by Bellefeuille et al. (2021). Eupeodes americanus has been able to use the banker plant system to feed and reproduce on it. Furthermore, adults that emerged from banker plant systems were able to locate and lay eggs on infested plants in the focal crop and finally control aphid populations (Bellefeuille et al. 2021). This demonstrates that E. americanus has several attributes to be an efficient biological control agent. However, basic information on the biology and ecology of this species is still missing.
Among the most fundamental aspects of the bioecology of potential biocontrol agents to describe are their life cycle, reproductive potential, and voracity (Coppel and Mertins 1977, Soleyman-Nezhadiyan and Laughlin 1998, Stiling and Cornelissen 2005, Hoddle and Van Driesche 2009). These characteristics are essential for the development of an effective biological control program and for the development of productive mass-rearing systems (Soleyman-Nezhadiyan and Laughlin 1998, Stiling and Cornelissen 2005). To this end, prior work helped to define the life cycle of E. americanus and compared it to A. aphidimyza, a commercially available biocontrol agent. The aphid midge, A. aphidimyza, is one of the most important predators used for aphid control, making it a good reference in comparative studies (Boulanger et al. 2019). Results demonstrated that E. americanus larval developmental time and adult longevity are clearly longer than A. aphidimyza and consequently have a higher potential for biological control (Ouattara et al. 2022). The voracity of E. americanus was found to be very high as 1 larva can consume around 2,000 aphids (Fauteux et al. 2024). This great voracity should generate a high killing rate which makes E. americanus a good predator, however, crucial information regarding its reproductive potential remains unavailable.
The objective of the present study was to determine the length of the preoviposition and oviposition periods, the fecundity, the egg-hatching rate, the fertility, and the adult longevity. These different factors were determined in E. americanus and compared with those of a commercially available biological control agent, A. aphidimyza. Our first hypothesis is that the longevity, lifetime fecundity, and daily fecundity of females E. americanus will be higher than that of females A. aphidimyza, since these parameters are usually correlated with body size (Elgar and Pierce 1988, Jikumaru et al. 1994, Branquart and Hemptinne 2000, García-Barros 2000, Šešlija and Tucić 2003). Our second hypothesis is that the oviposition and preoviposition periods of female E. americanus will be longer than those of female A. aphidimyza because these parameters are correlated positively with adult longevity in predators (Scott and Barlow 1984, Jikumaru et al. 1994, Coll 1996).
Materials and methods
Insects Rearing
Eupeodes americanus specimens came from an experimental colony held at the biocontrol laboratory of the Université du Québec à Montréal since 2014. These individuals were originally collected as wild adults on Phlox sp. L. (Polemoniaceae) flowers in Sainte-Agathe-de-Lotbinière (N 46°23ʹ726″, W 71°21ʹ446″), Québec, Canada. The laboratory colony was refreshed yearly with new wild individuals. The Fraser methodology was used to rear multiple generations in the laboratory (Fraser 1972). A rearing cage of dimension 81 × 53 × 60 cm covered with muslin was used to keep adults in a greenhouse at 22 °C during the day, 19 °C at night, at 60% RH and 16:8 (L:D) under high-pressure sodium lamps. Adults were fed through an artificial flower and a sugar:water mixture (1:10 v/v). Artificial flowers consisted of a wooden stick inserted inside a round cotton makeup remover pad saturated with a honey:water mixture (1:3 v/v) and covered with wildflower bee pollen. These food resources were replaced twice a week. Broad bean plants Vicia faba L. (Fabaceae) (Norseco, Quebec, Canada) infested with pea aphid Acyrthosiphon pisum (Harris) were replaced in the adult rearing cage twice a week in order to allow females to oviposit after mating. Larvae were collected and transferred to two 35 cm3 rearing cages covered with muslin maintained in a growth chamber (Conviron, Model E15, Canada) set at 24 °C, 70% RH and 16:8 (L:D) once each week. These larval cages contained barley plants Hordeum vulgare L. (Poaceae) (Sollio Agriculture, Quebec, Canada), infested with cereal aphids Rhopalosiphum padi (L.). When the larvae became adults, they were introduced into the adult rearing cage described previously.
Aphidoletes aphidimyza specimens were obtained from a commercial supplier, Anatis Bioprotection (Saint-André, Quebec, Canada) as pupae. They were reared in 35 × 35 × 35 cm rearing cages covered with muslin and put in the same growth chamber as hoverfly larvae. All life stages of A. aphidimyza were reared on green peach aphid M. persicae on potato plants Solanum tuberosum L. (Solanaceae) (var. Norland, Propur, Quebec, Canada). A sugar:water mixture (1:10 v/v) was used to feed adults.
Experiment on Preoviposition Period
Tests were done under controlled conditions of 24 °C, 70% RH and 16:8 (L:D). The temperature of 24 °C was chosen as it is in the optimal range of temperatures for our reference, A. aphidimyza (Havelka and Zemek 1999, Boulanger et al. 2019) but also for E. americanus. In this study, the reproduction parameters of 20 females in E. americanus and 15 females in A. aphidimyza were determined. After emergence, each female (less than 24 h old) was immediately placed with 2 males in rearing cages covered with muslin of 71 × 71 × 31 cm for E. americanus and 14.5 × 11 × 11 cm for A. aphidimyza and were reared as described above. Each rearing cage contained about one 10 cm high broad bean plant Vicia faba L. (Fabaceae) infested with about 85 A. pisum nymphs of the second and third stages. Females were observed daily until the first oviposition. The oviposition date was recorded, and the preoviposition period was determined as the period between female emergence and its first egg-laying (Dje et al. 2011).
Experiment on Fecundity, Fertility, Oviposition Period, Egg Hatching Rate, and Longevity of Females
As soon as the first egg was observed, the broad bean plant infested with aphids was removed and replaced by another one every 24 h in each cage. The number of eggs deposited by the female on the broad bean plant was noted daily. The experiment continued until the death of the female. Males were replaced if they died before the female. The longevity of males was not monitored in this study. Lifetime fecundity was determined as the sum of eggs laid per female. The egg hatching rate was determined for 258 eggs in E. americanus and for 165 eggs in A. aphidimyza randomly chosen among the eggs laid (number of offspring (L1) produced/total number of incubated fertile eggs × 100). The fertility of females or viability of eggs laid (Leather 1995) was assessed by taking into account both lifetime fecundity (number of eggs laid) and egg hatch rate. The date of the last oviposition was noted, and the oviposition period (the period between the first and the last oviposition) was determined in days. Then, the daily fecundity was determined by dividing the lifetime fecundity by the length of the oviposition period. Finally, female longevity was determined as the period between adult emergence and death (Dje et al. 2011).
Statistical Analysis
All statistical analyses were performed within the R statistical environment (v. 3.4.2, R Foundation for Statistical Computing 2017). For each test, the significance level was set at alpha = 0.05.
Prior to any subsequent analysis, assumptions for parametric analyses were fulfilled following a Shapiro–Wilk test of normality (P > 0.05) and with the inspection of diagnostic plots (residuals vs fitted, normal QQ plot, scale location, and constant leverage). The mean preoviposition period, oviposition period, longevity, lifetime fecundity, fertility, and daily fecundity did not follow a normal distribution. No transformation is allowed to meet a normal distribution. Then, those data were compared between predator’s species using a nonparametric Wilcoxon rank-sum test. A generalized linear mixed model (GLMM) was employed to investigate the oviposition across the 2 aphid predator species over time (‘lme4’ package in R) (Bates et al. 2015). The GLMM was specified with the number of eggs laid as the response variable. Fixed effects included time (in days), species (E. americanus or A. aphidimyza), and their interaction. A random factor was included to account for random variability in oviposition among individuals within species. Given the non-normal distribution and typical right-skewness of the count data, Poisson distribution was used, with a log link function. The validation of the model was assessed through residual diagnostics including Q–Q plots for the normality of residuals and plots of Pearson residuals against explanatory variables as well as Cooks distance plots to verify homoscedasticity of variance. The Spearman rank correlation test was conducted to measure the degree of relatedness between the oviposition period and longevity and between the oviposition period and fecundity for each predator. Finally, egg hatch rates were compared between predators using a Pearson χ2 analysis.
Results
Preoviposition Period, Oviposition Period, and Longevity of Females
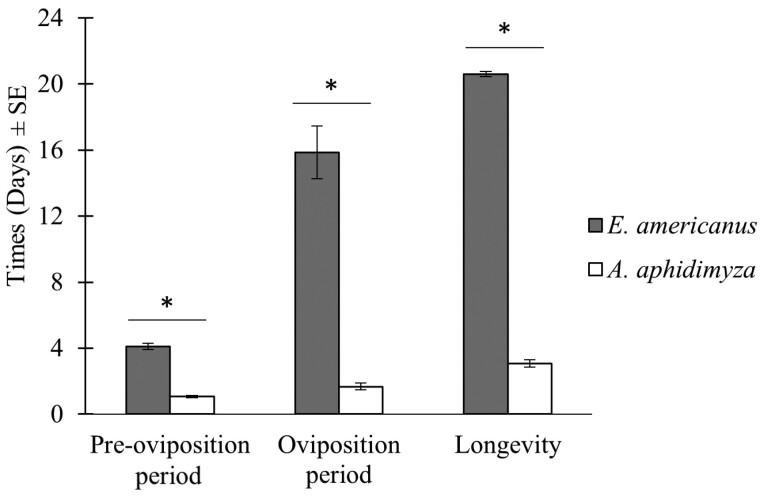
The preoviposition period varied from 3 to 6 days in length for E. americanus and from 1 to 2 days for A. aphidimyza. The mean preoviposition period of E. americanus was significantly longer than of A. aphidimyza with 4.1 ± 0.2 and 1.1 ± 0.1 days, respectively (Wilcoxon, W = 0; df = 1; P < 0.001; Fig. 1).
Fig. 1.
Mean preoviposition period; oviposition period and female adult longevity (±SE) for E. americanus (n = 20) and A. aphidimyza (n = 15). An asterisk (*) indicates a significant difference between species (P < 0.05).
The mean longevity of adult E. americanus and A. aphidimyza females were 20.6 ± 1.6and 3.1 ± 0.2 days, respectively, which were significantly different from one another (Wilcoxon, W = 0; df = 1; P < 0.001; Fig. 1). The oviposition period varied from 5 to 31 days for E. americanus and from 1 to 3 days for A. aphidimyza (Fig. 3). The mean oviposition period, 15.9 ± 1.6 days for E. americanus was drastically longer (9.5 times more) than for A. aphidimyza at only 1.7 ± 0.2 days long (Wilcoxon, W = 0; df = 1; P < 0.001; Fig. 1). The oviposition period in females was positively correlated with their longevity in both species (Spearman, S = 27.75; Rs = 0.98; P < 0.001 for E. americanus, S = 99.79; Rs = 0.82; P < 0.001 for A. aphidimyza).
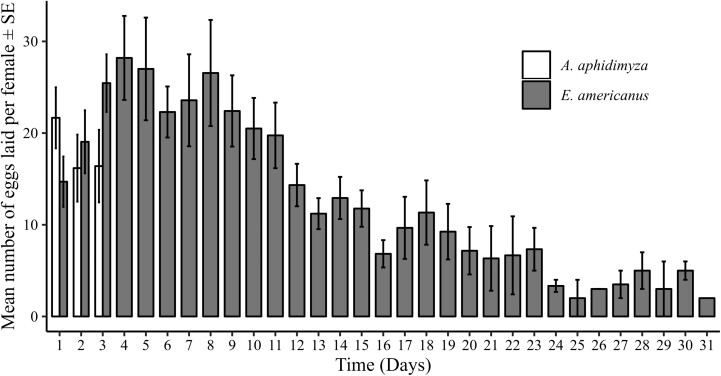
Fig. 3.
Evolution of the mean number of eggs laid per female (±SE) over time (in days) for E. americanus (n = 20) and A. aphidimyza (n = 15).
Lifetime Fecundity, Daily Fecundity, Egg Hatching Rate, and Fertility
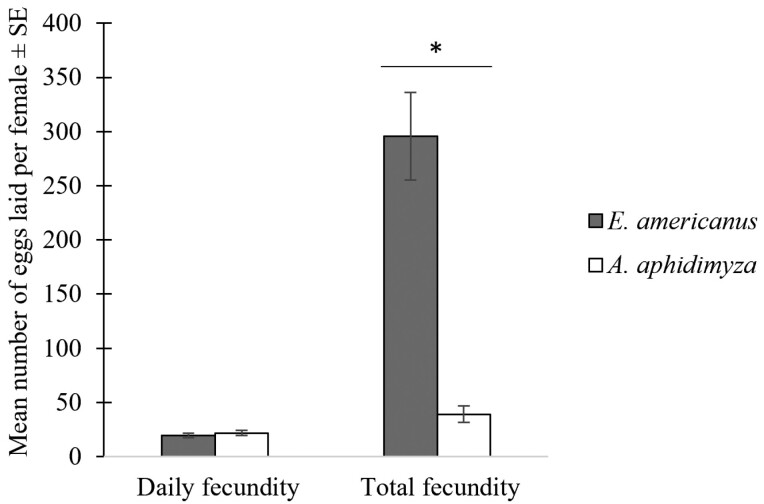
Among both species assessed, the highest overall number of eggs laid per individual was of 693 for E. americanus with a mean lifetime fecundity of 295.7 ± 40.4 eggs. The highest total number of eggs laid per A. aphidimyza female was 89 eggs with a mean lifetime fecundity of 39 ± 7.6 eggs. Eupeodes americanus had a lifetime fecundity 7.6 times higher than that of A. aphidimyza (Wilcoxon, W = 0; df = 1; P < 0.001; Fig. 2). Logically, females who had a longer oviposition period laid more eggs than those with shorter oviposition period in both species (Spearman, S = 456.38; Rs = 0.66; P = 0.002 for E. americanus, S = 103.38; Rs = 0.82; P < 0.001 for A. aphidimyza). The number of eggs laid daily per female varied from 0 to 105 in E. americanus and from 0 to 39 in A. aphidimyza. The mean daily fecundity was not significantly different between E. americanus and A. aphidimyza with respectively 19.4 ± 2.0 and 21.7 ± 2.3 eggs (Wilcoxon, W = 170; df = 1; P = 0.52; Fig. 2).
Fig. 2.
Mean daily fecundity and total fecundity (±SE) for female E. americanus (n = 20) and female A. aphidimyza (n = 15). An asterisk (*) indicates a significant difference between species (P < 0.05).
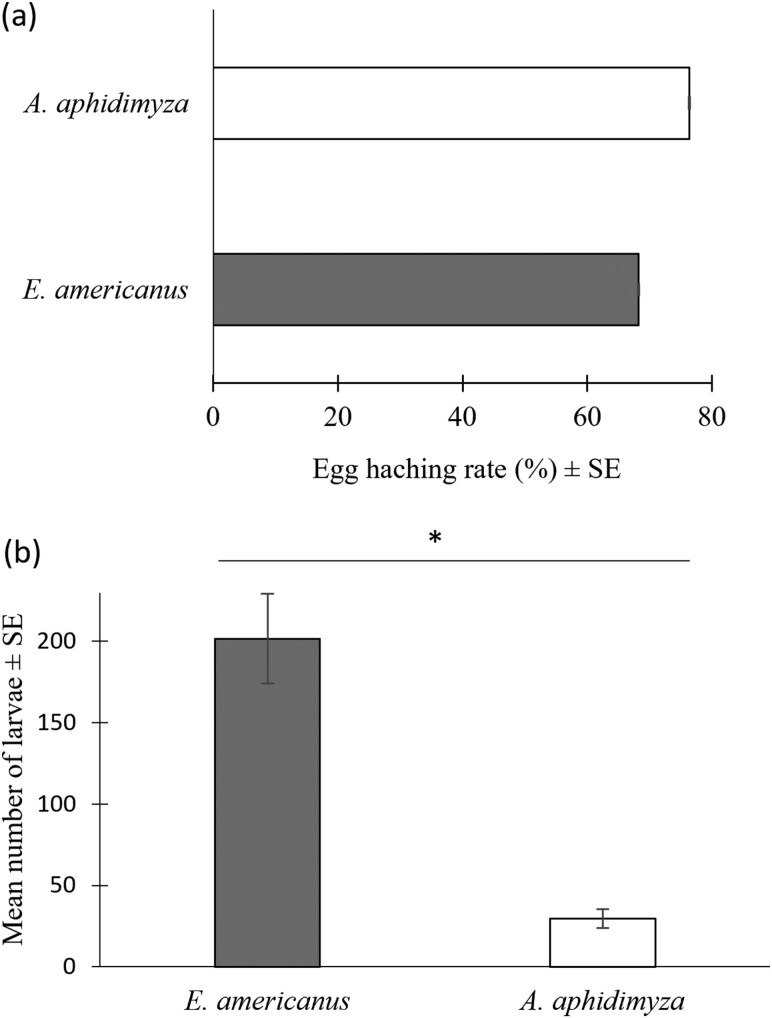
The GLMM analysis revealed significant effects of time and the interaction between time and species on the oviposition activity. Specifically, time had a notable negative impact on the number of eggs laid (Estimate = –0.27833, SE = 0.05838, z = –4.768, P < 0.001), indicating a decrease in egg-laying as time progressed (Fig. 3). The species effect, represented by the comparison between E. americanus and A. aphidimiza, was not statistically significant (estimate = –0.01659, SE = 0.10371, z = –0.160, P = 0.87), suggesting no initial difference in oviposition rates between the 2 species. However, the interaction between time and species was significant (estimate = 0.21616, SE = 0.05844, z = 3.699, P < 0.001;), demonstrating that E. americanus experienced a less pronounced decline in egg-laying over time compared to A. aphidimiza. There was no difference in egg hatch rate between E. americanus (68.2%) and A. aphidimyza (76.4%) (Pearson, χ2 = 2.88; df = 1; P = 0.09; Fig. 4a). The mean fertility was significantly superior in E. americanus than in A. aphidimyza, with respectively 201.7 and 29.8 larvae (Wilcoxon, W = 2; df = 1; P < 0.001; Fig. 4b).
Fig. 4.
(a) Egg hatching rate (±SE) (E. americanus n = 258; A. aphidimyza n = 165) and (b) mean total fertility (±SE) for E. americanus (n = 20) and A. aphidimyza (n = 15). An asterisk (*) indicates a significant difference (P < 0.05) between species.
Discussion
The reproductive aptitudes of potential predators may constitute key factors for deciding to apply agents within biocontrol programs. The objective of the present study was to determine the reproductive capacity of E. americanus and to compare it with that of a commercially available agent, A. aphidimyza, in order to evaluate its potential as a new aphidophagous biological control agent. Globally, considering the reproductive aspects of both species, our results demonstrate a high potential for biocontrol in the American hoverfly. Indeed, the results of this study showed that the longevity, the preoviposition period and the oviposition period of E. americanus females were significantly longer than those of A. aphidimyza. The fecundity and fertility of E. americanus were also higher than those of A. aphidimyza. These results therefore confirm our alternate hypotheses, which posited that adult longevity, oviposition, and preoviposition periods, as well as lifetime fecundity and daily fecundity of E. americanus will be higher than that of A. aphidimyza. Building on these findings, it is essential to consider them in conjunction with the body sizes of the 2 predators with E. americanus being considerably larger than A. aphidimyza. Indeed, literature has shown that female body size is correlated with higher fecundity, indicating that body size is a primary constraint on an insect’s potential fecundity (Honěk 1993).
The preoviposition period of E. americanus (4.1 days) was shorter or similar to those observed in other syrphid species. Indeed, the egg-laying period for Dioprosopa clavata (Fabricius) lasts an average of 6.6 days at 23 °C (Belliure and Michaud 2001), 8.8 days for E. balteatus at 20 °C (Guest 1984), 6.8 days for Melangyna viridiceps (Macquart), 5.6 days for Symosyrphus grandicornis (Macquart) at 20 °C (Soleyman-Nezhadiyan 1996) and 3.8 days for E. corollae at 20 °C (Lillo et al. 2021). Several factors can influence oviposition times in predators such as larval foraging, temperature, and food quantity and quality (Zheng et al. 1993, Gotoh et al. 2004, Jandricic et al. 2013). A longer preoviposition period may be detrimental for a biocontrol agent since it delays the time when the larvae will start consuming the pest.
Female longevity was approximately 7 times longer for E. americanus than for A. aphidimyza (20.6 and 3.1 days, respectively). Female longevity is a crucial factor in biological control that influences the dynamics of predator-prey populations (Laubertie 2007). Indeed, a longer adult stage can allow females to multiply their mating occasions and thus increase their oviposition rates and consequently the length of their reproductive period (Arnqvist and Nilsson 2000). Among syrphids, most species also have a longer female longevity than A. aphidimyza, lasting for example 11.6 days for Allograpta exotica (Wiedemann) at 25 °C (Arcaya et al. 2017), but was shorter than in E. americanus (20.6 days). The longevity of E. americanus was comparable to that reported for S. grandicornis at 20 °C (Soleyman-Nezhadiyan 1996) but was shorter than that for E. balteatus which is around 40 days at 21 °C (Branquart and Hemptinne 2000). However, adult syrphid nutrition, as indicated by pollen from different plant species, also influences their longevity (Laubertie et al. 2012, Pinheiro et al. 2013, 2015). Another study suggests that A. aphidimyza has an average longevity of 2.5 days on R. padi but 4 days on M. persicae at 20 °C (Higashida et al. 2016) which is close to our results (3.1 days). Of course, in a real situation, the longevity of biocontrol agents must be evaluated in combination with a careful consideration of realized mortality in the greenhouse environments.
The oviposition period of E. americanus was also drastically longer than in A. aphidimyza (15.9 and 1.7 days, respectively). This long oviposition period is a clear advantage in biological control because the overall oviposition is more spread out over time (Borges et al. 2013). This would keep the population of E. americanus in a greenhouse longer than A. aphidimyza which has a short oviposition period. This is of real interest for biological control since E. americanus could have a medium- or long-term biocontrol effect, while A. aphidimyza will rather have a short-term biocontrol effect. The oviposition period in E. americanus obtained in this study (15.9 days) is relatively shorter than that reported in E. corollae (18 days) at 28 °C (Benestad 1970), E. balteatus (19 days) at 20 °C (Guest 1984) and M. viridiceps (19.9 days) at 20 °C (Soleyman-Nezhadiyan 1996). It was however longer than that obtained in E. corollae (9.8 days) at 20 °C (Lillo et al. 2021) and S. grandicornis (13.8 days) at 20 °C (Soleyman-Nezhadiyan 1996). Our study indicated that there was a positive correlation between oviposition period and female adult longevity. This positive correlation has been previously demonstrated in several studies in Syrphidae (Scott and Barlow 1984) and other predatory species (Jikumaru et al. 1994, Coll 1996, Borges et al. 2013).
According to the size difference, logically, the lifetime fecundity, and fertility of E. americanus were also drastically higher than those of A. aphidimyza (respectively, 295.7 vs. 89 eggs per female and 201.7 vs. 29.8 larvae). Fertility is the ultimate index of the reproductive potential of a predatory species since the overall impact is a function of larval number and larval voracity. Fecundity is also a factor in inoculative biological control because it is an important determinant of the population size for a given predator species (Coppel and Mertins 1977). Thus, the combination of a greater fecundity and a longer oviposition period in E. americanus, along with a favorable egg-hatching rate, results in the production of more larvae (aphidophagous stage) over time (Chambers and Adams 1986, Rojo et al. 1996). Other syrphid species also have higher fecundity than A. aphidimyza (Geusen-Pfister 1987, Soleyman-Nezhadiyan 1996, Fathipour et al. 2006). In our study, the fecundity of females was positively correlated with their oviposition period as reported in predatory syrphids and other predator species (Scott and Barlow 1984, Coll 1996). Furthermore, E. americanus has a higher lifetime fecundity than do other syrphid species such as Scaeva albomaculata (Macquart) (95.5 eggs) feeding on M. persicae at 25 °C (Fathipour et al. 2006), S. scripta (195.2 eggs) on Aphis crassivora Koch at 22 °C (Moetamedinia et al. 2004), E. corollae (169 eggs) on M. persicae at 20 °C (Lillo et al. 2021) and M. viridiceps (288 eggs) on Macrosiphum rosae (L.) at 20 °C (Soleyman-Nezhadiyan 1996). However, it was lower than that obtained in D. clavata (421.3 eggs) on Aphis spiraecola Patch (Hemiptera: Aphididae) at 23 °C (Belliure and Michaud 2001), E. corollae (436 eggs) on M. persicae at 28 °C (Benestad 1970) and E. balteatus (780 eggs) on A. craccivora and A. pisum at 25 °C (Geusen-Pfister 1987). For A. aphidimyza, the mean lifetime fecundity in this study was higher than that obtained by Higashida et al. (2016) (19.9 eggs) on R. padi, but was similar to those reported for this species by Watanabe et al. (2014) (39 eggs) on A. gossypii and by Higashida et al. (2016) (40.1 eggs) on M. persicae at 25 °C. Lifetime fecundity and fertility in predatory syrphids and other predators depend not only on both larval and adult nutrition (Schneider 1969, Havelka and Růžička 1984, Hickman and Wratten 1996) but also on aphid density present in the host plants (Tenhumberg 1995). Indeed, the fecundity of females in syrphid species can be influenced by their adult diet, in particular, the availability and quality of pollen (Gilbert 1981, Amorós-Jiménez et al. 2014). Pollen is a crucial resource for syrphid females because it provides the protein necessary for sexual maturation and egg development (Schneider 1969, Haslett 1989, Pinheiro et al. 2013). This means that in a greenhouse environment devoid of border vegetation, it is advisable to introduce flowering plant resources along with syrphid biocontrol agents to support predator reproduction (Hickman and Wratten 1996, Landis et al. 2000, Pineda and Marcos-García 2008, Gillespie et al. 2011, Hogg et al. 2011, Leman et al. 2023). Variation in aphid prey species and aphid density offered to larval stages can also influence the fecundity of subsequent adults (Cornelius and Barlow 1980) because an aphid species with a low nutritional value or a low density of aphids offered to larval syrphid stages may induce the emergence of smaller syrphid adults at the next generation with lower fecundity (Jikumaru et al. 1994, Branquart and Hemptinne 2000) as well as those of other predator species (Dixon and Guo 1993, Zheng et al. 1993). However, the use of banker plants in inoculative biological control in greenhouses would reduce not only these larval nutritional problems but also the period of preoviposition in adults (Frank 2010, Huang et al. 2011). Finally, the fecundity of females is also influenced by the aphid density present in host plants. In many aphidophagous hoverfly species, an absence of aphids on host plants leads to egg resorption by adult females (Dixon 1959, Schneider 1969, Branquart and Hemptinne 2000, Orengo-Green et al. 2022). According to Gonzalez et al. (2023c), the number of eggs laid by E. americanus females increases with the abundance of aphids, as it does for A. aphidimyza. It was also shown that E. americanus responds to low densities of aphids early in the infestation (2–5 aphids) process and that its oviposition is better than that of A. aphidimyza on sweet pepper plants and similar on cucumber (Gonzalez et al. 2023c). From a practical standpoint, these findings are promising, as it is crucial that the minimum aphid density above which E. americanus females start to oviposit remains low in order to ensure its success in a biocontrol context. For mass-rearing purposes, it will be essential to determine the optimal aphid density above which the number of eggs laid per female decreases. For example, in S. grandicornis, the number of eggs laid by a female increased with densities up to 100 aphids, then decreased when the number of aphids exceeded 100 individuals (Soleyman-Nezhadiyan 1996).
In conclusion, our study has demonstrated the considerable potential of E. americanus as a new candidate biological control agent for combatting aphids in agroecosystems. The results also highlight, under our study condition, the superiority of the syrphid over A. aphidimyza with several especially important points: (1) the longer longevity and oviposition period for E. americanus and (2) the drastically higher lifetime fecundity and fertility in E. americanus. Furthermore, previous studies have also demonstrated other important characteristics of this biological control agent: (1) the great efficacy at low temperatures or short photoperiod of the syrphid (Bellefeuille et al. 2019, Gonzalez et al. 2023a), and its superiority over Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae) (Barriault et al. 2019), (2) the immature development time of E. americanus similar to that of A. aphidimyza and the larval development time significantly longer than in A. aphidimyza representing a longer predation period (Ouattara et al. 2022). All these demonstrate that E. americanus has great potential to be used as a biological control agent of aphid pests.
Acknowledgments
Our gratitude goes out to all the members of the Biocontrol Laboratory of the Université du Québec à Montréal. We would also like to thank Jill Vandermeerschen for her help with statistical analysis, and Nathan Morris for the English revision.
Contributor Information
Téné Yacine Ouattara, Laboratoire de Lutte Biologique, Département des Sciences Biologiques, Université du Québec à Montréal (UQAM), H3C 3P8 Montréal, Québec, Canada.
Marc Fournier, Laboratoire de Lutte Biologique, Département des Sciences Biologiques, Université du Québec à Montréal (UQAM), H3C 3P8 Montréal, Québec, Canada.
Noémie Gonzalez, Laboratoire de Lutte Biologique, Département des Sciences Biologiques, Université du Québec à Montréal (UQAM), H3C 3P8 Montréal, Québec, Canada.
Santos Rojo, Departamento de Ciencias Ambientales & Recursos Naturales, Universidad de Alicante, E-03080 Alicante, Spain.
Eric Lucas, Laboratoire de Lutte Biologique, Département des Sciences Biologiques, Université du Québec à Montréal (UQAM), H3C 3P8 Montréal, Québec, Canada.
Funding
This research was supported by the “Programme Canadien de Bourses de la Francophonie” (PCBF) and the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery Grants Program-Eric Lucas).
Author Contributions
Tene Yacine Ouattara (Conceptualization [Equal], Formal analysis [Equal], Funding acquisition [Equal], Investigation [Lead], Methodology [Equal], Visualization [Lead], Writing – original draft [Lead], Writing – review & editing [Equal]), Marc Fournier (Conceptualization [Equal], Formal analysis [Equal], Methodology [Equal], Writing – review & editing [Equal]), Noémie Gonzalez (Writing – original draft [Equal], Writing – review & editing [Equal]), Santos Rojo (Conceptualization [Equal], Methodology [Equal], Supervision [Equal], Writing – review & editing [Equal]), and Eric Lucas (Conceptualization [Equal], Funding acquisition [Equal], Methodology [Equal], Supervision [Equal], Writing – review & editing [Equal])
References
- Almohamad R, Verheggen F, Francis F, Haubruge E.. Evaluation of hoverfly Episyrphus balteatus De Geer (Diptera: Syrphidae) oviposition behaviour toward aphid-infested plants using a leaf disc system. Commun Agric Appl Biol Sci. 2006:71(2 Pt B):403–412. [PubMed] [Google Scholar]
- Alotaibi S. Mass production and utilization of the predatory midge, Aphidoletes aphidimyza Rondani for controlling aphids. Glob J Biotechnol Biochem. 2008:3(1):1–7. [Google Scholar]
- Amorós-Jiménez R, Pineda A, Fereres A, Marcos-García MA.. Prey availability and abiotic requirements of immature stages of the aphid predator Sphaerophoria rueppellii. Biol Control. 2012:63(1):17–24. 10.1016/j.biocontrol.2012.06.001 [DOI] [Google Scholar]
- Amorós-Jiménez R, Pineda A, Fereres A, Marcos-García MA.. Feeding preferences of the aphidophagous hoverfly Sphaerophoria rueppellii affect the performance of its offspring. BioControl. 2014:59(4):427–435. 10.1007/s10526-014-9577-8 [DOI] [Google Scholar]
- Arcaya E, Pérez-Bañón C, Mengual X, Zubcoff-Vallejo JJ, Rojo S.. Life table and predation rates of the syrphid fly Allograpta exotica, a control agent of the cowpea aphid Aphis craccivora. Biol Control. 2017:115:74–84. 10.1016/j.biocontrol.2017.09.009 [DOI] [Google Scholar]
- Arnqvist G, Nilsson T.. The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav. 2000:60(2):145–164. 10.1006/anbe.2000.1446 [DOI] [PubMed] [Google Scholar]
- Barriault S, Soares AO, Gaimari SD, Lucas E.. Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae), a new aphidophagous biocontrol agent; development, survival and comparison with Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae). Bull Entomol Res. 2019:109(4):472–478. 10.1017/S0007485318000767 [DOI] [PubMed] [Google Scholar]
- Bass C, Denholm I, Williamson MS, Nauen R.. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol. 2015:121:78–87. 10.1016/j.pestbp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S.. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015:67(1):1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bellefeuille Y, Fournier M, Lucas E.. Evaluation of two potential biological control agents against the foxglove aphid at low temperatures. J Insect Sci. 2019:19(1):1–8. 10.1093/jisesa/iey130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellefeuille Y, Fournier M, Lucas E.. Biological control of the foxglove aphid using a banker plant with Eupeodes americanus (Diptera: Syrphidae) in experimental and commercial greenhouses. Biol Control. 2021:155:104541. 10.1016/j.biocontrol.2021.104541 [DOI] [Google Scholar]
- Belliure B, Michaud JP.. Biology and behavior of Pseudodorus clavatus (Diptera: Syrphidae), an important predator of citrus aphids. Ann Entomol Soc Am. 2001:94(1):91–96. 10.1603/0013-8746(2001)094[0091:babopc]2.0.co;2 [DOI] [Google Scholar]
- Benestad E. Laboratory experiments on the biology of Syrphus corollae (Fabr.) (Dipt., Syrphidae). Norw J Entomol. 1970:17:77–85. [Google Scholar]
- Blackman RL, Eastop VF.. Taxonomic issues. In: Emden HFV, Harrington R, editors. Aphids as crop pests. 1st ed. UK: CABI; 2007. [accessed 2023 Sep 21]. p. 1–29. http://www.cabidigitallibrary.org/doi/10.1079/9780851998190.0001 [Google Scholar]
- Borges I, Hemptinne J-L, Soares AO.. Contrasting population growth parameters of the aphidophagous Scymnus nubilus and the coccidophagous Nephus reunioni. BioControl. 2013:58(3):351–357. 10.1007/s10526-012-9490-y [DOI] [Google Scholar]
- Boulanger F-X, Jandricic S, Bolckmans K, Wäckers FL, Pekas A.. Optimizing aphid biocontrol with the predator Aphidoletes aphidimyza, based on biology and ecology. Pest Manag Sci. 2019:75(6):1479–1493. 10.1002/ps.5270 [DOI] [PubMed] [Google Scholar]
- Branquart E, Hemptinne J-L, Hemptinne J-L.. Development of ovaries, allometry of reproductive traits and fecundity of Episyrphus balteatus (Diptera: Syrphidae). Eur J Entomol. 2000:97(2):165–170. 10.14411/eje.2000.031 [DOI] [Google Scholar]
- Cabrera LY. Pesticides: a case domain for environmental neuroethics. Camb Q Healthc Ethics. 2017:26(4):602–615. 10.1017/S0963180117000111 [DOI] [PubMed] [Google Scholar]
- Chambers RJ. Preliminary experiments on the potential of hoverflies (Dipt.: Syrphidae) for the control of aphids under glass. Entomophaga. 1986:31(2):197–204. 10.1007/bf02372371 [DOI] [Google Scholar]
- Chambers RJ, Adams THL.. Quantification of the impact of hoverflies (Diptera: Syrphidae) on cereal aphids in winter wheat: an analysis of field populations. J Appl Ecol. 1986:23(3):895–904. 10.2307/2403942 [DOI] [Google Scholar]
- Coll M. Feeding and Ovipositing on Plants by an Omnivorous Insect Predator. Oecologia. 1996:105(2):214–220. 10.1007/BF00328549 [DOI] [PubMed] [Google Scholar]
- Coppel HC, Mertins JW.. Organisms used in classical biological insect pest suppression. In: Coppel HC, Mertins JW, editors. Biological Insect Pest Suppression. Berlin, Heidelberg: Springer; 1977. [accessed 2023 Sep 21]. (Advanced Series in Agricultural Sciences). p. 73–165. 10.1007/978-3-642-66487-8_3 [DOI] [Google Scholar]
- Cornelius M, Barlow CA.. Effect of aphid consumption by larvae on development and reproductive efficiency of a flower fly, Syrphus corollae (Diptera: Syrphidae). The Can Entomol. 1980:112(10):989–992. 10.4039/ent112989-10 [DOI] [Google Scholar]
- Dixon AFG, Guo YQ.. Egg and cluster size in ladybird beetles (Coleoptera: Coccinellidae): the direct and indirect effects of aphid abundance. EJE. 1993:90(4):457–463. [Google Scholar]
- Dixon T. Studies on oviposition behavior of Syrphidea (Diptera). Trans R Entomol Soc Lond. 1959:111(3):57–80. 10.1111/j.1365-2311.1959.tb02276.x [DOI] [Google Scholar]
- Dje TKC, Aboua LRN, Seri-Kouassi BP, Ouali-N’Goran SWM, Allou K.. Etude de quelques paramètres biologiques de Pseudotheraptus devastans Distant (Heteroptera: Coreidae) sur les noix de Cocos nucifera L. de la variété PB 121+ à la station Marc Delorme (Côte d’Ivoire). Sci Nat. 2011:8(1–2):13–22. [Google Scholar]
- Dunn L, Lequerica M, Reid CR, Latty T.. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): pollinators and biological control agents. Pest Manag Sci. 2020:76(6):1973–1979. 10.1002/ps.5807 [DOI] [PubMed] [Google Scholar]
- Elgar MA, Pierce NE.. 1988. Mating success and fecundity in an ant-tended lycaenid butterfly. In: Clutton-Brock TH, editor. Reproductive success: studies of selection and adaptation in contrasting breeding systems. University of Chicago Press. Chicago. p. 59–75. [Google Scholar]
- Fathipour Y, Jalilian F, Asghar TA. . Biology and larval feeding rate of Scaeva albomaculata (Dip.: Syrphidae) on Myzus persicae (Hom.: Aphididae) at laboratory conditions. Iran J Agric Sci. 2006:37(2):249–254. [Google Scholar]
- Fauteux A, Soares AO, Lucas E.. Larval development and voracity of Eupeodes americanus (Diptera: Syrphidae): comparison of the focal prey Aphis gossypii (Hemiptera: Aphididae) and the banker prey Rhopalosiphum padi (Hemiptera: Aphididae). Insect Sci. 2024:31(2) 575–586 10.1111/1744-7917.13255 [DOI] [PubMed] [Google Scholar]
- Frank SD. Biological control of arthropod pests using banker plant systems: past progress and future directions. Biol Control. 2010:52(1):8–16. 10.1016/j.biocontrol.2009.09.011 [DOI] [Google Scholar]
- Fraser BD. A simple and efficient method of rearing aphidophagous hoverflies (Diptera: Syrphidae). J Entomol Soc BC. 1972:69:23–24. [Google Scholar]
- García-Barros E. Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera: Papilionoidea, Hesperioidea). Biol J Linn Soc. 2000:70(2):251–284. 10.1111/j.1095-8312.2000.tb00210.x [DOI] [Google Scholar]
- Geusen-Pfister H. Studies on the biology and reproductive capacity of Episyrphus balteatus Deg. (Dipt., Syrphidae) under greenhouse conditions. J Appl Entomol. 1987:104(3):261–270. [Google Scholar]
- Gilbert FS. Foraging ecology of hoverflies: morphology of the mouthparts in relation to feeding on nectar and pollen in some common urban species. Ecol Entomol. 1981:6(3):245–262. 10.1111/j.1365-2311.1981.tb00612.x [DOI] [Google Scholar]
- Gillespie M, Wratten S, Sedcole R, Colfer R.. Manipulating floral resources dispersion for hoverflies (Diptera: Syrphidae) in a California lettuce agro-ecosystem. Biol Control. 2011:59(2):215–220. 10.1016/j.biocontrol.2011.07.010 [DOI] [Google Scholar]
- Gonzalez N, Buitenhuis R, Lucas E.. Spotlight on Eupeodes americanus: Oviposition and fertility under HPS- and full spectrum LED-extended photoperiod in northern greenhouses. Biol Control. 2023a:187:105382. 10.1016/j.biocontrol.2023.105382 [DOI] [Google Scholar]
- Gonzalez N, Fauteux A, Louis J-C, Buitenhuis R, Lucas E.. Oviposition preference of the American hoverfly, Eupeodes americanus, between banker plants and target crops. Insects. 2023b:14(3):295. 10.3390/insects14030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, Fournier M, Buitenhuis R, Lucas E.. Evaluating a new aphid biocontrol agent: The role of aphid density in modulating oviposition behaviour in the American hoverfly, Eupeodes americanus, and the aphid midge, Aphidoletes aphidimyza. J Appl Entomol. 2023c:148(1):5–12. 10.1111/jen.13202 [DOI] [Google Scholar]
- Gotoh T, Yamaguchi K, Fukazawa M, Mori K.. Effect of temperature on life history traits of the predatory thrips, Scolothrips takahashii Priesner (Thysanoptera: Thripidae). Appl Entomol Zool. 2004:39(3):511–519. 10.1303/aez.2004.511 [DOI] [Google Scholar]
- Guest PJ. 1984. Oviposition strategies of aphidophagous Syrphidae. [Thesis]. London, England, UK: University of London. [Google Scholar]
- Haslett JR. Adult feeding by holometabolous insects: pollen and nectar as complementary nutrient sources for Rhingia campestris (Diptera: Syrphidae). Oecologia. 1989:81(3):361–363. 10.1007/BF00377084 [DOI] [PubMed] [Google Scholar]
- Havelka J, Růžička Z.. Selection of aphid species by ovipositing females and effects of larval food on the development and fecundity in Aphidoletes aphidimyza (Rondani) (Diptera, Cecidomyiidae). Z Angew Entomol. 1984:98(1–5):432–437. 10.1111/j.1439-0418.1984.tb02732.x [DOI] [Google Scholar]
- Havelka J, Zemek R.. Life table parameters and oviposition dynamics of various populations of the predacious gall-midge Aphidoletes aphidimyza. Entomol Exp Appl. 1999:91(3):483–486. 10.1046/j.1570-7458.1999.00517.x [DOI] [Google Scholar]
- Heiss EM. A Classification of the larvae and Puparia of the Syrphidae of illinois exclusive of aquatic forms. Ann Entomol Soc Am. 1938:31(4):475. 10.1093/aesa/31.4.475 [DOI] [Google Scholar]
- Herron GA, Powis K, Rophail J.. Insecticide resistance in Aphis gossypii Glover (Hemiptera: Aphididae), a serious threat to Australian cotton. Aust J Entomol. 2001:40(1):85–91. 10.1046/j.1440-6055.2001.00200.x [DOI] [Google Scholar]
- Hickman JM, Wratten SD.. Use of Phelia tanacetifolia strips to enhance biological control of aphids by overfly larvae in cereal fields. J Econ Entomol. 1996:89(4):832–840. 10.1093/jee/89.4.832 [DOI] [Google Scholar]
- Higashida K, Yano E, Nishikawa S, Ono S, Okuno N, Sakaguchi T.. Reproduction and oviposition selection by Aphidoletes aphidimyza (Diptera: Cecidomyiidae) on the banker plants with alternative prey aphids or crop plants with pest aphids. Appl Entomol Zool. 2016:51(3):445–456. 10.1007/s13355-016-0420-9 [DOI] [Google Scholar]
- Hoddle MS, Van Driesche R.. Biological control of insect pests. In: Resh VH, Cardé RT, editors. Encyclopedia of Insects. USA: Academic Press; 2009[accessed 2023 Sep 21]. p. 91–101. [Google Scholar]
- Hogg BN, Bugg RL, Daane KM.. Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol Control. 2011:56(1):76–84. 10.1016/j.biocontrol.2010.09.007 [DOI] [Google Scholar]
- Honěk A, Honek A.. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos. 1993:66(3):483–492. 10.2307/3544943 [DOI] [Google Scholar]
- Huang N, Enkegaard A, Osborne LS, Ramakers PMJ, Messelink GJ, Pijnakker J, Murphy G.. The banker plant method in biological control. Crit Rev Plant Sci. 2011:30(3):259–278. 10.1080/07352689.2011.572055 [DOI] [Google Scholar]
- Jandricic SE, Wraight SP, Gillespie DR, Sanderson JP.. Oviposition behavior of the biological control agent Aphidoletes aphidimyza (Diptera: Cecidomyiidae) in environments with multiple pest aphid species (Hemiptera: Aphididae). Biol Control. 2013:65(2):235–245. 10.1016/j.biocontrol.2013.01.013 [DOI] [Google Scholar]
- Jikumaru S, Togashi K, Taketsune A, Takahashi F.. Oviposition biology of Monochamus saltuarius (Coleoptera: Cerambycidae) at a constant temperature. Appl Entomol Zool. 1994:29(4):555–561. 10.1303/aez.29.555 [DOI] [Google Scholar]
- Kaiser ME, Noma T, Brewer MJ, Pike KS, Vockeroth JR, Gaimari SD.. Hymenopteran parasitoids and dipteran predators found using Soybean Aphid after its Midwestern United States invasion. Ann Entomol Soc Am. 2007:100(2):196–205. 10.1603/0013-8746(2007)100[196:hpadpf]2.0.co;2 [DOI] [Google Scholar]
- Kift NB, Mead A, Reynolds K, Sime S, Barber MD, Denholm I, Tatchell GM.. The impact of insecticide resistance in the currant-lettuce aphid, Nasonovia ribisnigri, on pest management in lettuce. Agric For Entomol. 2004:6(4):295–309. 10.1111/j.1461-9555.2004.00226.x [DOI] [Google Scholar]
- Landis DA, Wratten SD, Gurr GM.. Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann Rev Entomol. 2000:45(1):175–201. [DOI] [PubMed] [Google Scholar]
- Langer A, Boivin G, Hance T.. Oviposition, flight and walking capacity at low temperatures of four aphid parasitoid species (Hymenoptera: Aphidiinae). EJE. 2004:101(3):473–479. 10.14411/eje.2004.067 [DOI] [Google Scholar]
- Laubertie E. The role of resource subsidies in enhancing biological control of aphids by hoverflies (Diptera: Syrphidae); 2007. [accessed 2024 Mar 15]. Lincoln University.. https://hdl.handle.net/10182/984 [Google Scholar]
- Laubertie EA, Wratten SD, Hemptinne J-L.. The contribution of potential beneficial insectary plant species to adult hoverfly (Diptera: Syrphidae) fitness. Biol Control. 2012:61(1):1–6. 10.1016/j.biocontrol.2011.12.010 [DOI] [Google Scholar]
- Leather SR. 1995. Factors affecting fecundity, fertility, oviposition, and larviposition in insects. In: Leather SR, Hardie J, editors. Insect reproduction. CRC Press. p. 143–174; p. 32. [Google Scholar]
- Leman A, Mouratidis A, Pijnakker J, Vervoorn K, Wäckers F, Messelink GJ.. Sugar and pollen supply enhances aphid control by hoverflies in strawberry. Biol Control. 2023:186:105347. 10.1016/j.biocontrol.2023.105347 [DOI] [Google Scholar]
- Lillo I, Perez-Bañón C, Rojo S.. Life cycle, population parameters, and predation rate of the hover fly Eupeodes corollae fed on the aphid Myzus persicae. Entomol Exp Appl. 2021:169(11):1027–1038. 10.1111/eea.13090 [DOI] [Google Scholar]
- Lucas E, Brodeur J.. A fox in sheep’s clothing: furtive predators benefit from the communal defense of their prey. Ecology. 2001:82(11):3246–3250. 10.2307/2679847 [DOI] [Google Scholar]
- Meseguer R, Levi-Mourao A, Fournier M, Pons X, Lucas E.. May predator body-size hamper furtive predation strategy by aphidophagous insects? PLoS One. 2021:16(9):e0256991. 10.1371/journal.pone.0256991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messelink GJ, Calvo FJ, Marín F, Janssen D.. Cucurbits. In: Gullino ML, Albajes R, Nicot PC, editors. Integrated pest and disease management in greenhouse crops. Cham: Springer International Publishing. (Plant Pathology in the 21st Century); 2020. [accessed 2022 Oct 17]. p. 537–566. 10.1007/978-3-030-22304-5_19 [DOI] [Google Scholar]
- Moetamedinia B, Sahraghard A, Salehi L, Jalali-Sendi J.. Biology of Sphaerophoria scripta (Dip.: Syrphidae) in laboratory conditions. J Entomol Soc Iran. 2004:23(2):33–43. [Google Scholar]
- Noma T, Gratton C, Colunga-Garcia M, Brewer MJ, Mueller EE, Wyckhuys KAG, Heimpel GE, O’Neal ME.. Relationship of Soybean Aphid (Hemiptera: Aphididae) to Soybean plant nutrients, landscape structure, and natural enemies. Environ Entomol. 2010:39(1):31–41. 10.1603/EN09073 [DOI] [PubMed] [Google Scholar]
- Orengo-Green JJ, Casas JL, Marcos-García MA.. Effect of abiotic climatic factors on the gonadal maturation of the biocontrol agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Insects. 2022:13(7):573. 10.3390/insects13070573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara TY, Fournier M, Rojo S, Lucas E.. Development cycle of a potential biocontrol agent: the American hoverfly, Eupeodes americanus, and comparison with the commercial biocontrol agent Aphidoletes aphidimyza. Entomol Exp Appl. 2022:170(5):394–401. 10.1111/eea.13152 [DOI] [Google Scholar]
- Pekas A, De Craecker I, Boonen S, Wäckers FL, Moerkens R.. One stone; two birds: concurrent pest control and pollination services provided by aphidophagous hoverflies. Biol Control. 2020:149:104328. 10.1016/j.biocontrol.2020.104328 [DOI] [Google Scholar]
- Pineda A, Marcos-García MA.. Introducing barley as aphid reservoir in sweet-pepper greenhouses: effects on native and released hoverflies (Diptera: Syrphidae). EJE. 2008:105(3):531–535. 10.14411/eje.2008.070 [DOI] [Google Scholar]
- Pinheiro LA, Torres L, Raimundo J, Santos SAP.. Effect of floral resources on longevity and nutrient levels of Episyrphus balteatus (Diptera: Syrphidae). Biol Control. 2013:67(2):178–185. 10.1016/j.biocontrol.2013.07.010 [DOI] [Google Scholar]
- Pinheiro LA, Torres LM, Raimundo J, Santos SAP.. Effects of pollen, sugars and honeydew on lifespan and nutrient levels of Episyrphus balteatus. BioControl. 2015:60(1):47–57. 10.1007/s10526-014-9621-8 [DOI] [Google Scholar]
- Putra NS, Yasuda H.. Effects of prey species and its density on larval performance of two species of hoverfly larvae, Episyrphus balteatus de Geer and Eupeodes corollae Fabricius (Diptera: Syrphidae). Appl Entomol Zool. 2006:41(3):389–397. 10.1303/aez.2006.389 [DOI] [Google Scholar]
- R Foundation for Statistical Computing. R: A language and environment for statistical computing.Vienna, Austria: R Foundation for Statistical Computing. 2017https://www.Rproject.org/ [Google Scholar]
- Ramakers PMJ. PM Program for sweet pepper. In: Heinz KM, van Driesche RG, Parella MP, editors. Biocontrol in protected culture. Batavia: Ball Publishing; 2004. [accessed 2023 Nov 1]. https://www.cabdirect.org/cabdirect/abstract/20043186918 [Google Scholar]
- Rojo S, Fs G, Marcos-García M, Nieto JM, Mier Durante MP.. A world review of Predatory Hoverflies (Diptera, Syrphidae: Syrphinae) and their prey. Spain: CIBIOEdiciones; 2003. [Google Scholar]
- Rojo S, Hopper K, Marcos-García M.. Fitness of the hover flies Episyrphus balteatus and Eupeodes corollae faced with limited larval prey. Entomol Exp Appl. 1996:81(1):53–59. 10.1111/j.1570-7458.1996.tb02014.x [DOI] [Google Scholar]
- Sanchez JA, Cánovas F, Lacasa A.. Thresholds and management strategies for Aulacorthum solani (Hemiptera: Aphididae) in greenhouse pepper. J Econ Entomol. 2007:100(1):123–130. 10.1603/0022-0493(2007)100[123:tamsfa]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Schneider F. Bionomics and physiology of Aphidophagous Syrphidae. Annu Rev Entomol. 1969:14(1):103–124. 10.1146/annurev.en.14.010169.000535 [DOI] [Google Scholar]
- Scott SM, Barlow CA.. Effect of prey availability during development on the reproductive output of Metasyrphus corollae (Diptera: Syrphidae). Environ Entomol. 1984:13(3):669–674. 10.1093/ee/13.3.669 [DOI] [Google Scholar]
- Šešlija D, Tucić N.. Selection for developmental time in bean weevil (Acanthoscelides obtectus): correlated responses for other life history traits and genetic architecture of line differentiation. Entomol Exp Appl. 2003:106(1):19–35. 10.1046/j.1570-7458.2003.00007.x [DOI] [Google Scholar]
- Skevington JH, Locke MM, Young AD, Moran K, Crins WJ, Marshall SA.. 2019. Field guide to the flower flies of Northeastern North America. Princeton, New Jersey: Princeton University Press. [Google Scholar]
- Soleyman-Nezhadiyan E. The ecology of Melangyna viridiceps and Simosyrphus grandicornis (Diptera: Syrphidae) and their impact on populations of the rose aphid, Macrosiphum rosae/by Ebrahim Soleyman-Nezhadiyan [Thesis]. Adelaide, Australia: University of Adelaide; 1996. [accessed 2023 Sep 19]. https://digital.library.adelaide.edu.au/dspace/handle/2440/18976 [Google Scholar]
- Soleyman-Nezhadiyan E, Laughlin R.. Voracity of larvae, rate of development in eggs, larvae and pupae, and flight seasons of adults of the hoverflies Melangyna viridiceps Macquart and Symosyrphus grandicornis Macquart (Diptera: Syrphidae). Aust J Entomol. 1998:37(3):243–248. 10.1111/j.1440-6055.1998.tb01578.x [DOI] [Google Scholar]
- Sørensen CH, Toft S, Kristensen TN.. Cold-acclimation increases the predatory efficiency of the aphidophagous coccinellid Adalia bipunctata. Biol Control. 2013:65(1):87–94. 10.1016/j.biocontrol.2012.09.016 [DOI] [Google Scholar]
- Stiling P, Cornelissen T.. What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol Control. 2005:34(3):236–246. 10.1016/j.biocontrol.2005.02.017 [DOI] [Google Scholar]
- Tenhumberg B. Estimating Predatory Efficiency of Episyrphus balteatus (Diptera: Syrphidae) in Cereal Fields. Environ Entomol. 1995:24(3):687–691. 10.1093/ee/24.3.687 [DOI] [Google Scholar]
- van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A.. Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl. 2018:63(1):39–59. 10.1007/s10526-017-9801-4 [DOI] [Google Scholar]
- Vockeroth JR. The flower flies of the subfamily Syrphinae of Canada, Alaska, and Greenland: Diptera, Syrphidae. Ottawa (ON, Canada): Agriculture Canada (Insects and arachnids of Canada; 0706-7313;pt. 18); 1992. [accessed 2020 Apr 8]. https://catalog.hathitrust.org/Record/007471281 [Google Scholar]
- Watanabe H, Katayama N, Yano E, Sugiyama R, Nishikawa S, Endou T, Watanabe K, Takabayashi J, Ozawa R.. Effects of aphid honeydew sugars on the longevity and fecundity of the aphidophagous gall midge Aphidoletes aphidimyza. Biol Control. 2014:78:55–60. 10.1016/j.biocontrol.2014.07.007 [DOI] [Google Scholar]
- Zheng Y, Daane KM, Hagen KS, Mittler TE.. Influence of larval food consumption on the fecundity of the lacewing Chrysoperla carnea. Entomol Exp Appl. 1993:67(1):9–14. 10.1111/j.1570-7458.1993.tb01645.x [DOI] [Google Scholar]